Drosophila Homeodomain-Interacting Protein Kinase (Hipk) Phosphorylates the Homeodomain Proteins Homeobrain, Empty Spiracles, and Muscle Segment Homeobox
Abstract
1. Introduction
2. Results
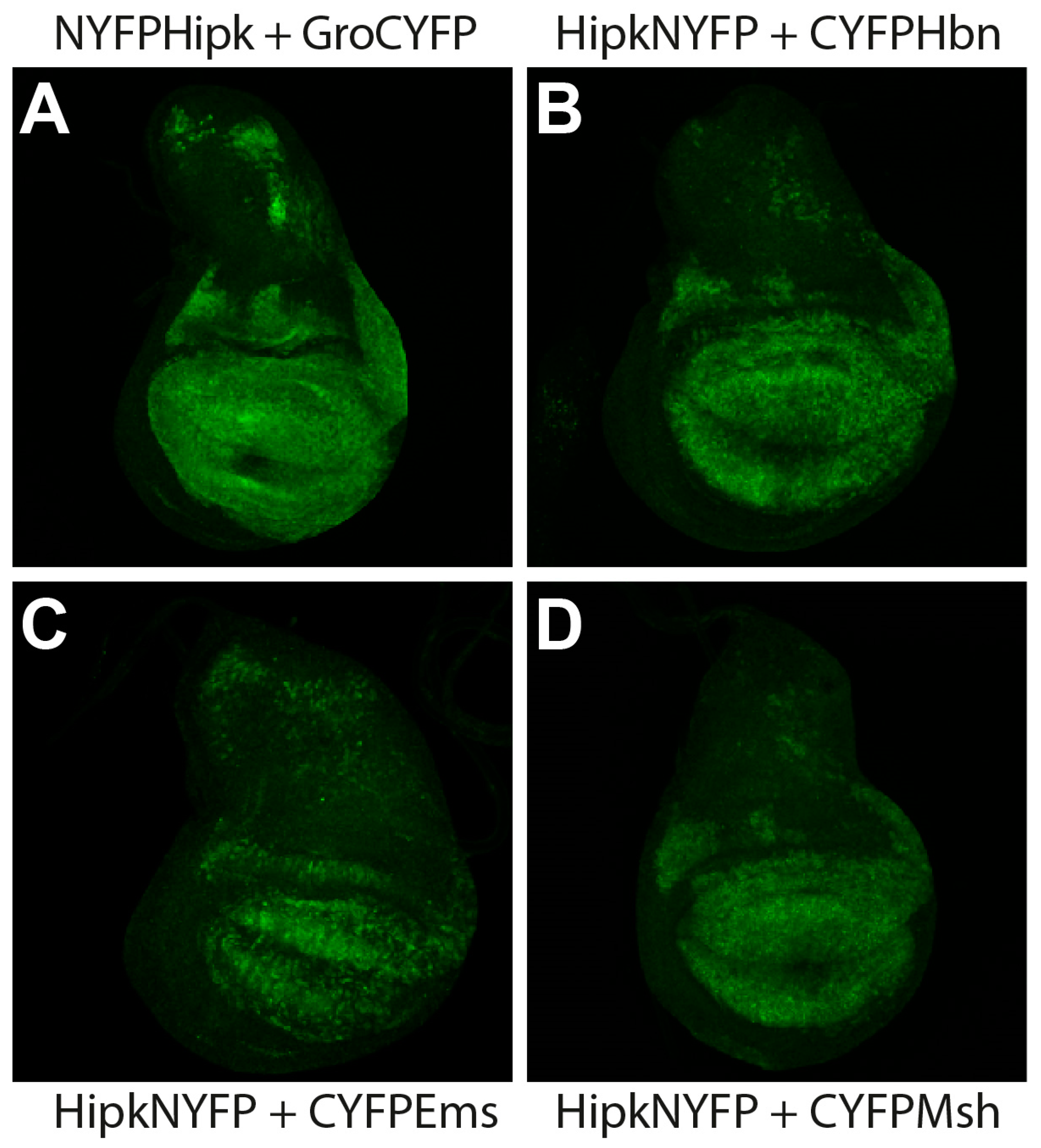
2.1. Hipk Interacts In Vivo with the Homeodomain Proteins Hbn, Ems, and Msh
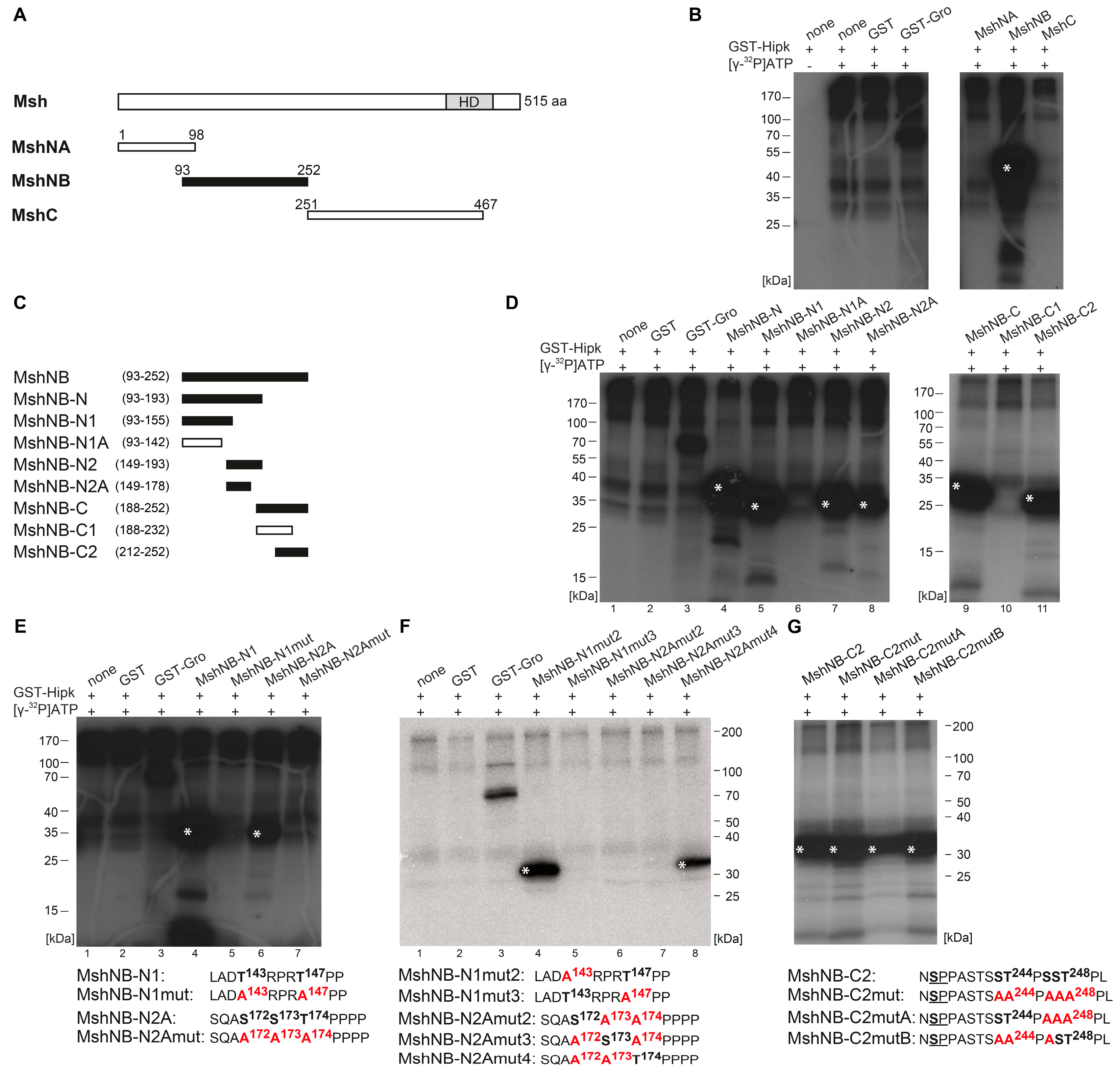
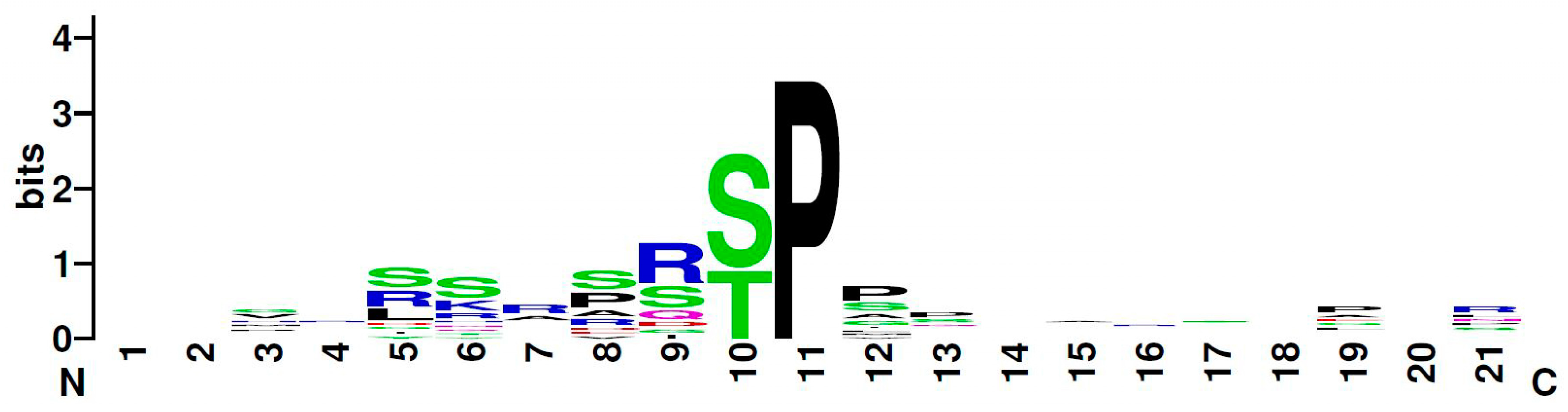
2.2. Hipk Phosphorylates Hbn, Ems, and Msh
3. Discussion
4. Materials and Methods
4.1. Bimolecular Fluorescence Complementation (BiFC)
4.2. Purification of Recombinant Proteins
4.3. In Vitro Mutagenesis
4.4. In Vitro Phosphorylation Assay
4.5. Immunostaining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HIPK/Hipk | Homeodomain-interacting protein kinase |
| HD | Homeodomain |
| VNC | Ventral nerve cord |
| aa | amino acids |
| GFP | Green fluorescent protein |
| YFP | Yellow fluorescent protein |
| GST | Glutathion S-transferase |
References
- Kim, Y.H.; Choi, C.Y.; Lee, S.-J.; Conti, M.A.; Kim, Y. Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J. Biol. Chem. 1998, 273, 25875–25879. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Choi, C.Y.; Kim, Y.H.; Kim, Y.O.; Park, S.J.; Kim, E.A.; Riemenschneider, W.; Gajewski, K.; Schulz, R.A.; Kim, Y. Phosphorylation by the DHIPK2 protein kinase modulates the corepressor activity of Groucho. J. Biol. Chem. 2005, 280, 21427–21436. [Google Scholar] [CrossRef] [PubMed]
- Link, N.; Chen, P.; Lu, W.J.; Pogue, K.; Chuong, A.; Mata, M.; Checketts, J.; Abrams, J.M. A collective form of cell death requires homeodomain interacting protein kinase. J. Cell Biol. 2007, 178, 567–574. [Google Scholar] [CrossRef]
- Lee, W.; Swarup, S.; Chen, J.; Ishitani, T.; Verheyen, E.M. Homeodomain-interacting protein kinases (Hipks) promote Wnt/Wg signaling through stabilization of β -catenin / Arm and stimulation of target gene expression. Development 2009, 136, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, C.; Prodosmo, A.; Siepi, F.; Soddu, S. HIPK2: A multitalented partner for transcription factors in DNA damage response and development. Biochem. Cell Biol. 2007, 85, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, C.; Siepi, F.; Prodosmo, A.; Soddu, S. HIPKs: Jack of all trades in basic nuclear activities. