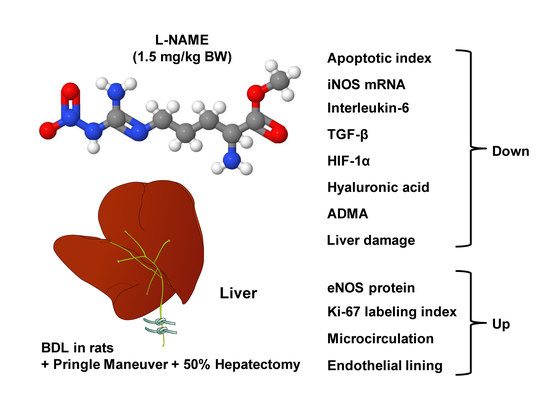
The Impact of a Nitric Oxide Synthase Inhibitor (L-NAME) on Ischemia–Reperfusion Injury of Cholestatic Livers by Pringle Maneuver and Liver Resection after Bile Duct Ligation in Rats
Abstract
1. Introduction
2. Results
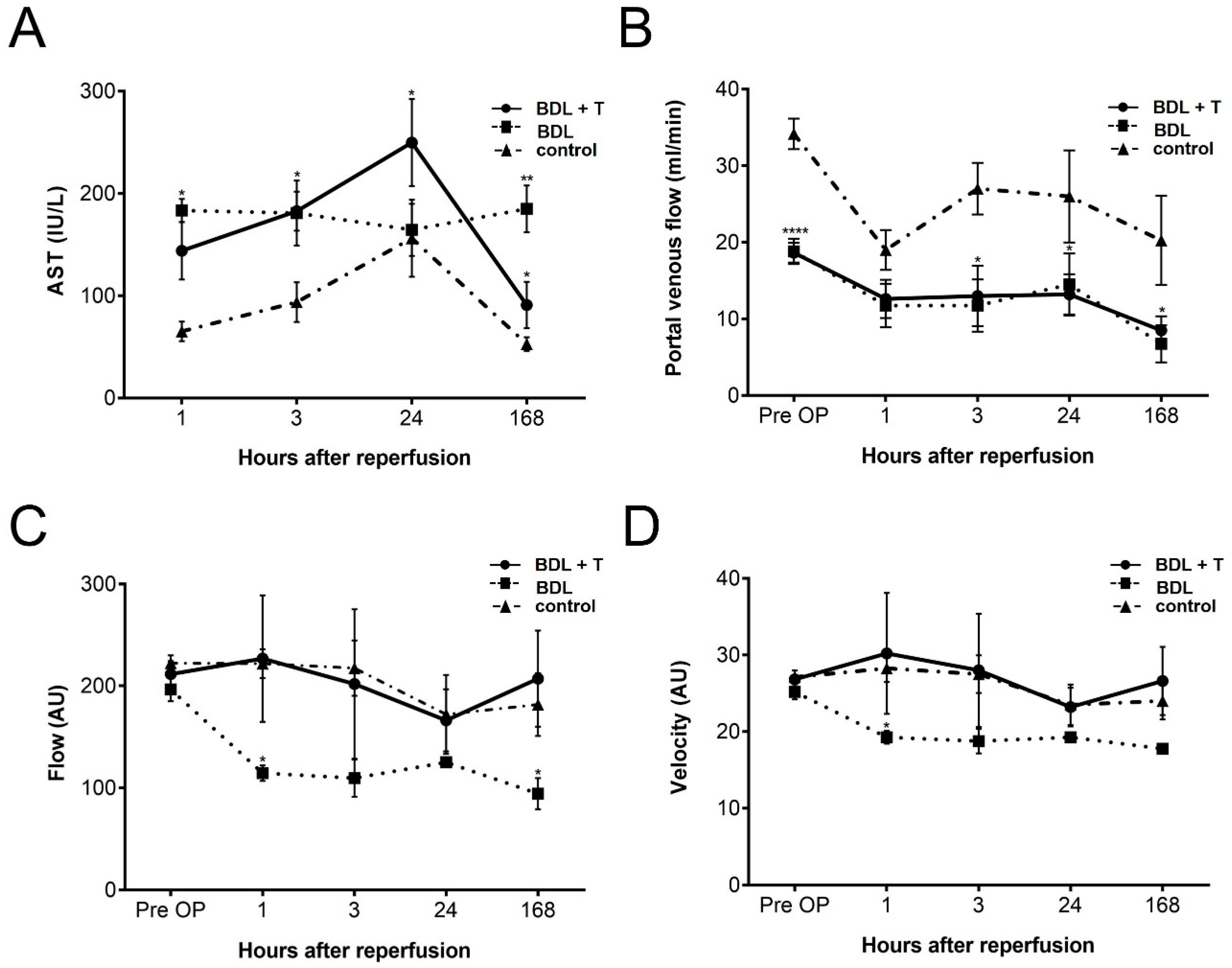
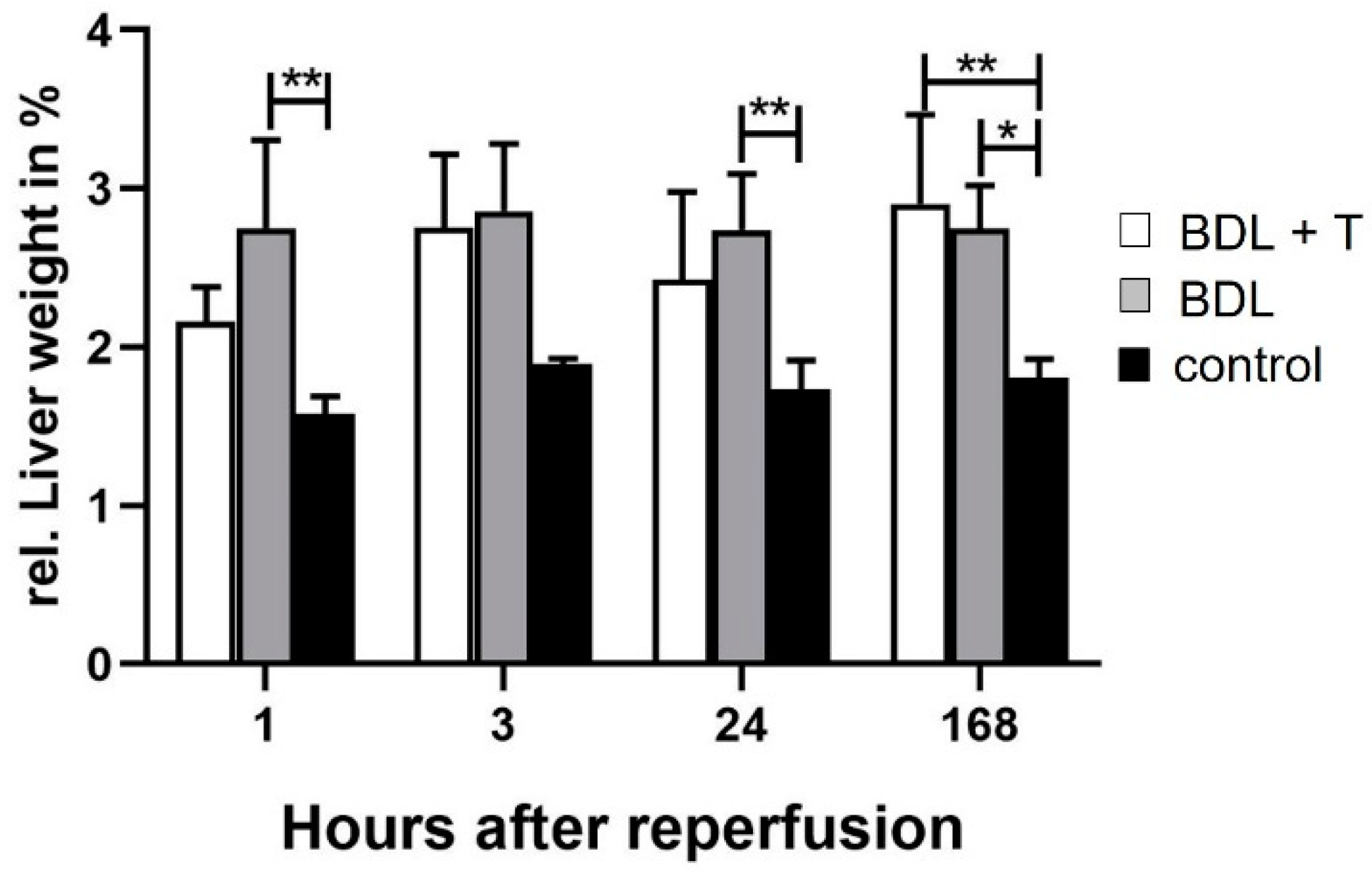
2.1. Hepatocellular Damage
2.2. Portal Venous Flow (PVF)
2.3. Microcirculation of the Liver
2.4. Lipid Peroxidation
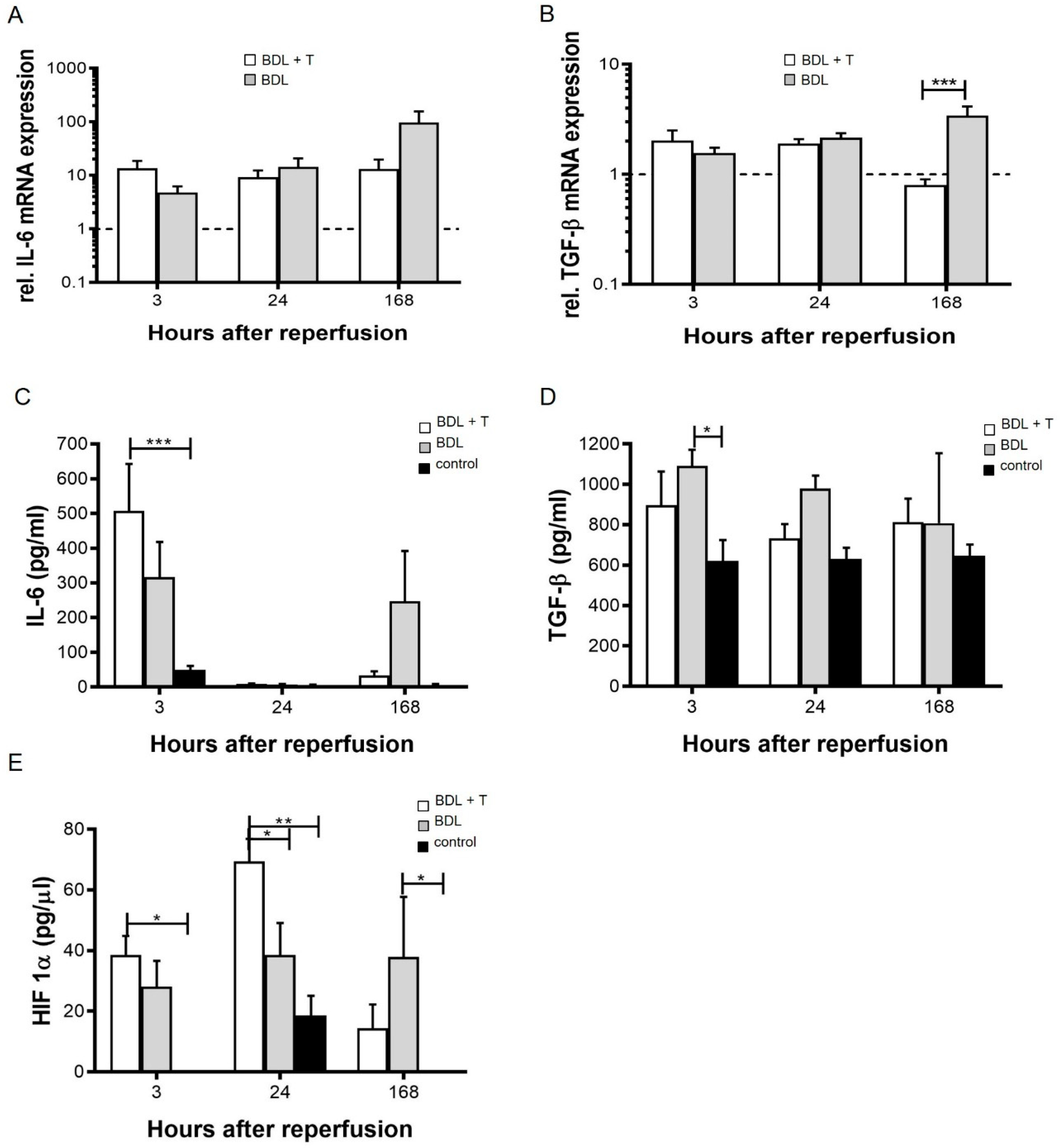
2.5. Pro-Inflammatory Cytokines
2.6. Serum HIF-1α Levels
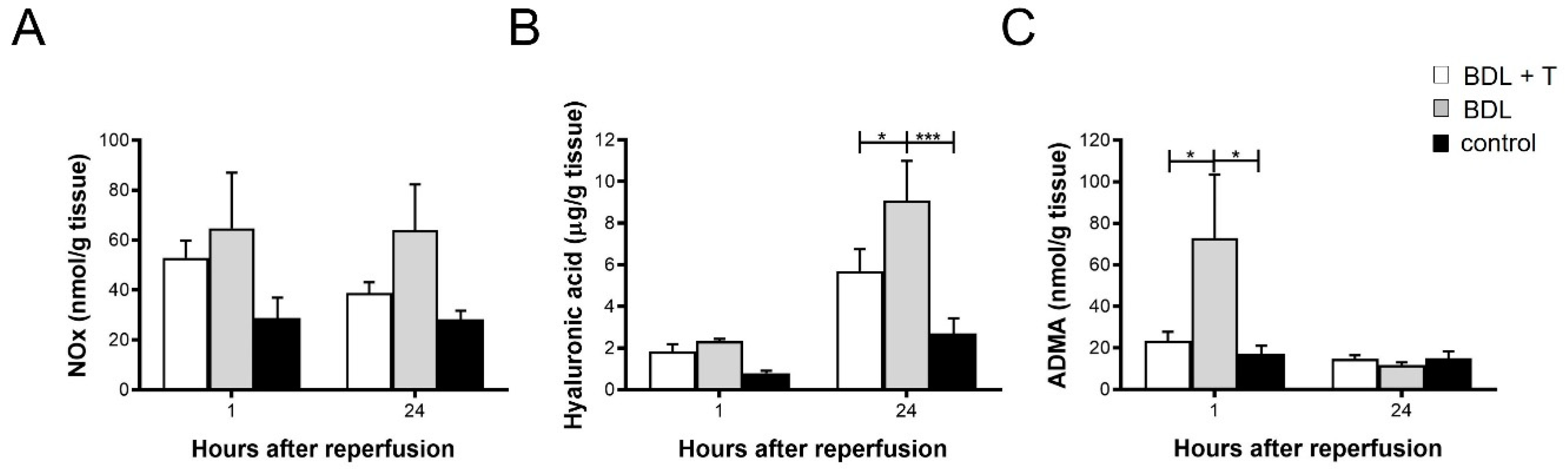
2.7. NOx Levels in Liver Tissue
2.8. Hepatic Hyaluronic Acid (HA) Levels
2.9. Asymmetric dimethylarginine (ADMA) in Liver Tissue
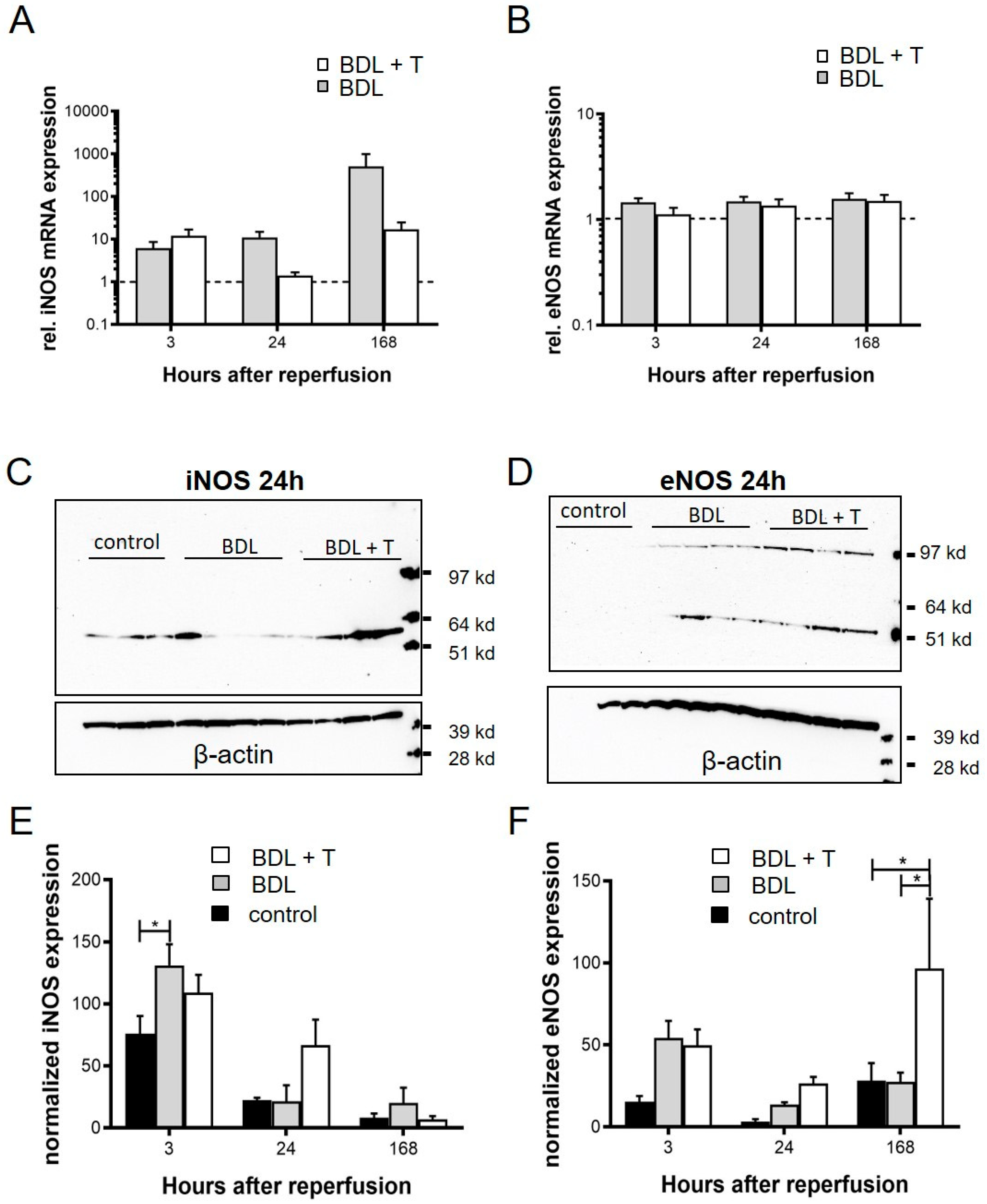
2.10. Expression of iNOS and eNOS
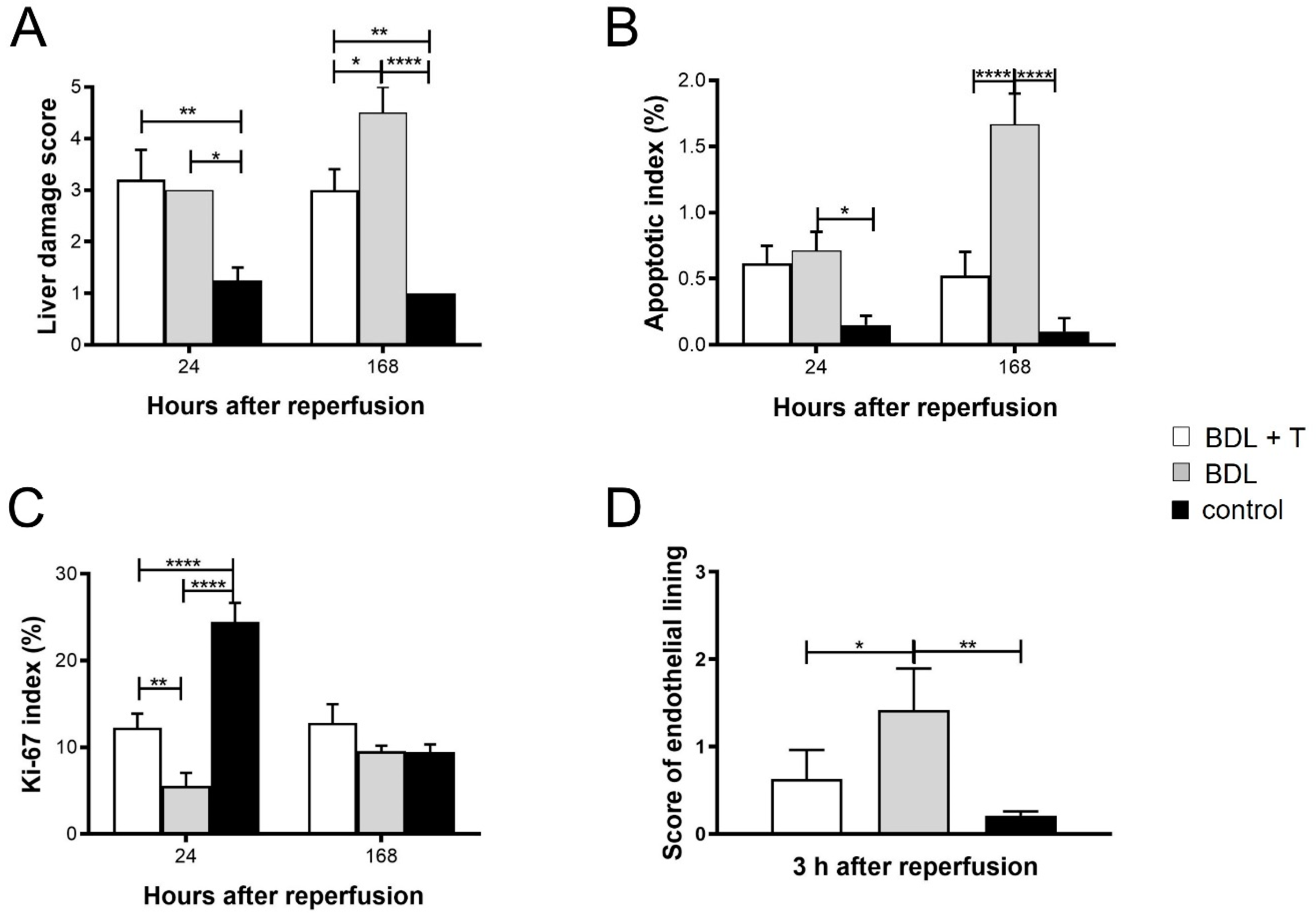
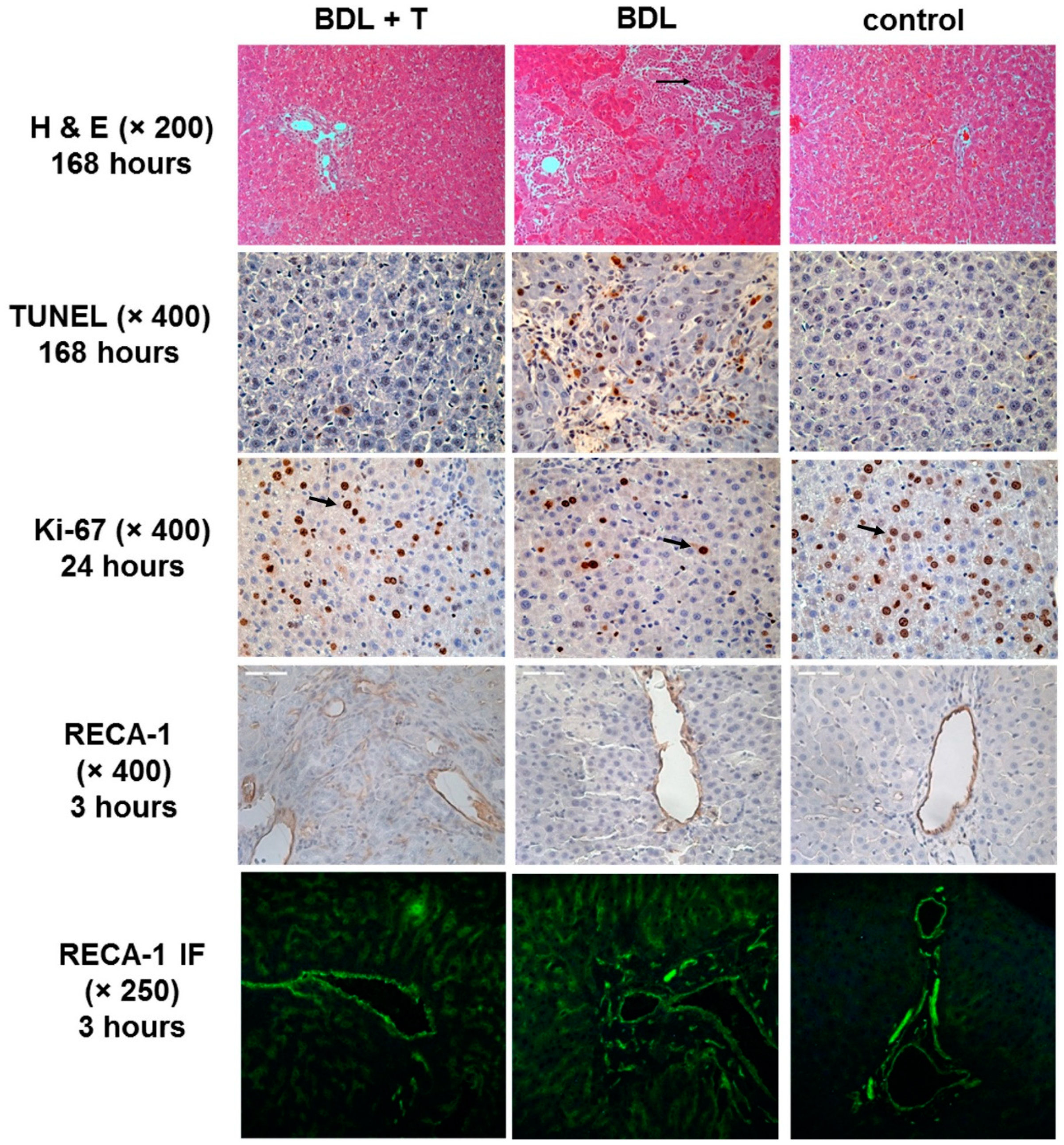
2.11. Histopathology and Immunohistochemistry
3. Discussion
4. Limitations of the Study
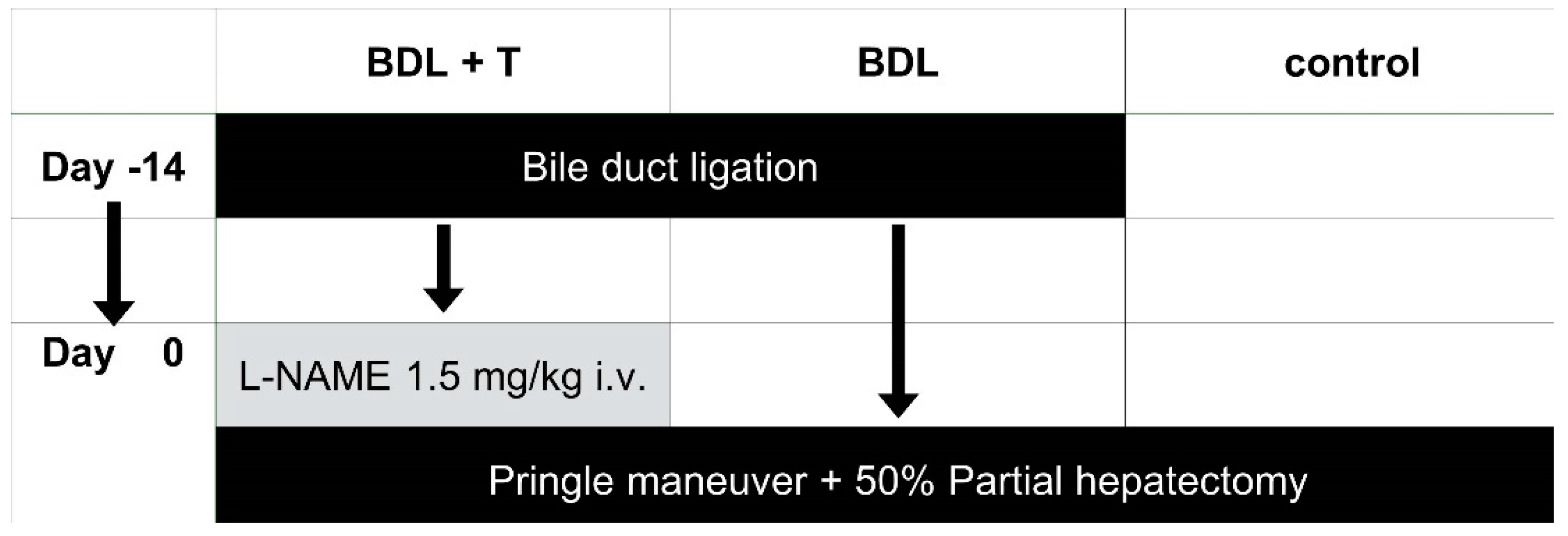
5. Materials and Methods
5.1. Ethical Approval
5.2. Animals
5.3. Surgical Procedure
5.3.1. Induction of Biliary Cirrhosis
5.3.2. Partial Hepatectomy and the IRI Experiment
5.4. Hepatocellular Damage
5.5. Measurement of Portal Venous Flow (PVF) and Microcirculation of Liver
5.6. Lipid Peroxidation
5.7. Pro-Inflammatory Cytokines
5.8. Serum Hypoxia-Inducible Factor-1α (HIF-1α) Levels
5.9. mRNA Expression Analysis of iNOS, eNOS, IL-6 and TGF-β by Quantitative Real-Time PCR
5.10. NOx, Hyaluronic Acid (HA) and Asymmetric dimethylarginine (ADMA) in Liver Tissue
5.11. SDS-PAGE and Western Blot Analysis
5.12. Histopathology
5.13. TUNEL-Staining for Apoptosis Measurement
5.14. Immunohistochemistry (IHC) for Ki-67 and RECA-1
5.15. Immunofluorescence (IF) Detection of RECA-1
5.16. Statistical Analyses
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| AST | aspartate aminotransferase |
| BDL | Bile duct ligation |
| BDL + T | Bile duct ligation + Treatment (L-NAME) |
| cNOS | constitutively expressed NOS |
| DAB | 3,3-Diaminobenzidine |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transition |
| eNOS | Endothelial NOS |
| HA | Hyaluronic acid |
| HIF-1α | Hypoxia-inducible factor-1α |
| HSC | Hepatic stellate cells |
| IF | Immunofluoresence |
| IHC | Immunohistochemistry |
| IL-6 | Interleukin 6 |
| iNOS | Inducible NOS |
| IRI | Ischemia–reperfusion injury |
| L-NAME | N-Nitroarginine methyl ester |
| MDA | Malondialdehyde |
| mRNA | Messenger ribonucleic acid |
| nNOS | Neuronal NOS |
| NO | Nitric oxide |
| NOS | NO synthase(s) |
| PM | Pringle maneuver |
| PVF | Portal venous flow |
| RT-qPCR | Quantitative real-time polymerase chain reaction |
| RECA-1 | Rat endothelial cell antigen-1 |
| TGF-β | Transforming growth factor β |
References
