Chiral Recognition of Hexahelicene on a Surface via the Forming of Asymmetric Heterochiral Trimers
Abstract
1. Introduction
2. Results and Discussion
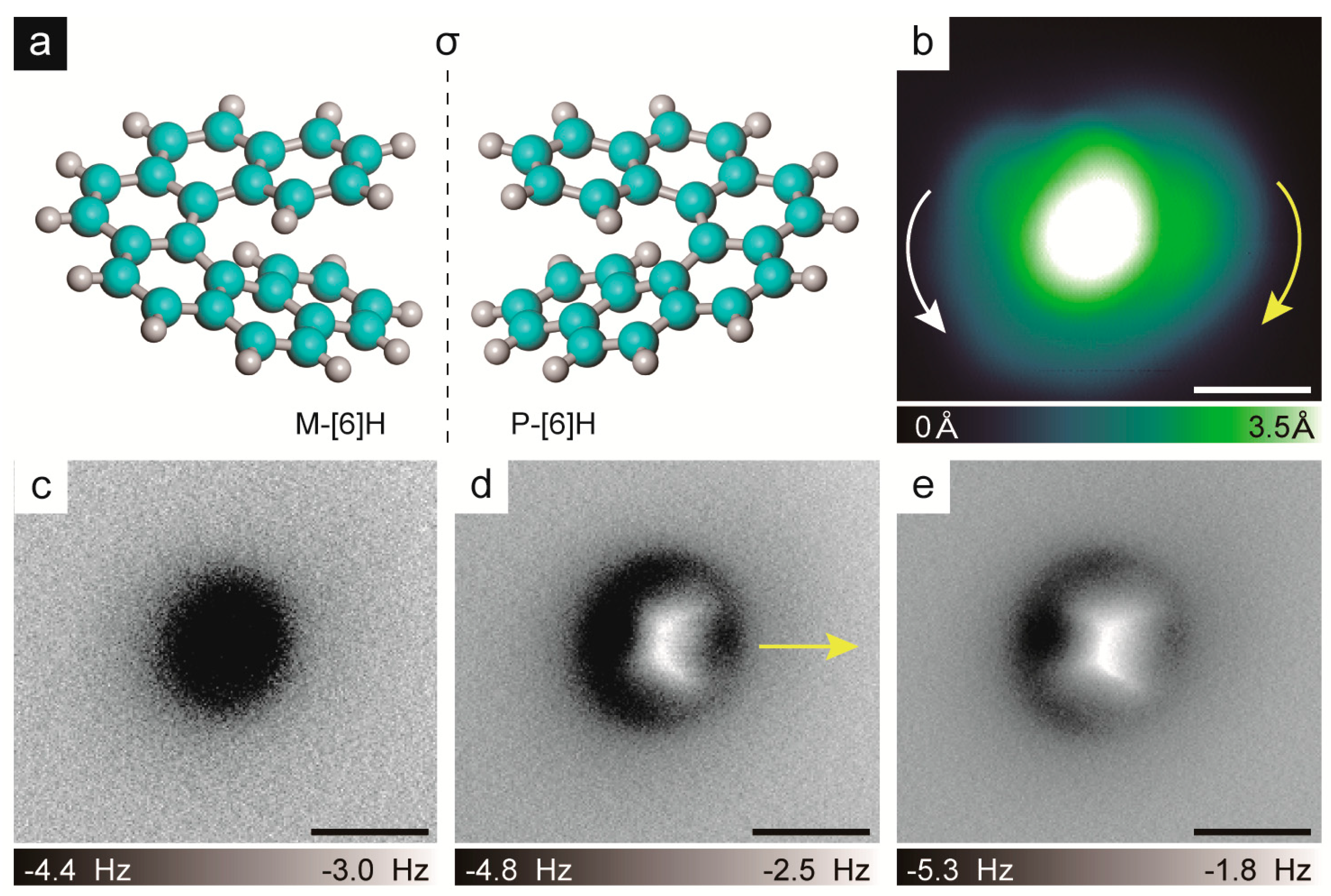
2.1. Chirality Determination Method
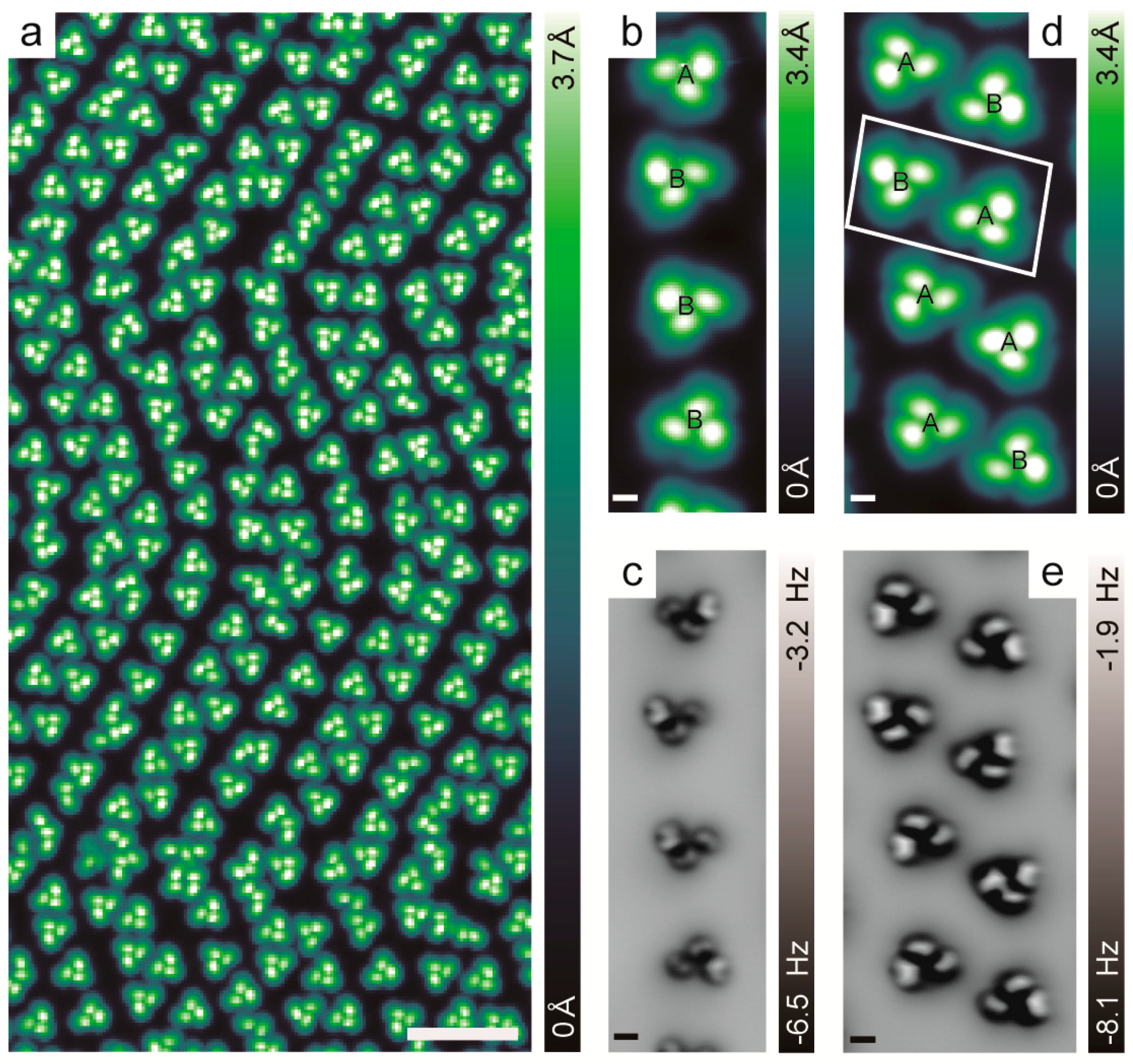
2.2. Chiral Recognition of Hexahelicene ([6]H)
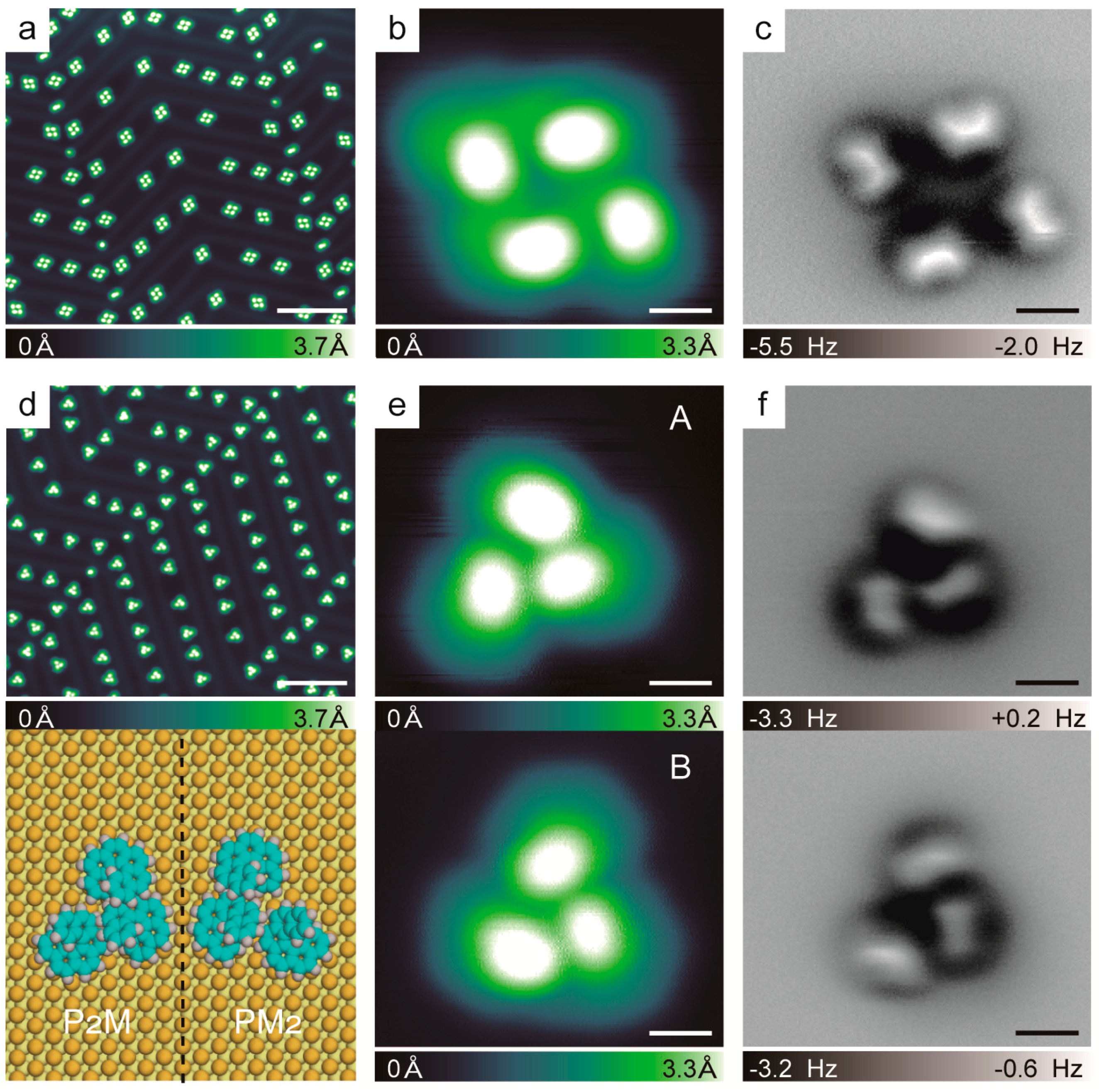
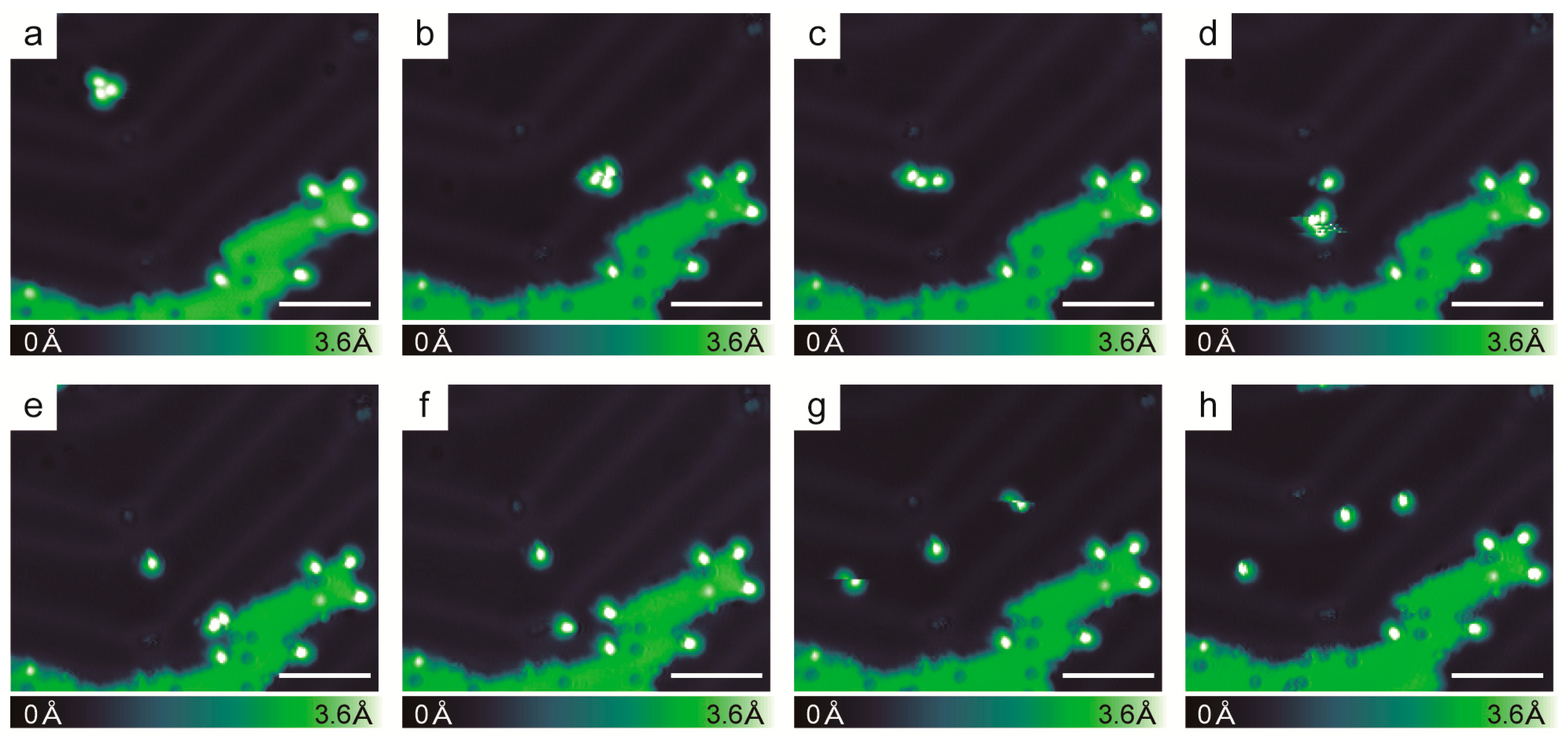
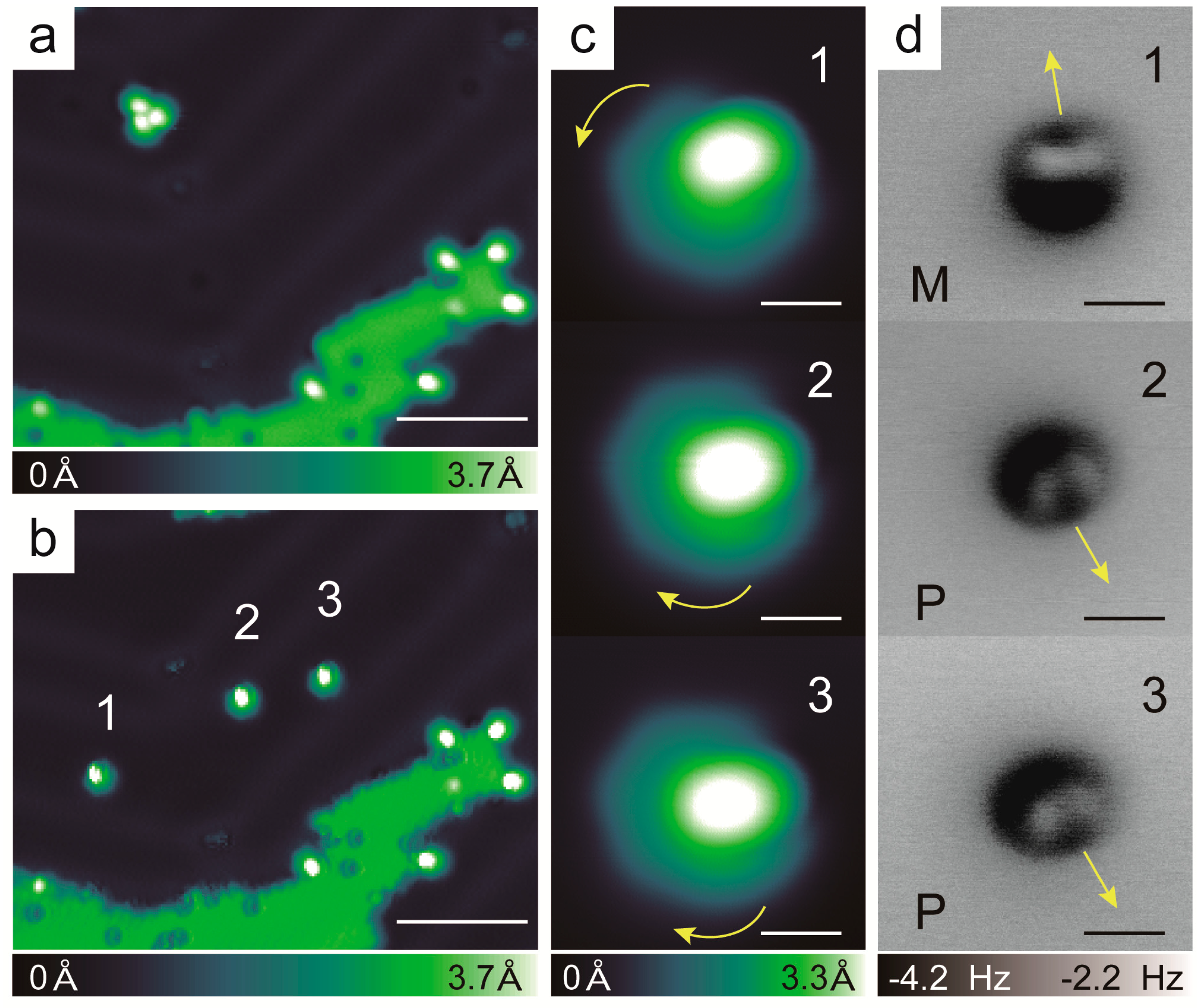
2.3. Chirality Composition of the Trimer
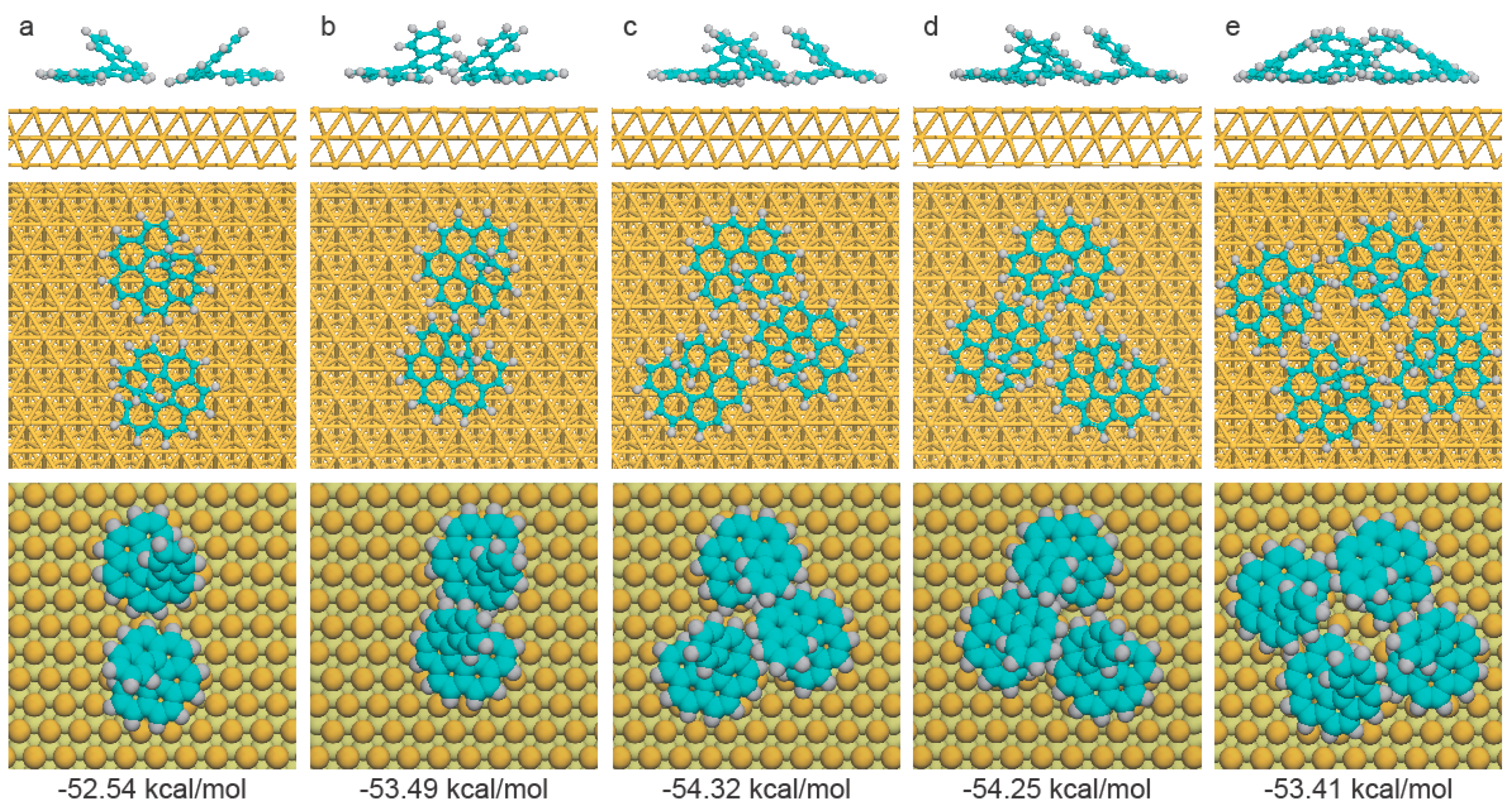
2.4. Theoretical Calculations
2.5. Racemic Hexahelicene Self-Assembly at High Coverage
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| [5]H | pentahelicene |
| [6]H | hexahelicene |
| [7]H | heptahelicene |
| rac | racemic |
| STM | scanning tunneling microscopy |
| nc-AFM | non-contact atomic force microscopy |
| DFT | density functional theory |
| SI | supplementary information |
References
- Cahn, R.S.; Ingold, C.K.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. Engl. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Gingras, M. One hundred years of helicene chemistry. Part 3: Applications and properties of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1051–1095. [Google Scholar] [CrossRef]
