The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma
Abstract
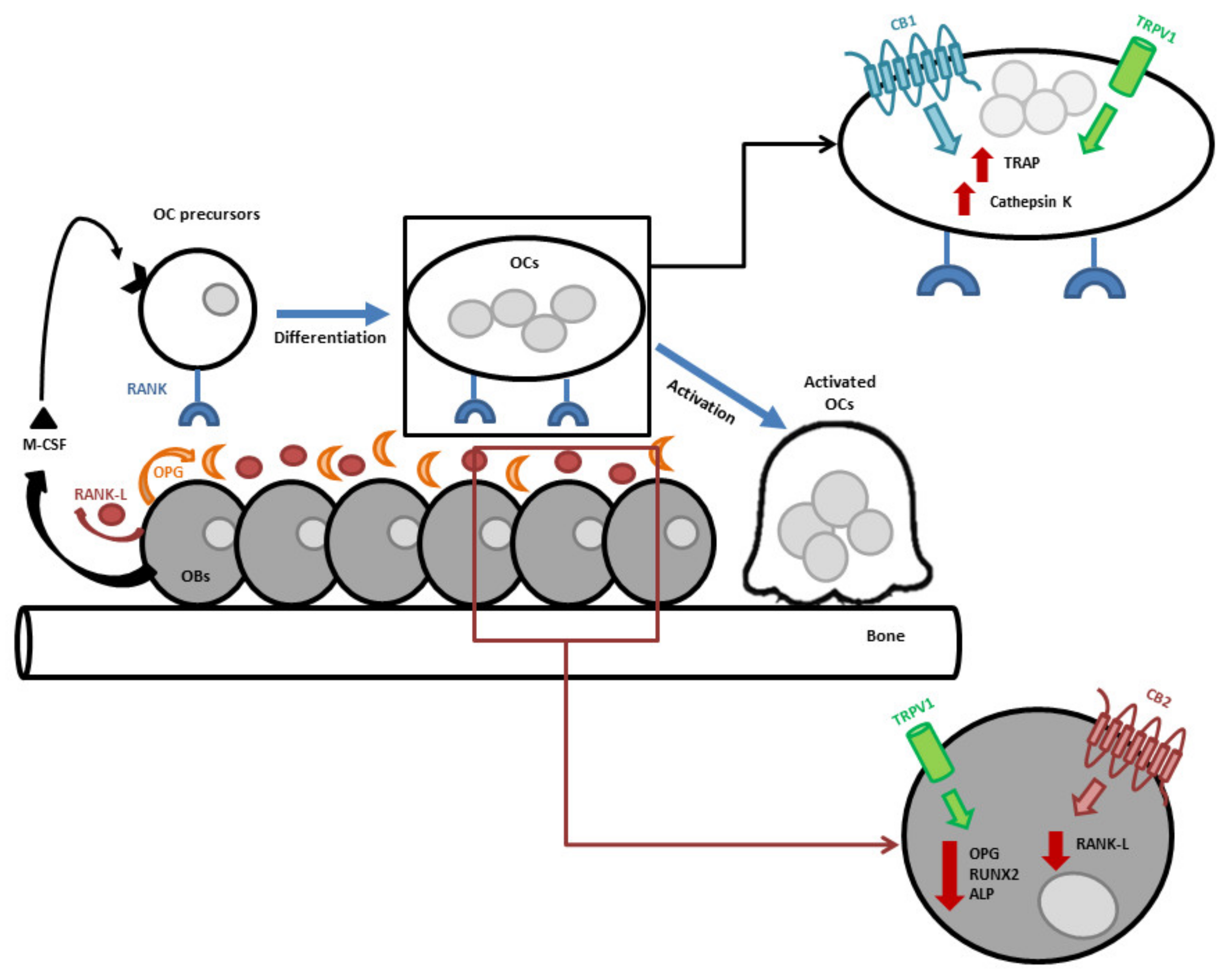
1. Endocannabinoid/Endovanilloid (EC/EV) System in Bone
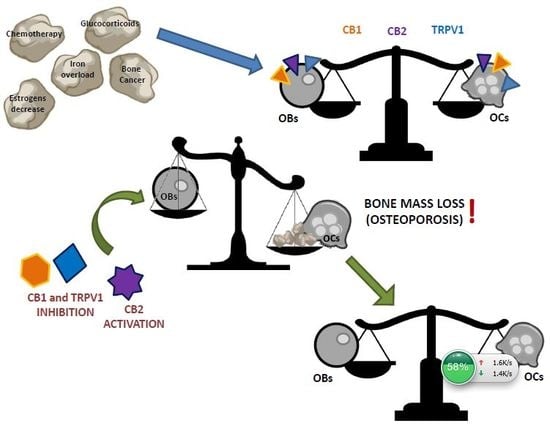
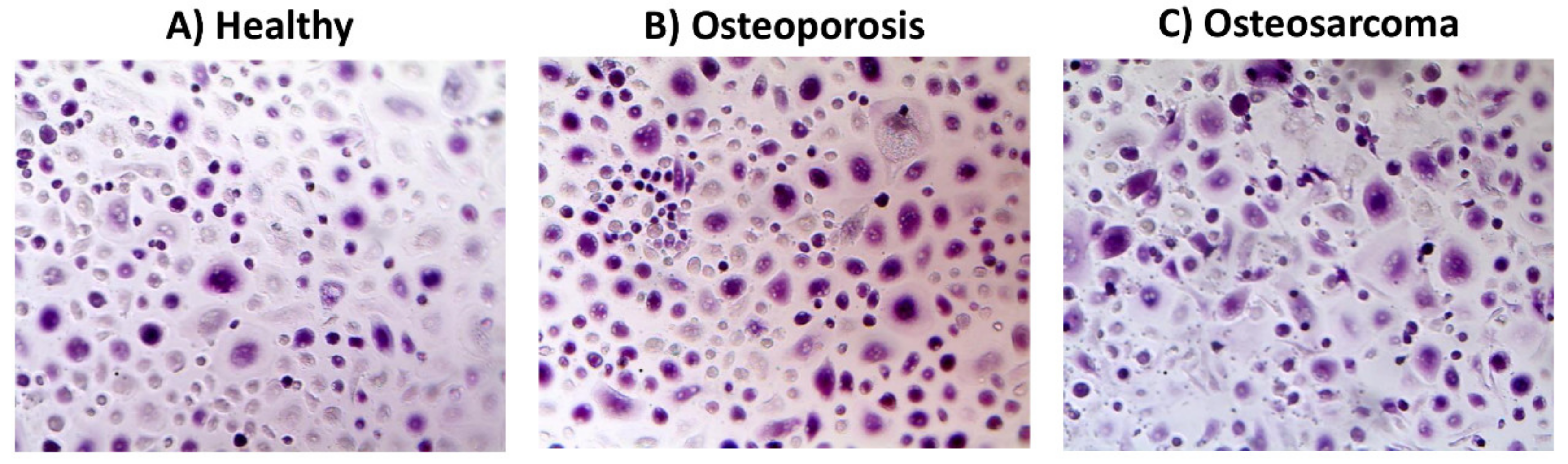
2. Endocannabinoid/Endovanilloid System in Osteoporosis
3. Endocannabinoid/Endovanilloid System in Bone Cancer
4. EC/EV System in Bone Cancer Pain
5. Conclusions
Funding
Conflicts of Interest
References
- Abdelgawad, M.E.; Delaisse, J.M.; Hinge, M.; Jensen, P.R.; Alnaimi, R.W.; Rolighed, L.; Engelholm, L.H.; Marcussen, N.; Andersen, T.L. Early reversal cells in adult human bone remodeling: Osteoblastic nature, catabolic functions and interactions with osteoclasts. Histochem. Cell Biol. 2016, 145, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, G.; Liu, X.; Dai, M.; Zhang, B. Icariin inhibits RANKL-induced osteoclastogenesis via modulation of the NF-kappaB and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2019, 508, 902–906. [Google Scholar] [CrossRef]
- Schneeweis, L.A.; Willard, D.; Milla, M.E. Functional dissection of osteoprotegerin and its interaction with receptor activator of NF-kappaB ligand. J. Biol. Chem. 2005, 280, 41155–41164. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C.; et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Penninger, J.M. The RANKL-RANK Story. Gerontology 2015, 61, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, P.A.; Verhoeven, Y.; Trinh, X.B.; Wouters, A.; Lardon, F.; Prenen, H.; Smits, E.; Baldewijns, M.; Lammens, M. RANK/RANKL signaling inhibition may improve the effectiveness of checkpoint blockade in cancer treatment. Crit. Rev. Oncol./Hematol. 2019, 133, 85–91. [Google Scholar] [CrossRef]
- Gonzalez-Suarez, E.; Sanz-Moreno, A. RANK as a therapeutic target in cancer. FEBS J. 2016, 283, 2018–2033. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Cristino, L. Why endocannabinoids are not all alike. Nat. Neurosci. 2008, 11, 124–126. [Google Scholar] [PubMed]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of Cannabinoids in Obesity. Int. Mol. Sci. 2018, 19, 2690. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Przewlocka, B. Modulation of neuropathic-pain-related behaviour by the spinal endocannabinoid/endovanilloid system. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012, 367, 3286–3299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Burkovskiy, I.; Yang, H.; Sardinha, J.; Lehmann, C. CB2 and GPR55 Receptors as Therapeutic Targets for Systemic Immune Dysregulation. Front. Pharmacol. 2016, 7, 264. [Google Scholar] [CrossRef]
- Velasco, G.; Sanchez, C.; Guzman, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, S23–S32. [Google Scholar] [PubMed]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77543–77557. [Google Scholar] [CrossRef]
- Spiller, K.J.; Bi, G.H.; He, Y.; Galaj, E.; Gardner, E.L.; Xi, Z.X. Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. Br. J. Pharmacol. 2019, 176, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Canseco-Alba, A.; Zhang, H.Y.; Tagliaferro, P.; Chung, M.; Dennis, E.; Sanabria, B.; Schanz, N.; Escosteguy-Neto, J.C.; Ishiguro, H.; et al. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci. Rep. 2017, 7, 17410. [Google Scholar] [CrossRef]