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, Y.; Nishida, K.; Akaike, Y.; Kurokawa, K.; Nishikawa, T.; Masuda, K.; Rokutan, K. Homeodomain-interacting protein kinase-2: A critical regulator of the DNA damage response and the epigenome. Int. J. Mol. Sci. 2016, 17, 1638. [Google Scholar] [CrossRef]
- Blaquiere, J.A.; Verheyen, E.M. Homeodomain-Interacting Protein Kinases: Diverse and Complex Roles in Development and Disease. Curr. Top. Dev. Biol. 2017, 123, 73–103. [Google Scholar]
- Schmitz, M.L.; Rodriguez-Gil, A.; Hornung, J. Integration of stress signals by homeodomain interacting protein kinases. Biol. Chem. 2014, 395, 375–386. [Google Scholar] [CrossRef]
- Gehring, W.J.; Affolter, M.; Bürglin, T. Homeodomain Proteins. Annu. Rev. Biochem. 1994, 63, 487–526. [Google Scholar] [CrossRef] [PubMed]
- Bürglin, T.R.; Affolter, M. Homeodomain proteins: An update. Chromosoma 2016, 125, 497–521. [Google Scholar] [CrossRef]
- Burri, M.; Tromvoukis, Y.; Bopp, D.; Frigerio, G.; Noll, M. Conservation of the paired domain in metazoans and its structure in three isolated human genes. EMBO J. 1989, 8, 1183–1190. [Google Scholar] [CrossRef]
- Smith, S.; Jaynes, J.B. A conserved region of engrailed, shared among all en-, gsc-, Nk1-, Nk2- and msh-class homeoproteins, mediates active transcriptional repression in vivo. Development 1996, 122, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.; Paroush, Z.; Ish-Horowicz, D. Groucho acts as a corepressor for a subset of negative regulators, including Hairy and Engrailed. Genes Dev. 1997, 11, 3072–3082. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.L.; Caudy, M. Groucho proteins: Transcriptional corepressors for specific subsets of DNA-binding transcription factors in vertebrates and invertebrates. Genes Dev. 1998, 12, 1931–1940. [Google Scholar] [CrossRef][Green Version]
- Lord, P.C.W.; Lin, M.H.; Hales, K.H.; Storti, R.V. Normal expression and the effects of ectopic expression of the Drosophila muscle segment homeobox (msh) gene suggest a role in differentiation and patterning of embryonic muscles. Dev. Biol. 1995. [Google Scholar] [CrossRef]