- Jarnagin, W.R.; Gonen, M.; Fong, Y.; DeMatteo, R.P.; Ben-Porat, L.; Little, S.; Corvera, C.; Weber, S.; Blumgart, L.H. Improvement in perioperative outcome after hepatic resection: Analysis of 1,803 consecutive cases over the past decade. Ann. Surg. 2002, 236, 397–406. [Google Scholar] [CrossRef]
- Pringle, J.H.V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann. Surg. 1908, 48, 541–549. [Google Scholar] [CrossRef]
- Belghiti, J.; Noun, R.; Malafosse, R.; Jagot, P.; Sauvanet, A.; Pierangeli, F.; Marty, J.; Farges, O. Continuous versus intermittent portal triad clamping for liver resection: A controlled study. Ann. Surg. 1999, 229, 369–375. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Yoshimoto, J.; Miwa, K.; Sugo, H.; Kawasaki, S. Safety of prolonged intermittent pringle maneuver during hepatic resection. Arch. Surg. 2006, 141, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Teoh, N.C. Hepatic ischemia reperfusion injury: Contemporary perspectives on pathogenic mechanisms and basis for hepatoprotection-the good, bad and deadly. J. Gastroenterol Hepatol. 2011, 26 (Suppl. 1), 180–187. [Google Scholar] [CrossRef]
- Van Wagensveld, B.A.; van Gulik, T.M.; Gelderblom, H.C.; Scheepers, J.J.; Bosma, A.; Endert, E.; Obertop, H.; Gouma, D.J. Continuous or intermittent vascular clamping during hemihepatectomy in pigs: Hyaluronic acid kinetics in the assessment of early microvascular liver damage. Eur. J. Surg. 2000, 166, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Nakashima, K.; Tada, I.; Kawano, K.; Kobayashi, M. Prolonged normothermic ischaemia of human cirrhotic liver during hepatectomy: A preliminary report. Br. J. Surg. 1993, 80, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.T.; Lai, E.C.; Lo, C.M.; Ng, I.O.; Wong, J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch. Surg. 1995, 130, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Hwang, C.R.; Liu, T.J.; P’eng, F.K. Effects and limitations of prolonged intermittent ischaemia for hepatic resection of the cirrhotic liver. Br. J. Surg. 1996, 83, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Kopincová, J.; Púzserová, A.; Bernátová, I. L-NAME in the cardiovascular system -nitric oxide synthase activator? Pharmacol Rep. 2012, 64, 511–520. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef]
- Canbakan, B.; Akin, H.; Tahan, G.; Tarcin, O.; Eren, F.; Atug, O.; Tahan, V.; Imeryuz, N.; Yapicier, O.; Avsar, E.; Tozun, N. The effects of pegylated interferon alpha 2b on bile-duct ligation induced liver fibrosis in rats. Ann. Hepatol. 2009, 8, 234–240. [Google Scholar] [PubMed]
- Coban, S.; Yildiz, F.; Terzi, A.; Al, B.; Aksoy, N.; Bitiren, M.; Celik, H. The effects of Nigella sativa on bile duct ligation induced-liver injury in rats. Cell Biochem. Funct. 2010, 28, 83–88. [Google Scholar] [CrossRef]