- Minke, B. The history of the Drosophila TRP channel: The birth of a new channel superfamily. J. Neurogenet. 2010, 24, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Ralston, S.H. Cannabinoids and bone: Friend or foe? Calcif. Tissue Int. 2010, 87, 285–297. [Google Scholar] [CrossRef]
- Rossi, F.; Siniscalco, D.; Luongo, L.; De Petrocellis, L.; Bellini, G.; Petrosino, S.; Torella, M.; Santoro, C.; Nobili, B.; Perrotta, S.; et al. The endovanilloid/endocannabinoid system in human osteoclasts: Possible involvement in bone formation and resorption. Bone 2009, 44, 476–484. [Google Scholar] [CrossRef]
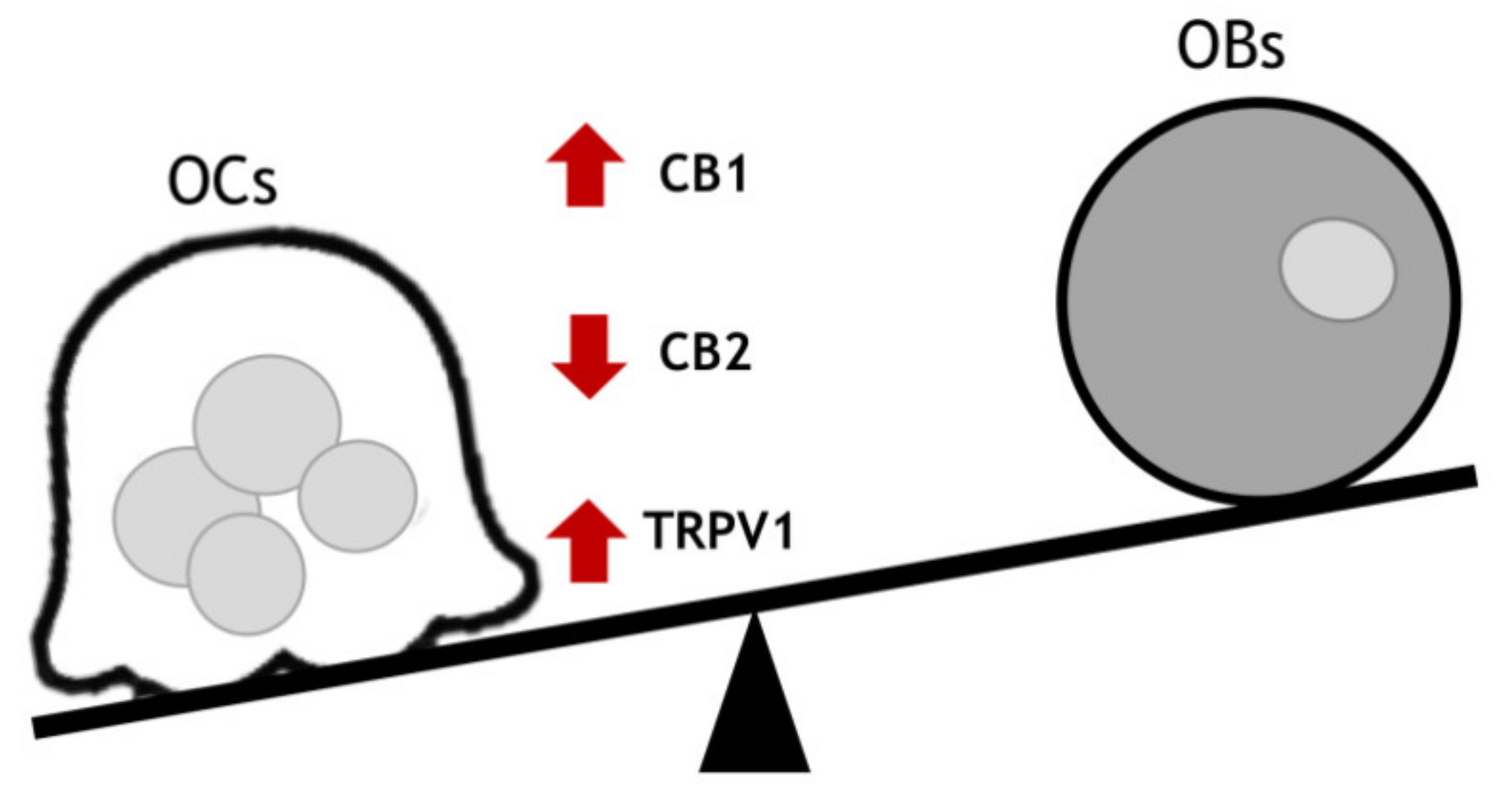
- Rossi, F.; Bellini, G.; Tortora, C.; Bernardo, M.E.; Luongo, L.; Conforti, A.; Starc, N.; Manzo, I.; Nobili, B.; Locatelli, F.; et al. CB(2) and TRPV(1) receptors oppositely modulate in vitro human osteoblast activity. Pharmacol. Res. 2015, 99, 194–201. [Google Scholar] [CrossRef]
- Zimmer, A. A collaboration investigating endocannabinoid signalling in brain and bone. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacological actions of cannabinoids. In Handbook of Experimental Pharmacology; Springer-Verlag: Berlin, Germany, 2005; pp. 1–51. [Google Scholar]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Sophocleous, A.; Landao-Bassonga, E.; Canals, M.; Milligan, G.; Baker, D.; van’t Hof, R.J.; Ralston, S.H. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab. 2009, 10, 139–147. [Google Scholar] [CrossRef]
- Evans, R.M.; Scott, R.H.; Ross, R.A. Chronic exposure of sensory neurones to increased levels of nerve growth factor modulates CB1/TRPV1 receptor crosstalk. Br. J. Pharmacol. 2007, 152, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.; Urban, L.; Bevan, S.; Nagy, I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur. J. Neurosci. 2003, 17, 2611–2618. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Bisogno, T.; Davis, J.B.; Pertwee, R.G.; Di Marzo, V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: Inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000, 483, 52–56. [Google Scholar] [CrossRef]
- Kanaya, K.; Iba, K.; Dohke, T.; Okazaki, S.; Yamashita, T. TRPV1, ASICs and P2X2/3 expressed in bone cells simultaneously regulate bone metabolic markers in ovariectomized mice. J. Musculoskelet. Neuronal Interact. 2016, 16, 145–151. [Google Scholar]
- He, L.H.; Liu, M.; He, Y.; Xiao, E.; Zhao, L.; Zhang, T.; Yang, H.Q.; Zhang, Y. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation. Sci. Rep. 2017, 7, 42385. [Google Scholar] [CrossRef]
- Idris, A.I.; Ralston, S.H. Role of cannabinoids in the regulation of bone remodeling. Front. Endocrinol. 2012, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I. The promise and dilemma of cannabinoid therapy: Lessons from animal studies of bone disease. BoneKEy Rep. 2012, 1, 224. [Google Scholar] [CrossRef][Green Version]
- Idris, A.I.; van’t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar] [CrossRef]
