Evaluation of Biological Response of STRO-1/c-Kit Enriched Human Dental Pulp Stem Cells to Titanium Surfaces Treated with Two Different Cleaning Systems
Abstract
1. Introduction
2. Results
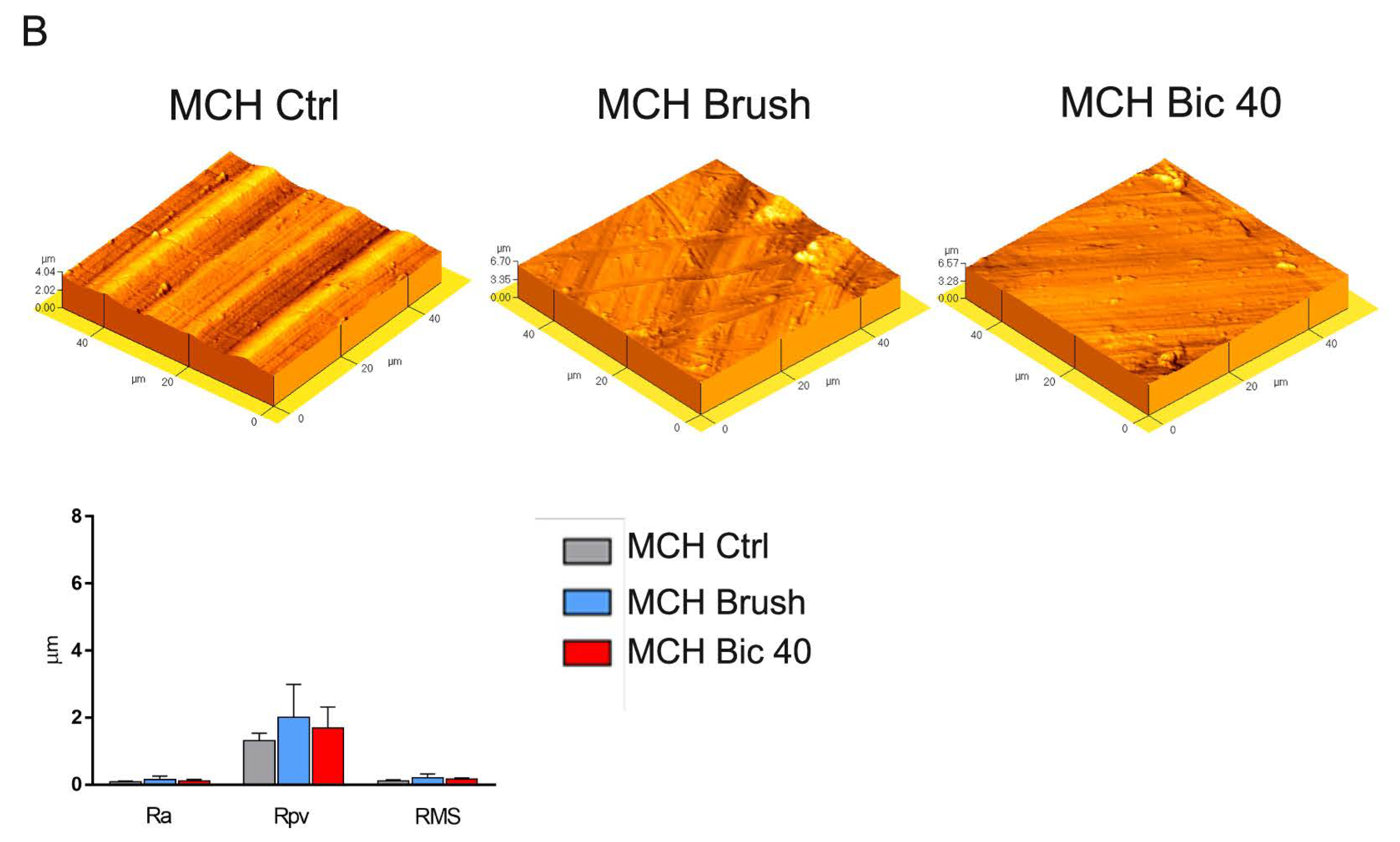