- Tag, C.G.; Weiskirchen, S.; Hittatiya, K.; Tacke, F.; Tolba, R.H.; Weiskirchen, R. Induction of experimental obstructive cholestasis in mice. Lab Anim. 2015, 49 (Suppl. 1), 70–80. [Google Scholar] [CrossRef]
- Tag, C.G.; Sauer-Lehnen, S.; Weiskirchen, S.; Borkham-Kamphorst, E.; Tolba, R.H.; Tacke, F.; Weiskirchen, R. Bile duct ligation in mice: Induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J. Vis. Exp. 2015, 96, e52438. [Google Scholar] [CrossRef]
- Giraldez, R.R.; Panda, A.; Xia, Y.; Sanders, S.P.; Zweier, J.L. Decreased nitric-oxide synthase activity causes impaired endothelium-dependent relaxation in the postischemic heart. J. Biol. Chem. 1997, 272, 21420–21426. [Google Scholar] [CrossRef]
- Goldstein, M.J.; Salame, E.; Kapur, S.; Kinkhabwala, M.; LaPointe-Rudow, D.; Harren, N.P.P.; Lobritto, S.J.; Russo, M.; Brown, R.S., Jr.; Cataldegirmen, G.; et al. Analysis of failure in living donor liver transplantation: Differential outcomes in children and adults. World J. Surg. 2003, 27, 356–364. [Google Scholar] [CrossRef]
- Glanemann, M.; Eipel, C.; Nussler, A.K.; Vollmar, B.; Neuhaus, P. Hyperperfusion syndrome in small-for-size livers. Eur. Surg. Res. 2005, 37, 335–341. [Google Scholar] [CrossRef]
- Eipel, C.; Glanemann, M.; Nuessler, A.K.; Menger, M.D.; Neuhaus, P.; Vollmar, B. Ischemic preconditioning impairs liver regeneration in extended reduced-size livers. Ann. Surg. 2005, 241, 477–484. [Google Scholar] [CrossRef]
- Dahm, F.; Georgiev, P.; Clavien, P.A. Small-for-size syndrome after partial liver transplantation: Definition, mechanisms of disease and clinical implications. Am. J. Transplant. 2005, 5, 2605–2610. [Google Scholar] [CrossRef]
- Demetris, A.J.; Kelly, D.M.; Eghtesad, B.; Fontes, P.; Wallis, M.J.; Tom, K.; Tan, H.P.; Shaw-Stiffel, T.; Boig, L.; Novelli, P.; et al. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am. J. Surg. Pathol. 2006, 30, 986–993. [Google Scholar] [CrossRef]
- Pan, G.D.; Yan, L.N. Problems in adult living donor liver transplantation using the right hepatic lobe. Hepatobiliary Pancreat. Dis. Int. 2006, 5, 345–349. [Google Scholar] [PubMed]
- Lo, C.M.; Liu, C.L.; Fan, S.T. Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft-successful treatment with splenic artery ligation. Liver Transpl. 2003, 9, 626–628. [Google Scholar] [CrossRef]
- Troisi, R.; Ricciardi, S.; Smeets, P.; Petrovic, M.; Van Maele, G.; Colle, I.; Van Vlierberghe, H.; De Hemptinne, B. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am. J. Transplant. 2005, 5, 1397–1404. [Google Scholar] [CrossRef]
- Koeppel, T.A.; Thies, J.C.; Schemmer, P.; Trauner, M.; Gebhard, M.M.; Otto, G.; Post, S. Inhibition of nitric oxide synthesis in ischemia/reperfusion of the rat liver is followed by impairment of hepatic microvascular blood flow. J. Hepatol. 1997, 27, 163–169. [Google Scholar] [CrossRef]