- Idris, A.I.; Sophocleous, A.; Landao-Bassonga, E.; van’t Hof, R.J.; Ralston, S.H. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology 2008, 149, 5619–5626. [Google Scholar] [CrossRef] [PubMed]
- Bab, I.; Ofek, O.; Tam, J.; Rehnelt, J.; Zimmer, A. Endocannabinoids and the regulation of bone metabolism. J. Neuroendocrinol. 2008, 20 (Suppl. 1), 69–74. [Google Scholar] [CrossRef] [PubMed]
- Karsak, M.; Cohen-Solal, M.; Freudenberg, J.; Ostertag, A.; Morieux, C.; Kornak, U.; Essig, J.; Erxlebe, E.; Bab, I.; Kubisch, C.; et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum. Mol. Genet. 2005, 14, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Karsak, M.; Malkin, I.; Toliat, M.R.; Kubisch, C.; Nurnberg, P.; Zimmer, A.; Livshits, G. The cannabinoid receptor type 2 (CNR2) gene is associated with hand bone strength phenotypes in an ethnically homogeneous family sample. Hum. Genet. 2009, 126, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Iolascon, G.; Cianferotti, L.; Masi, L.; Marcucci, G.; Giusti, F.; Marini, F.; Parri, S.; Feola, M.; Rao, C.; et al. Clinical guidelines for the prevention and treatment of osteoporosis: Summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J. Orthopaed. Traumatol. Off. J. Ital. Soc. Orthop. Traumatol. 2017, 18 (Suppl. 1), 3–36. [Google Scholar] [CrossRef]
- Zethraeus, N.; Borgstrom, F.; Strom, O.; Kanis, J.A.; Jonsson, B. Cost-effectiveness of the treatment and prevention of osteoporosis—A review of the literature and a reference model. Osteoporosis Int. 2007, 18, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; McClure, L.A.; Delzell, E.; Howard, V.J.; Orwoll, E.; Saag, K.G.; Safford, M.; Howard, G. Population-based fracture risk assessment and osteoporosis treatment disparities by race and gender. J. Gen. Intern. Med. 2009, 24, 956–962. [Google Scholar] [CrossRef]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M. Treating osteoporosis to prevent fractures: Current concepts and future developments. J. Intern. Med. 2019, 285, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef]
- Wasnich, R.D.; Bagger, Y.Z.; Hosking, D.J.; McClung, M.R.; Wu, M.; Mantz, A.M.; Yates, J.J.; Ross, P.D.; Alexandersen, P.; Ravn, P.; et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause 2004, 11, 622–630. [Google Scholar] [CrossRef]
- Riggs, B.L.; Khosla, S.; Melton, L.J., 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Russow, G.; Jahn, D.; Appelt, J.; Mardian, S.; Tsitsilonis, S.; Keller, J. Anabolic Therapies in Osteoporosis and Bone Regeneration. Int. J. Mol. Sci. 2018, 20, 83. [Google Scholar] [CrossRef]
- Mirza, F.; Canalis, E. Management of endocrine disease: Secondary osteoporosis: Pathophysiology and management. Eur. J. Endocrinol. 2015, 173, R131–R151. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Luongo, L.; Torella, M.; Mancusi, S.; De Petrocellis, L.; Petrosino, S.; Siniscalco, D.; Orlando, P.; Scafuro, M.; et al. The endovanilloid/endocannabinoid system: A new potential target for osteoporosis therapy. Bone 2011, 48, 997–1007. [Google Scholar] [CrossRef]