- Nose, A.; Isshiki, T.; Takeichi, M. Regional specification of muscle progenitors in Drosophila: The role of the msh homeobox gene. Development 1998, 125, 215–223. [Google Scholar]
- Isshiki, T.; Takeichi, M.; Nose, A. The role of the msh homeobox gene during Drosophila neurogenesis: Implication for the dorsoventral specification of the neuroectoderm. Development 1997, 124, 3099–3109. [Google Scholar]
- Seibert, J.; Urbach, R. Role of en and novel interactions between msh, ind, and vnd in dorsoventral patterning of the Drosophila brain and ventral nerve cord. Dev. Biol. 2010, 346, 332–345. [Google Scholar] [CrossRef][Green Version]
- D’Alessio, M.; Frasch, M. msh may play a conserved role in dorsoventral patterning of the neuroectoderm and mesoderm. Mech. Dev. 1996, 58, 217–231. [Google Scholar] [CrossRef]
- Zhao, G.; Wheeler, S.R.; Skeath, J.B. Genetic control of dorsoventral patterning and neuroblast specification in the Drosophila Central Nervous System. Int. J. Dev. Biol. 2007, 51, 107–115. [Google Scholar] [CrossRef]
- Dalton, D.; Chadwick, R.; McGinnis, W. Expression and embryonic function of empty spiracles: A Drosophila homeo box gene with two patterning functions on the anterior-posterior axis of the embryo. Genes Dev. 1989, 3, 1940–1956. [Google Scholar] [CrossRef]
- Walldorf, U.; Gehring, W.J. Empty spiracles, a gap gene containing a homeobox involved in Drosophila head development. EMBO J. 1992, 11, 2247–2259. [Google Scholar] [CrossRef]
- Hartmann, B.; Hirth, F.; Walldorf, U.; Reichert, H. Expression, regulation and function of the homeobox gene empty spiracles in brain and ventral nerve cord development of Drosophila. Mech. Dev. 2000, 90, 143–153. [Google Scholar] [CrossRef]
- Hirth, F.; Therianos, S.; Loop, T.; Gehring, W.J.; Reichert, H.; Furukubo-Tokunaga, K. Developmental Defects in Brain Segmentation Caused by Mutations of the Homeobox Genes orthodenticle and empty spiracles in Drosophila. Neuron 1995, 15, 769–778. [Google Scholar] [CrossRef]