- Caban, A.; Oczkowicz, G.; Abdel-Samad, O.; Cierpka, L. Influence of ischemic preconditioning and nitric oxide on microcirculation and the degree of rat liver injury in the model of ischemia and reperfusion. Transplant. Proc. 2006, 38, 196–198. [Google Scholar] [CrossRef]
- Huang, T.P.; Nishida, T.; Kamike, W.; Kosaka, H.; Seiyama, A.; Morimoto, Y.; Tanaka, S.; Obunai, S.; Takei, Y.; Shiga, T.; et al. Role of nitric oxide in oxygen transport in rat liver sinusoids during endotoxemia. Hepatology 1997, 26, 336–342. [Google Scholar] [CrossRef]
- Meguro, M.; Katsuramaki, T.; Nagayama, M.; Kimura, H.; Isobe, M.; Kimura, Y.; Matsuno, T.; Nui, A.; Hirata, K. A novel inhibitor of inducible nitric oxide synthase (ONO-1714) prevents critical warm ischemia-reperfusion injury in the pig liver. Transplantation 2002, 73, 1439–1446. [Google Scholar] [CrossRef]
- Kukita, K.; Katsuramaki, T.; Kikuchi, H.; Meguro, M.; Nagayama, M.; Kimura, H.; Isobe, M.; Hirata, K. Remnant liver injury after hepatectomy with the pringle maneuver and its inhibition by an iNOS inhibitor (ONO-1714) in a pig model. J. Surg. Res. 2005, 125, 78–87. [Google Scholar] [CrossRef]
- Isobe, M.; Katsuramaki, T.; Hirata, K.; Kimura, H.; Nagayama, M.; Matsuno, T. Beneficial effects of inducible nitric oxide synthase inhibitor on reperfusion injury in the pig liver. Transplantation 1999, 68, 803–813. [Google Scholar] [CrossRef]
- Liu, P.; Xu, B.; Spokas, E.; Lai, P.S.; Wong, P.Y. Role of endogenous nitric oxide in TNF-alpha and IL-1beta generation in hepatic ischemia-repefusion. Shock 2000, 13, 217–223. [Google Scholar] [CrossRef]
- Shah, V.; Chen, A.F.; Cao, S.; Hendrickson, H.; Weiler, D.; Smith, L.; Yao, J.; Katusic, Z.S. Gene transfer of recombinant endothelial nitric oxide synthase to liver in vivo and in vitro. Am. J. Physiol. Gastrointest Liver Physiol. 2000, 279, G1023–G1030. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kawashima, S.; Ohashi, Y.; Ozaki, M.; Rikitake, Y.; Inoue, N.; Hirata, K.; Akita, H.; Yokoyama, M. Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. Hypertension 2000, 36, 97–102. [Google Scholar] [CrossRef]
- Rockey, D.C.; Chung, J.J. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: Endothelial dysfunction in portal hypertension. Gastroenterology 1998, 114, 344–351. [Google Scholar] [CrossRef]
- Shah, V.; Toruner, M.; Haddad, F.; Cadelina, G.; Papapetropoulos, A.; Choo, K.; Sessa, W.C.; Groszmann, R.J. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 1999, 117, 1222–1228. [Google Scholar] [CrossRef]
- Sarela, A.I.; Mihaimeed, F.M.; Batten, J.J.; Davidson, B.R.; Mathie, R.T. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut 1999, 44, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Meguro, M.; Katsuramaki, T.; Kimura, H.; Isobe, M.; Nagayama, M.; Kukita, K.; Nui, A.; Hirata, K. Apoptosis and necrosis after warm ischemia-reperfusion injury of the pig liver and their inhibition by ONO-1714. Transplantation 2003, 75, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Katsuramaki, T.; Isobe, M.; Nagayama, M.; Meguro, M.; Kukita, K.; Nui, A.; Hirata, K. Role of inducible nitric oxide synthase in pig liver transplantation. J. Surg. Res. 2003, 111, 28–37. [Google Scholar] [CrossRef]