- Frenkel, B.; Hong, A.; Baniwal, S.K.; Coetzee, G.A.; Ohlsson, C.; Khalid, O.; Gabet, Y. Regulation of adult bone turnover by sex steroids. J. Cell. Physiol. 2010, 224, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J. Bone Miner. Res. 1996, 11, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Mauriello-Romanazzi, G.; Berrendero, F.; Ramos, J.A.; Franzoni, M.F.; Fernandez-Ruiz, J. Decreased cannabinoid CB1 receptor mRNA levels and immunoreactivity in pituitary hyperplasia induced by prolonged exposure to estrogens. Pituitary 2000, 3, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Luongo, L.; Mancusi, S.; Torella, M.; Tortora, C.; Manzo, I.; Guida, F.; Nobili, B.; de Novellis, V.; et al. The 17-beta-oestradiol inhibits osteoclast activity by increasing the cannabinoid CB2 receptor expression. Pharmacol. Res. 2013, 68, 7–15. [Google Scholar] [CrossRef]
- Scutt, A.; Williamson, E.M. Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB2 receptors. Calcif. Tissue Int. 2007, 80, 50–59. [Google Scholar] [CrossRef]
- Bab, I.; Zimmer, A. Cannabinoid receptors and the regulation of bone mass. Br. J. Pharmacol. 2008, 153, 182–188. [Google Scholar] [CrossRef]
- Bab, I.A. Regulation of skeletal remodeling by the endocannabinoid system. Ann. N. Y. Acad. Sci. 2007, 1116, 414–422. [Google Scholar] [CrossRef]
- Gimble, J.M.; Zvonic, S.; Floyd, Z.E.; Kassem, M.; Nuttall, M.E. Playing with bone and fat. J. Cell. Biochem. 2006, 98, 251–266. [Google Scholar] [CrossRef]
- Samir, S.M.; Malek, H.A. Effect of cannabinoid receptors 1 modulation on osteoporosis in a rat model of different ages. J. Physiol. Pharmacol. 2014, 65, 687–694. [Google Scholar] [PubMed]
- Sophocleous, A.; Marino, S.; Kabir, D.; Ralston, S.H.; Idris, A.I. Combined deficiency of the Cnr1 and Cnr2 receptors protects against age-related bone loss by osteoclast inhibition. Aging Cell 2017, 16, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Bellini, G.; Torella, M.; Tortora, C.; Manzo, I.; Giordano, C.; Guida, F.; Luongo, L.; Papale, F.; Rosso, F.; et al. The genetic ablation or pharmacological inhibition of TRPV1 signalling is beneficial for the restoration of quiescent osteoclast activity in ovariectomized mice. Br. J. Pharmacol. 2014, 171, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Landao-Bassonga, E.; Ralston, S.H. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone 2010, 46, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Ko, J.Y.; Weng, L.H.; Yeh, D.W.; Ke, H.J.; Wu, S.L. Inhibition of glycogen synthase kinase-3beta attenuates glucocorticoid-induced bone loss. Life Sci. 2009, 85, 685–692. [Google Scholar] [CrossRef]
- Wang, F.S.; Ko, J.Y.; Yeh, D.W.; Ke, H.C.; Wu, H.L. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology 2008, 149, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, F.; Mackintosh, J.; Hayes, B.P.; McLaren, A.; Uings, I.J.; Salmon, P.; Humphreys, J.; Meldrum, E.; Farrow, S.N. Glucocorticoid-induced osteopenia in the mouse as assessed by histomorphometry, microcomputed tomography, and biochemical markers. Bone 2002, 30, 924–930. [Google Scholar] [CrossRef]