- Das, A.; Sen, S.; Lichtneckert, R.; Okada, R.; Ito, K.; Rodrigues, V.; Reichert, H. Drosophila olfactory local interneurons and projection neurons derive from a common neuroblast lineage specified by the empty spiracles gene. Neural Dev. 2008, 3, 1–17. [Google Scholar] [CrossRef]
- Lichtneckert, R.; Nobs, L.; Reichert, H. empty spiracles is required for the development of olfactory projection neuron circuitry in Drosophila. Development 2008, 135, 2415–2424. [Google Scholar] [CrossRef][Green Version]
- Sen, S.; Hartmann, B.; Reichert, H.; Rodrigues, V. Expression and function of the empty spiracles gene in olfactory sense organ development of Drosophila melanogaster. Development 2010, 137, 3687–3695. [Google Scholar] [CrossRef][Green Version]
- Walldorf, U.; Kiewe, A.; Wickert, M.; Ronshaugen, M.; McGinnis, W. Homeobrain, a novel paired-like homeobox gene is expressed in the Drosophila brain. Mech. Dev. 2000, 96, 141–144. [Google Scholar] [CrossRef]
- Simeone, A.; D’Apice, M.R.; Nigro, V.; Casanova, J.; Graziani, F.; Acampora, D.; Avantaggiato, V. Orthopedia, a novel homeobox-containing gene expressed in the developing CNS of both mouse and Drosophila. Neuron 1994, 13, 83–101. [Google Scholar] [CrossRef]
- Eggert, T.; Hauck, B.; Hildebrandt, N.; Gehring, W.J.; Walldorf, U. Isolation of a Drosophila homolog of the vertebrate homeobox gene Rx and its possible role in brain and eye development. Proc. Natl. Acad. Sci. USA 1998, 95, 2343–2348. [Google Scholar] [CrossRef]
- Lee, W.; Andrews, B.C.; Faust, M.; Walldorf, U.; Verheyen, E.M. Hipk is an essential protein that promotes Notch signal transduction in the Drosophila eye by inhibition of the global co-repressor Groucho. Dev. Biol. 2009, 325, 263–272. [Google Scholar] [CrossRef]
- Dewald, D.N.; Steinmetz, E.L.; Walldorf, U. Homeodomain-interacting protein kinase (Hipk) phosphorylates the small SPOC family protein Spenito. Insect Mol. Biol. 2014, 23, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Gohl, C.; Banovic, D.; Grevelhörster, A.; Bogdan, S. WAVE forms hetero- and homo-oligomeric complexes at integrin junctions in Drosophila visualized by bimolecular fluorescence complementation. J. Biol. Chem. 2010, 285, 40171–40179. [Google Scholar] [CrossRef] [PubMed]