- Duranski, M.R.; Elrod, J.W.; Calvert, J.W.; Bryan, N.S.; Feelisch, M.; Lefer, D.J. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2980–H2986. [Google Scholar] [CrossRef] [PubMed]
- Behar-Cohen, F.F.; Heydolph, S.; Faure, V.; Droy-Lefaix, M.T.; Courtois, Y.; Goureau, O. Peroxynitrite cytotoxicity on bovine retinal pigmented epithelial cells in culture. Biochem. Biophys. Res. Commun. 1996, 226, 842–849. [Google Scholar] [CrossRef]
- Fan, C.; Zwacka, R.M.; Engelhardt, J.F. Therapeutic approaches for ischemia/reperfusion injury in the liver. J. Mol. Med. (Berl.) 1999, 77, 577–592. [Google Scholar] [CrossRef]
- Li, J.; Billiar, T.R. Nitric Oxide. IV. Determinants of nitric oxide protection and toxicity in liver. Am. J. Physiol. 1999, 276, G1069–G1073. [Google Scholar] [CrossRef]
- Suzuki, Y.; Deitch, E.A.; Mishima, S.; Lu, Q.; Xu, D. Inducible nitric oxide synthase gene knockout mice have increased resistance to gut injury and bacterial translocation after an intestinal ischemia-reperfusion injury. Crit. Care Med. 2000, 28, 3692–3696. [Google Scholar] [CrossRef]
- Schoen, J.M.; Wang, H.H.; Minuk, G.Y.; Lautt, W.W. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric. Oxide 2001, 5, 453–464. [Google Scholar] [CrossRef]
- Schoen, J.M.; Lautt, W.W. iNOS is not involved in shear stress-induced nitric oxide release, which triggers the liver regeneration cascade. Proc. West Pharmacol. Soc. 2001, 44, 181–182. [Google Scholar] [PubMed]
- Uzun, H.; Simsek, G.; Aydin, S.; Unal, E.; Karter, Y.; Yelmen, N.K.; Vehid, S.; Curgunlu, A.; Kaya, S. Potential effects of L-NAME on alcohol-induced oxidative stress. World J. Gastroenterol 2005, 11, 600–604. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Invest. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar] [CrossRef]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 2005, 115, 56–65. [Google Scholar] [CrossRef]
- Rivera, C.A.; Bradford, B.U.; Hunt, K.J.; Adachi, Y.; Schrum, L.W.; Koop, D.R.; Burchardt, E.R.; Rippe, R.A.; Thurman, R.G. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am. J. Physiol. Gastrointest Liver Physiol. 2001, 281, G200–G207. [Google Scholar] [CrossRef]
- Friedman, S.L.; Arthur, M.J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J. Clin. Invest. 1989, 84, 1780–1785. [Google Scholar] [CrossRef]
- Gabele, E.; Brenner, D.A.; Rippe, R.A. Liver fibrosis: Signals leading to the amplification of the fibrogenic hepatic stellate cell. Front. Biosci. 2003, 8, d69–d77. [Google Scholar] [CrossRef]