- Guler-Yuksel, M.; Hoes, J.N.; Bultink, I.E.M.; Lems, W.F. Glucocorticoids, Inflammation and Bone. Calcif. Tissue Int. 2018, 102, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Whittier, X.; Saag, K.G. Glucocorticoid-induced Osteoporosis. Rheum. Dis. Clin. North Am. 2016, 42, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Natsui, K.; Tanaka, K.; Suda, M.; Yasoda, A.; Sakuma, Y.; Ozasa, A.; Ozaki, S.; Nakao, K. High-dose glucocorticoid treatment induces rapid loss of trabecular bone mineral density and lean body mass. Osteoporosis Int. 2006, 17, 105–108. [Google Scholar] [CrossRef]
- Sosa, M.; Jodar, E.; Saavedra, P.; Navarro, M.C.; Gomez de Tejada, M.J.; Martin, A.; Pena, P.; Gomez, J. Postmenopausal Canarian women receiving oral glucocorticoids have an increased prevalence of vertebral fractures and low values of bone mineral density measured by quantitative computer tomography and dual X-ray absorptiometry, without significant changes in parathyroid hormone. Eur. J. Intern. Med. 2008, 19, 51–56. [Google Scholar]
- Migliaccio, S.; Brama, M.; Fornari, R.; Greco, E.A.; Spera, G.; Malavolta, N. Glucocorticoid-induced osteoporosis: An osteoblastic disease. Aging Clin. Exp. Res. 2007, 19 (Suppl. 3), 5–10. [Google Scholar]
- Bellini, G.; Torella, M.; Manzo, I.; Tortora, C.; Luongo, L.; Punzo, F.; Colacurci, N.; Nobili, B.; Maione, S.; Rossi, F. PKCbetaII-mediated cross-talk of TRPV1/CB2 modulates the glucocorticoid-induced osteoclast overactivity. Pharmacol. Res. 2017, 115, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J.; et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Jeney, V.; Arosio, P.; Poli, M.; Zavaczki, E.; Balla, G.; Balla, J. Ferritin ferroxidase activity: A potent inhibitor of osteogenesis. J. Bone Miner. Res. 2010, 25, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Mariani, R.; Trombini, P.; Pozzi, M.; Piperno, A. Iron metabolism in thalassemia and sickle cell disease. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009006. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Tolnai, E.; Nagy, B., Jr.; Nagy, B.; Balla, G.; Balla, J.; Jeney, V. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim. Biophys. Acta 2016, 1862, 1640–1649. [Google Scholar] [CrossRef]
- Rossi, F.; Perrotta, S.; Bellini, G.; Luongo, L.; Tortora, C.; Siniscalco, D.; Francese, M.; Torella, M.; Nobili, B.; Di Marzo, V.; et al. Iron overload causes osteoporosis in thalassemia major patients through interaction with transient receptor potential vanilloid type 1 (TRPV1) channels. Haematologica 2014, 99, 1876–1884. [Google Scholar] [CrossRef]
- Rizzoli, R.; Body, J.J.; Brandi, M.L.; Cannata-Andia, J.; Chappard, D.; El Maghraoui, A.; Gluer, C.C.; Kendler, D.; Napoli, N.; Papaioannou, A.; et al. Cancer-associated bone disease. Osteoporosis Int. 2013, 24, 2929–2953. [Google Scholar] [CrossRef] [PubMed]