- Dewald, D.N. Developmental Biology, Saarland University, Homburg/Germany. Unpublished results. 2014. [Google Scholar]
- Kim, E.A.; Noh, Y.T.; Ryu, M.-J.; Kim, H.-T.; Lee, S.-E.; Kim, C.-H.; Lee, C.; Kim, Y.H.; Choi, C.Y. Phosphorylation and transactivation of Pax6 by homeodomain-interacting protein kinase 2. J. Biol. Chem. 2006, 281, 7489–7497. [Google Scholar] [CrossRef]
- Steinmetz, E.L.; Dewald, D.N.; Walldorf, U. Homeodomain-interacting protein kinase phosphorylates the Drosophila Paired box protein 6 (Pax6) homologues Twin of eyeless and Eyeless. Insect Mol. Biol. 2018, 27, 198–211. [Google Scholar] [CrossRef]
- Zhai, B.; Villén, J.; Beausoleil, S.A.; Mintseris, J.; Gygi, S.P. Phosphoproteome analysis of Drosophila melanogaster embryos. J. Proteome Res. 2008, 7, 1675–1682. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. NCBI GenBank FTP Site\nWebLogo: A sequence logo generator. Genome Res 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Calzado, M.A.; Renner, F.; Roscic, A.; Schmitz, M.L. HIPK2: A versatile switchboard regulating the transcription machinery and cell death. Cell Cycle 2007, 6, 139–143. [Google Scholar] [CrossRef]
- Wiggins, A.K.; Wei, G.; Doxakis, E.; Wong, C.; Tang, A.A.; Zang, K.; Luo, E.J.; Neve, R.L.; Reichardt, L.F.; Huang, E.J. Interaction of Brn3a and HIPK2 mediates transcriptional repression of sensory neuron survival. J. Cell Biol. 2004, 167, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, P. Die Bedeutung des Homeoboxgens homeobrain für die Embryonale Gehirnentwicklung von Drosophila melanogaster. Ph.D. Thesis, Saarland University, Saarbrücken, Germany, 2008. [Google Scholar]
- Paul, L.K.; Brown, W.S.; Adolphs, R.; Tyszka, J.M.; Richards, L.J.; Mukherjee, P.; Sherr, E.H. Agenesis of the corpus callosum: Genetic, developmental and functional aspects of connectivity. Nat. Rev. Neurosci. 2007, 8, 287–299. [Google Scholar] [CrossRef]
- Dobyns, W.B.; Berry-Kravis, E.; Havernick, N.J.; Holden, K.R.; Viskochil, D. X-Linked Lissencephaly With Absent Corpus Callosum and Abnormal Genitalia. Am. J. Med. Genet. 1999, 86, 331–337. [Google Scholar] [CrossRef]