- Milani, S.; Herbst, H.; Schuppan, D.; Kim, K.Y.; Riecken, E.O.; Stein, H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology 1990, 98, 175–184. [Google Scholar] [CrossRef]
- Marra, F. Hepatic stellate cells and the regulation of liver inflammation. J. Hepatol. 1999, 31, 1120–1130. [Google Scholar] [CrossRef]
- Gressner, A.M.; Weiskirchen, R.; Breitkopf, K.; Dooley, S. Roles of TGF-beta in hepatic fibrosis. Front. Biosci. 2002, 7, d793–d807. [Google Scholar] [CrossRef]
- Schnabl, B.; Kweon, Y.O.; Frederick, J.P.; Wang, X.F.; Rippe, R.A.; Brenner, D.A. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology 2001, 34, 89–100. [Google Scholar] [CrossRef]
- Dooley, S.; Hamzavi, J.; Breitkopf, K.; Wiercinska, E.; Said, H.M.; Lorenzen, J.; Ten, D.P.; Gressner, A.M. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology 2003, 125, 178–191. [Google Scholar] [CrossRef]
- Shek, F.W.; Benyon, R.C. How can transforming growth factor beta be targeted usefully to combat liver fibrosis? Eur. J. Gastroenterol Hepatol. 2004, 16, 123–126. [Google Scholar] [CrossRef]
- Tolba, R.H.; Schildberg, F.A.; Decker, D.; Abdullah, Z.; Buttner, R.; Minor, T.; von Ruecker, A. Mechanisms of improved wound healing in Murphy Roths Large (MRL) mice after skin transplantation. Wound Repair Regen. 2010, 18, 662–670. [Google Scholar] [CrossRef]
- Nanashima, A.; Yamaguchi, H.; Shibasaki, S.; Sawai, T.; Yamaguchi, E.; Yasutake, T.; Tsuji, T.; Jibiki, M.; Nakagoe, T.; Ayabe, H. Measurement of serum hyaluronic acid level during the perioperative period of liver resection for evaluation of functional liver reserve. J. Gastroenterol Hepatol 2001, 16, 1158–1163. [Google Scholar] [CrossRef]
- Pungpapong, S.; Nunes, D.P.; Krishna, M.; Nakhleh, R.; Chambers, K.; Ghabril, M.; Dickson, R.C.; Hughes, C.B.; Steers, J.; Nguyen, J.H.; et al. Serum fibrosis markers can predict rapid fibrosis progression after liver transplantation for hepatitis C. Liver Transpl. 2008, 14, 1294–1302. [Google Scholar] [CrossRef]
- Valva, P.; Casciato, P.; Carrasco, J.M.D.; Gadano, A.; Galdame, O.; Galoppo, M.C.; Mullen, E.; De, M.E.; Preciado, M.V. The role of serum biomarkers in predicting fibrosis progression in pediatric and adult hepatitis C virus chronic infection. PLoS ONE 2011, 6, e23218. [Google Scholar] [CrossRef]
- Loor, G.; Schumacker, P.T. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ. 2008, 15, 686–690. [Google Scholar] [CrossRef]
- Olson, N.; van der Vliet, A. Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric. Oxide 2011, 25, 125–137. [Google Scholar] [CrossRef]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Cohen, C.D.; et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 2007, 117, 3810–3820. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Harokopos, V.; Paparountas, T.; Oikonomou, N.; Chatziioannou, A.; Vilaras, G.; Tsiambas, E.; Karameris, A.; Bouros, D.; Aidinis, V. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis. Am. J. Respir. Crit. Care Med. 2007, 176, 1108–1119. [Google Scholar] [CrossRef]