- Field, J.E.; Muller, G.; Riehl, J.P.; Venkataraman, D. Circularly Polarized Luminescence from Bridged Triarylamine Helicenes. J. Am. Chem. Soc. 2003, 125, 11808–11809. [Google Scholar] [CrossRef] [PubMed]
- Kiran, V.; Mathew, S.P.; Cohen, S.R.; Hernandez Delgado, I.; Lacour, J.; Naaman, R. Helicenes—A New Class of Organic Spin Filter. Adv. Mater. 2016, 28, 1957–1962. [Google Scholar] [CrossRef]
- De Feyter, S.; De Schryver, F.C. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 2003, 32, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.-H. Stereochemical Recognition of Helicenes on Metal Surfaces. Acc. Chem. Res. 2016, 49, 1182–1190. [Google Scholar] [CrossRef]
- Stroscio, J.A.; Eigler, D.M. Atomic and molecular manipulation with the scanning tunneling microscope. Science 1991, 254, 1319–1326. [Google Scholar] [CrossRef]
- Ernst, K.-H.; Baumann, S.; Lutz, C.P.; Seibel, J.; Zoppi, L.; Heinrich, A.J. Pasteur’s Experiment Performed at the Nanoscale: Manual Separation of Chiral Molecules, One by One. Nano Lett. 2015, 15, 5388–5392. [Google Scholar] [CrossRef] [PubMed]