- Kitamura, K.; Yanazawa, M.; Sugiyama, N.; Miura, H.; Iizuka-Kogo, A.; Kusaka, M.; Omichi, K.; Suzuki, R.; Kato-Fukui, Y.; Kamiirisa, K.; et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat. Genet. 2002, 32, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mattiske, T.; Tan, M.H.; Dearsley, O.; Cloosterman, D.; Hii, C.S.; Gécz, J.; Shoubridge, C. Regulating transcriptional activity by phosphorylation: A new mechanism for the ARX homeodomain transcription factor. PLoS ONE 2018, 13, e0206914. [Google Scholar] [CrossRef] [PubMed]
- Simeone, A.; Gulisano, M.; Acampora, D.; Stornaiuolo, A.; Rambaldi, M.; Boncinelli, E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992, 11, 2541–2550. [Google Scholar] [CrossRef]
- Simeone, A.; Acampora, D.; Gulisano, M.; Stornaiuolo, A.; Boncinelli, E. Nested expression domains of four homeobox genes in developing rostral brain. Nature 1992, 358, 687–690. [Google Scholar] [CrossRef]
- Qiu, M.; Anderson, S.; Chen, S.; Meneses, J.J.; Hevner, R.; Kuwana, E.; Pedersen, R.A.; Rubenstein, J.L.R. Mutation of the Emx-1 homeobox gene disrupts the corpus callosum. Dev. Biol. 1996, 178, 174–178. [Google Scholar] [CrossRef]
- Pellegrini, M.; Mansouri, A.; Simeone, A.; Boncinelli, E.; Gruss, P. Dentate gyrus formation requires Emx2. Development 1996, 122, 3893–3898. [Google Scholar] [PubMed]
- Yoshida, M.; Suda, Y.; Matsuo, I.; Miyamoto, N.; Takeda, N.; Kuratani, S.; Aizawa, S. Emx1 and Emx2 functions in development of dorsal telencephalon. Development 1997, 124, 101–111. [Google Scholar]
- Cecchi, C.; Boncinelli, E. Emx homeogenes and mouse brain development. Trends Neurosci. 2000, 23, 347–352. [Google Scholar] [CrossRef]
- Bishop, K.M.; Garel, S.; Nakagawa, Y.; Rubenstein, J.L.; O’Leary, D. Toward a unified model of vertebrate taste bud development. J. Comp. Neurol. 2003, 457, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, L.; Brand, A.H. Dorsal-Ventral Differences in Neural Stem Cell Quiescence Are Induced by p57KIP2/Dacapo. Dev. Cell 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Whitmarsh, A.J.; Davis, R.J. Regulation of transcription factor function by phosphorylation. Cell. Mol. Life Sci. 2000, 57, 1172–1183. [Google Scholar] [CrossRef]