- Shahrokni, A.; Wu, A.J.; Carter, J.; Lichtman, S.M. Long-term Toxicity of Cancer Treatment in Older Patients. Clin. Geriatr. Med. 2016, 32, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and cancer: Therapeutic implication. Br. J. Pharmacol. 2011, 163, 1447–1463. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic activity of cannabinoids. J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Carchman, R.A.; Harris, L.S.; Munson, A.E. The inhibition of DNA synthesis by cannabinoids. Cancer Res. 1976, 36, 95–100. [Google Scholar] [PubMed]
- Cunningham, C.W. Plant-Based Modulators of Endocannabinoid Signaling. J. Nat. Prod. 2019, 82, 636–646. [Google Scholar] [CrossRef]
- Kogan, N.M. Cannabinoids and cancer. Mini Rev. Med. Chem. 2005, 5, 941–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Afaq, F.; Mukhtar, H. Cannabinoids for cancer treatment: Progress and promise. Cancer Res. 2008, 68, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [CrossRef]
- Niaudet, C.; Bonnaud, S.; Guillonneau, M.; Gouard, S.; Gaugler, M.H.; Dutoit, S.; Ripoche, N.; Dubois, N.; Trichet, V.; Corre, I.; et al. Plasma membrane reorganization links acid sphingomyelinase/ceramide to p38 MAPK pathways in endothelial cells apoptosis. Cell. Signal. 2017, 33, 10–21. [Google Scholar] [CrossRef]
- Chien, C.S.; Ma, K.H.; Lee, H.S.; Liu, P.S.; Li, Y.H.; Huang, Y.S.; Chueh, S.H. Dual effect of capsaicin on cell death in human osteosarcoma G292 cells. Eur. J. Pharmacol. 2013, 718, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Cordova, C.; Marchant, I.; Zuniga, R.; Ochova, P.; Ramirez-Barrantes, R.; Gonzalez-Arriagada, W.A.; Rodriguez, B.; Olivero, P. Intracellular aggregated TRPV1 is associated with lower survival in breast cancer patients. Breast Cancer 2018, 10, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Barrantes, R.; Cordova, C.; Gatica, S.; Rodriguez, B.; Lozano, C.; Marchant, I.; Echeverria, C.; Simon, F.; Olivero, P. Transient Receptor Potential Vanilloid 1 Expression Mediates Capsaicin-Induced Cell Death. Front. Physiol. 2018, 9, 682. [Google Scholar] [CrossRef]
- Naziroglu, M.; Cig, B.; Blum, W.; Vizler, C.; Buhala, A.; Marton, A.; Katona, R.; Josvay, K.; Schwaller, B.; Olah, Z.; et al. Targeting breast cancer cells by MRS1477, a positive allosteric modulator of TRPV1 channels. PLoS ONE 2017, 12, e0179950. [Google Scholar] [CrossRef] [PubMed]
- Bovee, J.V.; Hogendoorn, P.C.; Wunder, J.S.; Alman, B.A. Cartilage tumours and bone development: Molecular pathology and possible therapeutic targets. Nat. Rev. Cancer 2010, 10, 481–488. [Google Scholar] [CrossRef]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; de Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone microenvironment signals in osteosarcoma development. Cell. Mol. Life Sci. 2015, 72, 3097–3113. [Google Scholar] [CrossRef]
- Zamborsky, R.; Kokavec, M.; Harsanyi, S.; Danisovic, L. Identification of Prognostic and Predictive Osteosarcoma Biomarkers. Med. Sci. 2019, 7, 28. [Google Scholar] [CrossRef]