- Stetsovych, O.; Svec, M.; Vacek, J.; Chocholousova, J.V.; Jancarik, A.; Rybacek, J.; Kosmider, K.; Stara, I.G.; Jelinek, P.; Stary, I. From helical to planar chirality by on-surface chemistry. Nat. Chem. 2017, 9, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Shiotari, A.; Tanaka, K.; Nakae, T.; Mori, S.; Okujima, T.; Uno, H.; Sakaguchi, H.; Sugimoto, Y. Chiral Discrimination and Manipulation of Individual Heptahelicene Molecules on Cu(001) by Noncontact Atomic Force Microscopy. J. Phys. Chem. C 2018, 122, 4997–5003. [Google Scholar] [CrossRef]
- Ernst, K.-H. Amplification of chirality at solid surfaces. Orig. Life Evol. Biosph. 2010, 40, 41–50. [Google Scholar] [CrossRef]
- Ernst, K.-H. Molecular chirality at surfaces. Phys. Status Solidi B 2012, 249, 2057–2088. [Google Scholar] [CrossRef]
- Ernst, K.H. Stereochemistry of 2D Molecular Crystallization. Chimia 2014, 68, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Fasel, R.; Parschau, M.; Ernst, K.H. Chirality transfer from single molecules into self-assembled monolayers. Angew. Chem. Int. Ed. Engl. 2003, 42, 5178–5181. [Google Scholar] [CrossRef] [PubMed]