- Davidson, D. The function and evolution of Msx genes pointers and paradoxes. Trend Genet. 1995, 11, 405–411. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Xu, H.; Lufkin, T. Msx3: A novel murine homologue of the Drosophila msh homeobox gene restricted to the dorsal embryonic central nervous system. Mech. Dev. 1996, 58, 203–215. [Google Scholar] [CrossRef]
- Alappat, S.; Zhang, Z.Y.; Chen, Y.P. Msx homeobox gene family and craniofacial development. Cell Res. 2003, 13, 429–442. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Helms, A.W.; Johnson, J.E. Distinct activities of Msx1 and Msx3 in dorsal neural tube development. Development 2004, 131, 1017–1028. [Google Scholar] [CrossRef]
- Ramos, C.; Robert, B. msh/Msx gene family in neural development. Trends Genet. 2005, 21, 624–632. [Google Scholar] [CrossRef]
- Foerst-Potts, L.; Sadler, T.W. Disruption of Msx-1 and Msx-2 reveals roles for these genes in craniofacial, eye, and axial development. Dev. Dyn. 1997, 209, 70–84. [Google Scholar] [CrossRef]
- Ruhin-Poncet, B.; Ghoul-Mazgar, S.; Hotton, D.; Capron, F.; Jaafoura, M.H.; Goubin, G.; Berdal, A. Msx and Dlx homeogene expression in epithelial odontogenic tumors. J. Histochem. Cytochem. 2009, 57, 69–78. [Google Scholar] [CrossRef]
- Lanigan, F.; Gremel, G.; Hughes, R.; Brennan, D.J.; Martin, F.; Jirström, K.; Gallagher, W.M. Homeobox transcription factor muscle segment homeobox 2 (Msx2) correlates with good prognosis in breast cancer patients and induces apoptosis in vitro. Breast Cancer Res. 2010, 12, R59. [Google Scholar] [CrossRef]
- Tao, H.; Guo, L.; Chen, L.; Qiao, G.; Meng, X.; Xu, B.; Ye, W. MSX1 inhibits cell migration and invasion through regulating the Wnt/β-catenin pathway in glioblastoma. Tumor Biol. 2016, 37, 1097–1104. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Zhang, C.; Zhu, H.; Xu, Q.; Bu, Y.; Lei, Y. Redox imbalance in the development of colorectal cancer. J. Cancer 2017, 8, 1586–1597. [Google Scholar] [CrossRef]
- Wei, G.; Ku, S.; Ma, G.K.; Saito, S.; Tang, A.A.; Zhang, J.; Mao, J.; Appella, E.; Balmain, A.; Huang, E.J. HIPK2 represses beta-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 13040–13045. [Google Scholar] [CrossRef]