- Carnovali, M.; Ottria, R.; Pasqualetti, S.; Banfi, G.; Ciuffreda, P.; Mariotti, M. Effects of bioactive fatty acid amide derivatives in zebrafish scale model of bone metabolism and disease. Pharmacol. Res. 2016, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Watson, J.E.; Hong, I.S.; Fan, T.M.; Das, A. Antitumorigenic Properties of Omega-3 Endocannabinoid Epoxides. J. Med. Chem. 2018, 61, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, F.F.; Han, Y.C.; Jia, B.; Ding, Y. Cannabinoid receptor CB2 is involved in tetrahydrocannabinol-induced anti-inflammation against lipopolysaccharide in MG-63 cells. Mediat. Inflamm. 2015, 2015, 362126. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, Z.I.; Lee, S.; Kirkland, R.; Ross, M.; de La Serre, C.B. Cannabinoid receptor type-1 partially mediates metabolic endotoxemia-induced inflammation and insulin resistance. Physiol. Behav. 2019, 199, 282–291. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Taylor, L.; Feuerborn, A.; Valaris, S.; Hussain, M.T.; Rainger, G.E.; Greaves, D.R.; Iqbal, A.J. Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. FASEB J. 2019, fj201802524R. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Gentile, A.; Iezzi, E.; Zagaglia, S.; Musella, A.; Simonelli, I.; Gilio, L.; Furlan, R.; Finardi, A.; Marfia, G.A.; et al. Transient Receptor Potential Vanilloid 1 Modulates Central Inflammation in Multiple Sclerosis. Front. Neurol. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F.; Tortora, C.; Di Pinto, D.; Manzo, I.; Bellini, G.; Casale, F.; Rossi, F. Anti-proliferative, pro-apoptotic and anti-invasive effect of EC/EV system in human osteosarcoma. Oncotarget 2017, 8, 54459–54471. [Google Scholar] [CrossRef] [PubMed]
- Mohseny, A.B.; Machado, I.; Cai, Y.; Schaefer, K.L.; Serra, M.; Hogendoorn, P.C.; Llombart-Bosch, A.; Cleton-Jansen, A.M. Functional characterization of osteosarcoma cell lines provides representative models to study the human disease. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 1195–1205. [Google Scholar] [CrossRef]
- Punzo, F.; Tortora, C.; Di Pinto, D.; Pota, E.; Argenziano, M.; Di Paola, A.; Casale, F.; Rossi, F. Bortezomib and endocannabinoid/endovanilloid system: A synergism in osteosarcoma. Pharmacol. Res. 2018, 137, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.S.; Huang, C.J.; Cheng, H.H.; Chou, C.T.; Lee, H.Y.; Wang, J.L.; Chen, I.S.; Liu, S.I.; Lu, Y.C.; Chang, H.T.; et al. Anandamide-induced Ca2+ elevation leading to p38 MAPK phosphorylation and subsequent cell death via apoptosis in human osteosarcoma cells. Toxicology 2007, 231, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bellini, G.; Di Pinto, D.; Tortora, C.; Manzo, I.; Punzo, F.; Casale, F.; Rossi, F. The Role of Mifamurtide in Chemotherapy-induced Osteoporosis of Children with Osteosarcoma. Curr. Cancer Drug Targets 2017, 17, 650–656. [Google Scholar] [CrossRef]
- Marino, S.; Idris, A.I. Emerging therapeutic targets in cancer induced bone disease: A focus on the peripheral type 2 cannabinoid receptor. Pharmacol. Res. 2017, 119, 391–403. [Google Scholar] [CrossRef]
- Lozano-Ondoua, A.N.; Hanlon, K.E.; Symons-Liguori, A.M.; Largent-Milnes, T.M.; Havelin, J.J.; Ferland, H.L., 3rd; Chandramouli, A.; Owusu-Ankomah, M.; Nikolich-Zugich, T.; Bloom, A.P.; et al. Disease modification of breast cancer-induced bone remodeling by cannabinoid 2 receptor agonists. J. Bone Miner. Res. 2013, 28, 92–107. [Google Scholar] [CrossRef]