- Moon, J.O.; Welch, T.P.; Gonzalez, F.J.; Copple, B.L. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am. J. Physiol. Gastrointest Liver Physiol. 2009, 296, G582–G592. [Google Scholar] [CrossRef]
- Albina, J.E.; Reichner, J.S. Oxygen and the regulation of gene expression in wounds. Wound Repair Regen. 2003, 11, 445–451. [Google Scholar] [CrossRef]
- Botusan, I.R.; Sunkari, V.G.; Savu, O.; Catrina, A.I.; Grunler, J.; Lindberg, S.; Pereira, T.; Yla-Herttuala, S.; Poellinger, L.; Brismar, K.; et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. USA 2008, 105, 19426–19431. [Google Scholar] [CrossRef]
- Willis, B.C.; Borok, Z. TGF-beta-induced EMT: Mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L525–L534. [Google Scholar] [CrossRef]
- Copple, B.L. Hypoxia stimulates hepatocyte epithelial to mesenchymal transition by hypoxia-inducible factor and transforming growth factor-beta-dependent mechanisms. Liver Int. 2010, 30, 669–682. [Google Scholar] [CrossRef]
- Zeisberg, M.; Yang, C.; Martino, M.; Duncan, M.B.; Rieder, F.; Tanjore, H.; Kalluri, R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem. 2007, 282, 23337–23347. [Google Scholar] [CrossRef]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Teerlink, T.; Siroen, M.P.; van Lambalgen, A.A.; Rauwerda, J.A.; van Leeuwen, P.A. The liver is an important organ in the metabolism of asymmetrical dimethylarginine (ADMA). Clin. Nutr. 2003, 22, 17–22. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Teerlink, T.; Van Der, H.B.; Siroen, M.P.; Kuik, D.J.; Rauwerda, J.A.; van Leeuwen, P.A. Asymmetrical dimethylarginine (ADMA) in critically ill patients: High plasma ADMA concentration is an independent risk factor of ICU mortality. Clin. Nutr. 2003, 22, 23–30. [Google Scholar] [CrossRef]
- Ferrigno, A.; Palladini, G.; Bianchi, A.; Rizzo, V.; Di Pasqua, L.G.; Perlini, S.; Richelmi, P.; Vairetti, M. Lobe-specific heterogeneity in assymetric dimethlyarginines and matriy metalloproteinase levels in a rat model of obstructive cholestasis. Biomed. Res. Int. 2014, 327537. [Google Scholar]
- Ferrigno, A.; Di Pasqua, L.G.; Berardo, C.; Rizzo, V.; Richelmi, P.; Vairetti, M. Changes in Biliary Levels of Arginien and its Methylated Derviates after Hepatic Ischaemia/Reperfusion. Basic Clin. Pharmacol. Toxicol. 2016, 119, 101–109. [Google Scholar] [CrossRef]
- Laleman, W.; Omasta, A.; Van de Casteele, M.; Zeegers, M.; Vander Elst, I.; Van Landeghem, L.; Severi, T.; van Pelt, J.; Roskams, T.; Fevery, J.; et al. A role for asymmetric dimethylarginine in the pathophysiology of portal hypertension in rats with biliary cirrhosis. Hepatology 2005, 42, 1382–1390. [Google Scholar] [CrossRef]