- D’Orazi, G.; Rinaldo, C.; Soddu, S. Updates on HIPK2: A resourceful oncosuppressor for clearing cancer. J. Exp. Clin. Cancer Res. 2012, 31, 63. [Google Scholar] [CrossRef]
- Hofmann, T.G.; Glas, C.; Bitomsky, N. HIPK2: A tumour suppressor that controls DNA damage-induced cell fate and cytokinesis. BioEssays 2013, 35, 55–64. [Google Scholar] [CrossRef]
- Jacq, B.; Gehring, W.J. Department of Cell Biology, Biozentrum, University of Basel, Switzerland. Unpublished results. 1987. [Google Scholar]
- Venken, K.J.T.; Bellen, H.J. Transgenesis upgrades for Drosophila melanogaster. Development 2007, 134, 3571–3584. [Google Scholar] [CrossRef]
- Pfeiffer, B.D.; Jenett, A.; Hammonds, A.S.; Ngo, T.T.; Misra, S.; Murphy, C.; Scully, A.; Carlson, J.W.; Wan, K.H.; Laverty, T.R.; et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 9715–9720. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinmetz, E.L.; Dewald, D.N.; Luxem, N.; Walldorf, U. Drosophila Homeodomain-Interacting Protein Kinase (Hipk) Phosphorylates the Homeodomain Proteins Homeobrain, Empty Spiracles, and Muscle Segment Homeobox. Int. J. Mol. Sci. 2019, 20, 1931. https://doi.org/10.3390/ijms20081931
Steinmetz EL, Dewald DN, Luxem N, Walldorf U. Drosophila Homeodomain-Interacting Protein Kinase (Hipk) Phosphorylates the Homeodomain Proteins Homeobrain, Empty Spiracles, and Muscle Segment Homeobox. International Journal of Molecular Sciences. 2019; 20(8):1931. https://doi.org/10.3390/ijms20081931
Chicago/Turabian StyleSteinmetz, Eva Louise, Denise Nicole Dewald, Nadine Luxem, and Uwe Walldorf. 2019. "Drosophila Homeodomain-Interacting Protein Kinase (Hipk) Phosphorylates the Homeodomain Proteins Homeobrain, Empty Spiracles, and Muscle Segment Homeobox" International Journal of Molecular Sciences 20, no. 8: 1931. https://doi.org/10.3390/ijms20081931
APA StyleSteinmetz, E. L., Dewald, D. N., Luxem, N., & Walldorf, U. (2019). Drosophila Homeodomain-Interacting Protein Kinase (Hipk) Phosphorylates the Homeodomain Proteins Homeobrain, Empty Spiracles, and Muscle Segment Homeobox. International Journal of Molecular Sciences, 20(8), 1931. https://doi.org/10.3390/ijms20081931