- Seibel, J.; Parschau, M.; Ernst, K.-H. Two-Dimensional Crystallization of Enantiopure and Racemic Heptahelicene on Ag(111) and Au(111). J. Phys. Chem. C 2014, 118, 29135–29141. [Google Scholar] [CrossRef]
- Seibel, J.; Zoppi, L.; Ernst, K.H. 2D conglomerate crystallization of heptahelicene. Chem. Commun. 2014, 50, 8751–8753. [Google Scholar] [CrossRef] [PubMed]
- Fasel, R.; Parschau, M.; Ernst, K.H. Amplification of chirality in two-dimensional enantiomorphous lattices. Nature 2006, 439, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Seibel, J.; Parschau, M.; Ernst, K.H. From Homochiral Clusters to Racemate Crystals: Viable Nuclei in 2D Chiral Crystallization. J. Am. Chem. Soc. 2015, 137, 7970–7973. [Google Scholar] [CrossRef]
- Stohr, M.; Boz, S.; Schar, M.; Nguyen, M.T.; Pignedoli, C.A.; Passerone, D.; Schweizer, W.B.; Thilgen, C.; Jung, T.A.; Diederich, F. Self-assembly and two-dimensional spontaneous resolution of cyano-functionalized [7]helicenes on Cu111. Angew. Chem. Int. Ed. Engl. 2011, 50, 9982–9986. [Google Scholar] [CrossRef]
- Mairena, A.; Zoppi, L.; Seibel, J.; Troster, A.F.; Grenader, K.; Parschau, M.; Terfort, A.; Ernst, K.H. Heterochiral to Homochiral Transition in Pentahelicene 2D Crystallization Induced by Second-Layer