- Srinivasan, P.K.; Yagi, S.; Doorschodt, B.; Nagai, K.; Afify, M.; Uemoto, S.; Tolba, R. Impact of venous systemic oxygen persufflation supplemented with nitric oxide gas on cold-stored, warm ischemia-damaged experimental liver grafts. Liver Transpl. 2012, 18, 219–225. [Google Scholar] [CrossRef]
- Yagi, S.; Doorschodt, B.M.; Afify, M.; Klinge, U.; Kobayashi, E.; Uemoto, S.; Tolba, R.H. Improved preservation and microcirculation with POLYSOL after partial liver transplantation in rats. J. Surg. Res. 2011, 167, e375–e383. [Google Scholar] [CrossRef]
- Nagai, K.; Yagi, S.; Afify, M.; Bleilevens, C.; Uemoto, S.; Tolba, R.H. Impact of venous-systemic oxygen persufflation with nitric oxide gas on steatotic grafts after partial orthotopic liver transplantation in rats. Transplantation 2013, 95, 78–84. [Google Scholar] [CrossRef] [PubMed]
- ImageJ. Available online: https://imagej.net (accessed on 19 April 2019).
- Song, S.W.; Tolba, R.H.; Yonezawa, K.; Manekeller, S.; Minor, T. Exogenous superoxide dismutase prevents peroxynitrite-induced apoptosis in non-heart-beating donor livers. Eur. Surg. Res. 2008, 41, 353–361. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, J.; Afify, M.; Bleilevens, C.; Klinge, U.; Weiskirchen, R.; Steitz, J.; Vogt, M.; Yagi, S.; Nagai, K.; Uemoto, S.; et al. The Impact of a Nitric Oxide Synthase Inhibitor (L-NAME) on Ischemia–Reperfusion Injury of Cholestatic Livers by Pringle Maneuver and Liver Resection after Bile Duct Ligation in Rats. Int. J. Mol. Sci. 2019, 20, 2114. https://doi.org/10.3390/ijms20092114
Iwasaki J, Afify M, Bleilevens C, Klinge U, Weiskirchen R, Steitz J, Vogt M, Yagi S, Nagai K, Uemoto S, et al. The Impact of a Nitric Oxide Synthase Inhibitor (L-NAME) on Ischemia–Reperfusion Injury of Cholestatic Livers by Pringle Maneuver and Liver Resection after Bile Duct Ligation in Rats. International Journal of Molecular Sciences. 2019; 20(9):2114. https://doi.org/10.3390/ijms20092114
Chicago/Turabian StyleIwasaki, Junji, Mamdouh Afify, Christian Bleilevens, Uwe Klinge, Ralf Weiskirchen, Julia Steitz, Michael Vogt, Shintaro Yagi, Kazuyuki Nagai, Shinji Uemoto, and et al. 2019. "The Impact of a Nitric Oxide Synthase Inhibitor (L-NAME) on Ischemia–Reperfusion Injury of Cholestatic Livers by Pringle Maneuver and Liver Resection after Bile Duct Ligation in Rats" International Journal of Molecular Sciences 20, no. 9: 2114. https://doi.org/10.3390/ijms20092114
APA StyleIwasaki, J., Afify, M., Bleilevens, C., Klinge, U., Weiskirchen, R., Steitz, J., Vogt, M., Yagi, S., Nagai, K., Uemoto, S., & Tolba, R. H. (2019). The Impact of a Nitric Oxide Synthase Inhibitor (L-NAME) on Ischemia–Reperfusion Injury of Cholestatic Livers by Pringle Maneuver and Liver Resection after Bile Duct Ligation in Rats. International Journal of Molecular Sciences, 20(9), 2114. https://doi.org/10.3390/ijms20092114