- Wang, D.; Ren, B.X.; Liu, C.L.; Zhu, J.; Zhang, J.; Zhang, W.; Mei, F.M.; Gu, X.P.; Ma, Z.L. [Role of cannabinoid 2 receptor in the development of bone cancer pain]. Zhonghua Yi Xue Za Zhi 2012, 92, 440–443. [Google Scholar]
- Romero-Sandoval, A.; Eisenach, J.C. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology 2007, 106, 787–794. [Google Scholar] [CrossRef]
- Yao, B.B.; Hsieh, G.C.; Frost, J.M.; Fan, Y.; Garrison, T.R.; Daza, A.V.; Grayson, G.K.; Zhu, C.Z.; Pai, M.; Chandran, P.; et al. In vitro and in vivo characterization of A-796260: A selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br. J. Pharmacol. 2008, 153, 390–401. [Google Scholar] [CrossRef]
- Leichsenring, A.; Andriske, M.; Backer, I.; Stichel, C.C.; Lubbert, H. Analgesic and antiinflammatory effects of cannabinoid receptor agonists in a rat model of neuropathic pain. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 379, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Curto-Reyes, V.; Llames, S.; Hidalgo, A.; Menendez, L.; Baamonde, A. Spinal and peripheral analgesic effects of the CB2 cannabinoid receptor agonist AM1241 in two models of bone cancer-induced pain. Br. J. Pharmacol. 2010, 160, 561–573. [Google Scholar] [CrossRef]
- Lozano-Ondoua, A.N.; Wright, C.; Vardanyan, A.; King, T.; Largent-Milnes, T.M.; Nelson, M.; Jimenez-Andrade, J.M.; Mantyh, P.W.; Vanderah, T.W. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sci. 2010, 86, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, G.; Gao, Y.; Du, M.; Yang, L.; Kong, X.; Zheng, H.; Hou, W.; Hua, B. Topical treatment with Xiaozheng Zhitong Paste alleviates bone cancer pain by inhibiting proteinase-activated receptor 2 signaling pathway. Oncol. Rep. 2015, 34, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, Y.; Kawamata, T.; Yamamoto, J.; Omote, K.; Namiki, A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience 2007, 148, 560–572. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, F.; Tortora, C.; Punzo, F.; Bellini, G.; Argenziano, M.; Di Paola, A.; Torella, M.; Perrotta, S. The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. Int. J. Mol. Sci. 2019, 20, 1919. https://doi.org/10.3390/ijms20081919
Rossi F, Tortora C, Punzo F, Bellini G, Argenziano M, Di Paola A, Torella M, Perrotta S. The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. International Journal of Molecular Sciences. 2019; 20(8):1919. https://doi.org/10.3390/ijms20081919
Chicago/Turabian StyleRossi, Francesca, Chiara Tortora, Francesca Punzo, Giulia Bellini, Maura Argenziano, Alessandra Di Paola, Marco Torella, and Silverio Perrotta. 2019. "The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma" International Journal of Molecular Sciences 20, no. 8: 1919. https://doi.org/10.3390/ijms20081919
APA StyleRossi, F., Tortora, C., Punzo, F., Bellini, G., Argenziano, M., Di Paola, A., Torella, M., & Perrotta, S. (2019). The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. International Journal of Molecular Sciences, 20(8), 1919. https://doi.org/10.3390/ijms20081919