Nucleation. ACS Nano 2017, 11, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Balandina, T.; van der Meijden, M.W.; Ivasenko, O.; Cornil, D.; Cornil, J.; Lazzaroni, R.; Kellogg, R.M.; De Feyter, S. Self-assembly of an asymmetrically functionalized [6]helicene at liquid/solid interfaces. Chem. Commun. 2013, 49, 2207–2209. [Google Scholar] [CrossRef] [PubMed]
- Ascolani, H.; van der Meijden, M.W.; Cristina, L.J.; Gayone, J.E.; Kellogg, R.M.; Fuhr, J.D.; Lingenfelder, M. Van der Waals interactions in the self-assembly of 5-amino[6]helicene on Cu(100) and Au(111). Chem. Commun. 2014, 50, 13907–13909. [Google Scholar] [CrossRef]
- Talele, H.R.; Chaudhary, A.R.; Patel, P.R.; Bedekar, A.V. Expeditious synthesis of helicenes using an improved protocol of photocyclodehydrogenation of stilbenes. Arkivoc 2011. [Google Scholar] [CrossRef]
- Gross, L.; Mohn, F.; Moll, N.; Liljeroth, P.; Meyer, G. The Chemical Structure of a Molecule Resolved by Atomic Force Microscopy. Science 2009, 325, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Pavlicek, N.; Fleury, B.; Neu, M.; Niedenfuhr, J.; Herranz-Lancho, C.; Ruben, M.; Repp, J. Atomic force microscopy reveals bistable configurations of dibenzo[a,h]thianthrene and their interconversion pathway. Phys. Rev. Lett. 2012, 108, 086101. [Google Scholar] [CrossRef] [PubMed]
- Dienel, T.; Kawai, S.; Sode, H.; Feng, X.; Mullen, K.; Ruffieux, P.; Fasel, R.; Groning, O. Resolving Atomic Connectivity in Graphene Nanostructure Junctions. Nano Lett. 2015, 15, 5185–5190. [Google Scholar] [CrossRef]
- Su, X.; Xue, Z.; Li, G.; Yu, P. Edge State Engineering of Graphene Nanoribbons. Nano Lett. 2018, 18, 5744–5751. [Google Scholar] [CrossRef]
- Bürgi, T.; Urakawa, A.; Behzadi, B.; Ernst, K.-H.; Baiker, A. The absolute configuration of heptahelicene: aVCD spectroscopy study. New J. Chem. 2004, 28, 332–334. [Google Scholar] [CrossRef]
- Gross, L.; Mohn, F.; Moll, N.; Schuler, B.; Criado, A.; Guitian, E.; Pena, D.; Gourdon, A.; Meyer, G. Bond-Order Discrimination by Atomic Force Microscopy. Science 2012, 337, 1326–1329. [Google Scholar] [CrossRef]
- Kawai, S.; Nishiuchi, T.; Kodama, T.; Spijker, P.; Pawlak, R.; Meier, T.; Tracey, J.; Kubo, T.; Meyer, E.; Foster, A.S. Direct quantitative measurement of the C=O⋅⋅⋅H–C bond by atomic force microscopy. Sci. Adv. 2017, 3, e1603258. [Google Scholar] [CrossRef]
- Dion, M.; Rydberg, H.; Schroder, E.; Langreth, D.C.; Lundqvist, B.I. van der Waals density functional for general geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Liu, W.; Michaelides, A.; Tkatchenko, A. Insight into the description of van der Waals forces for benzene adsorption on transition metal (111) surfaces. J. Chem. Phys. 2014, 140, 084704. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Hafner, J. Ab-Initio Simulations of Materials Using VASP: Density-Functional Theory and Beyond. J. Comput. Chem. 2008, 29, 2044. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Bohringer, M.; Morgenstern, K.; Schneider, W.D.; Berndt, R.; Mauri, F.; De Vita, A.; Car, R. Two-dimensional self-assembly of supramolecular clusters and chains. Phys. Rev. Lett. 1999, 83, 324–327. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Gao, L.; Deng, Z.T.; Jiang, N.; Liu, Q.; Shi, D.X.; Du, S.X.; Guo, H.M.; Gao, H.J. Adsorption behavior of iron phthalocyanine on Au(111) surface at submonolayer coverage. J. Phys. Chem. C 2007, 111, 9240–9244. [Google Scholar] [CrossRef]
- BÖHringer, M.; Schneider, W.-D.; Berndt, R. Two-dimensional self-assembly of supermolecular structures. Surf. Rev. Lett. 2000, 7, 661–666. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, H.; Shen, C.; Gan, F.; Su, X.; Qiu, H.; Yang, B.; Yu, P. Chiral Recognition of Hexahelicene on a Surface via the Forming of Asymmetric Heterochiral Trimers. Int. J. Mol. Sci. 2019, 20, 2018. https://doi.org/10.3390/ijms20082018
Zhang H, Liu H, Shen C, Gan F, Su X, Qiu H, Yang B, Yu P. Chiral Recognition of Hexahelicene on a Surface via the Forming of Asymmetric Heterochiral Trimers. International Journal of Molecular Sciences. 2019; 20(8):2018. https://doi.org/10.3390/ijms20082018
Chicago/Turabian StyleZhang, Hong, Hong Liu, Chengshuo Shen, Fuwei Gan, Xuelei Su, Huibin Qiu, Bo Yang, and Ping Yu. 2019. "Chiral Recognition of Hexahelicene on a Surface via the Forming of Asymmetric Heterochiral Trimers" International Journal of Molecular Sciences 20, no. 8: 2018. https://doi.org/10.3390/ijms20082018
APA StyleZhang, H., Liu, H., Shen, C., Gan, F., Su, X., Qiu, H., Yang, B., & Yu, P. (2019). Chiral Recognition of Hexahelicene on a Surface via the Forming of Asymmetric Heterochiral Trimers. International Journal of Molecular Sciences, 20(8), 2018. https://doi.org/10.3390/ijms20082018