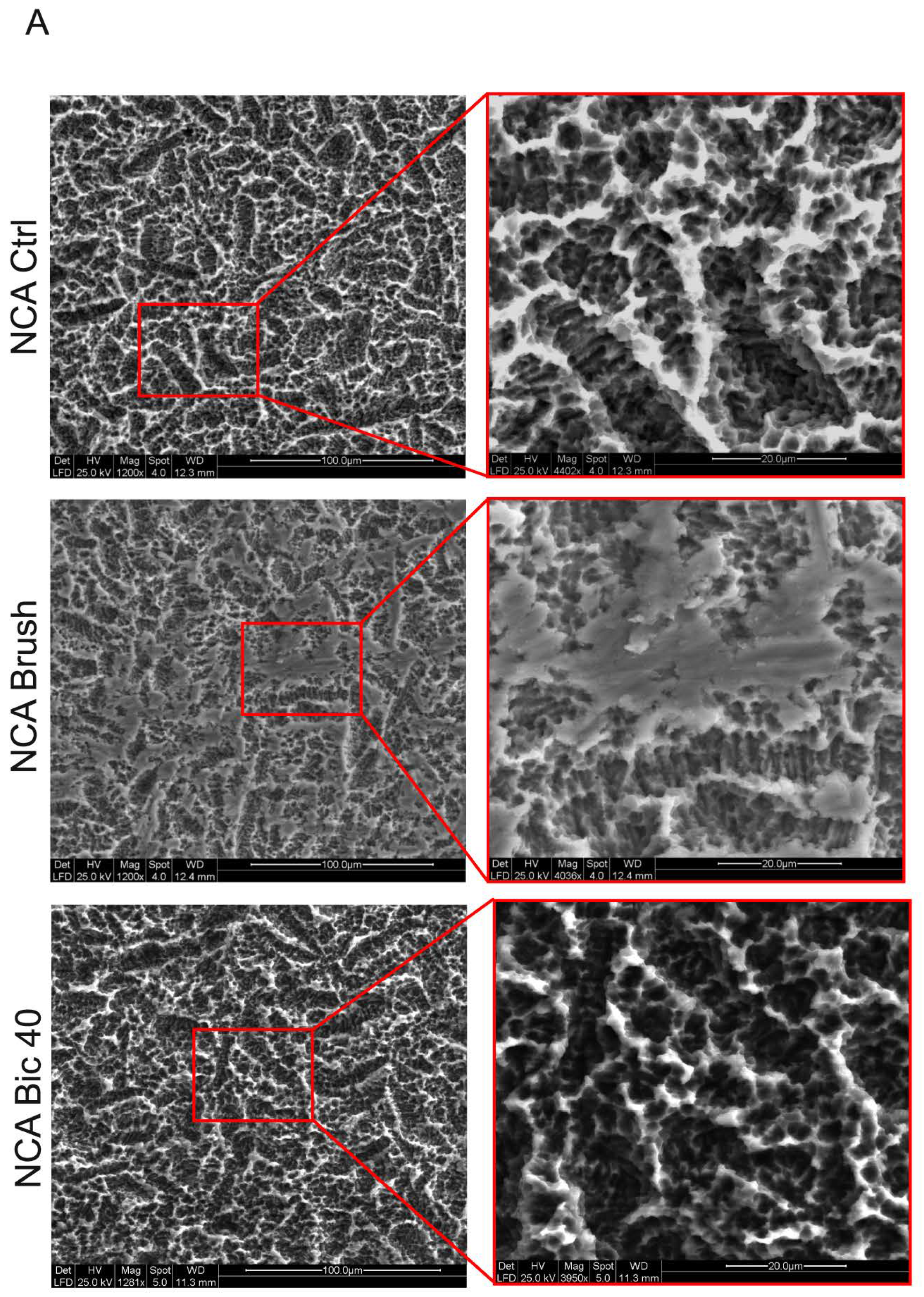
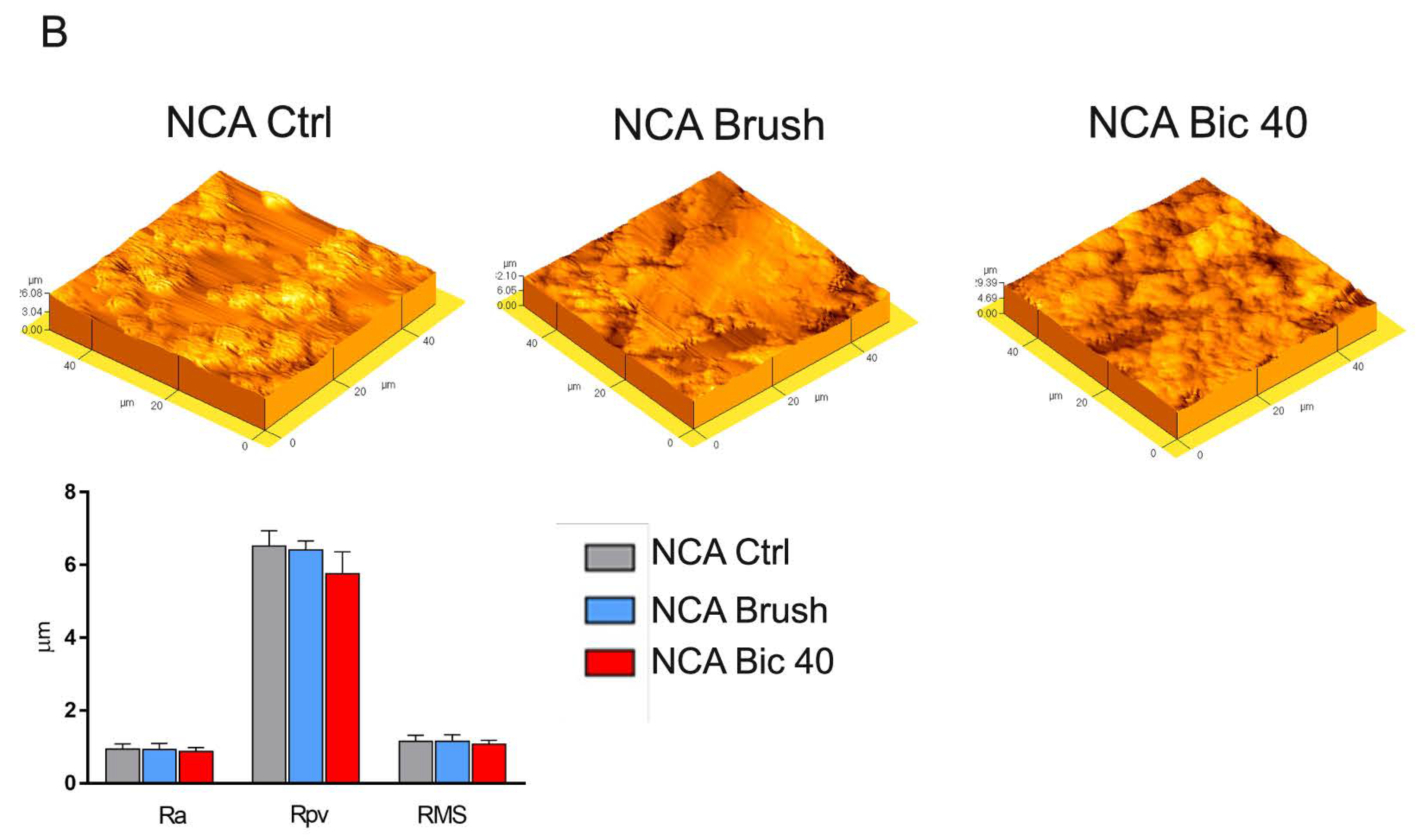
2.1. Titanium Surface Characterization
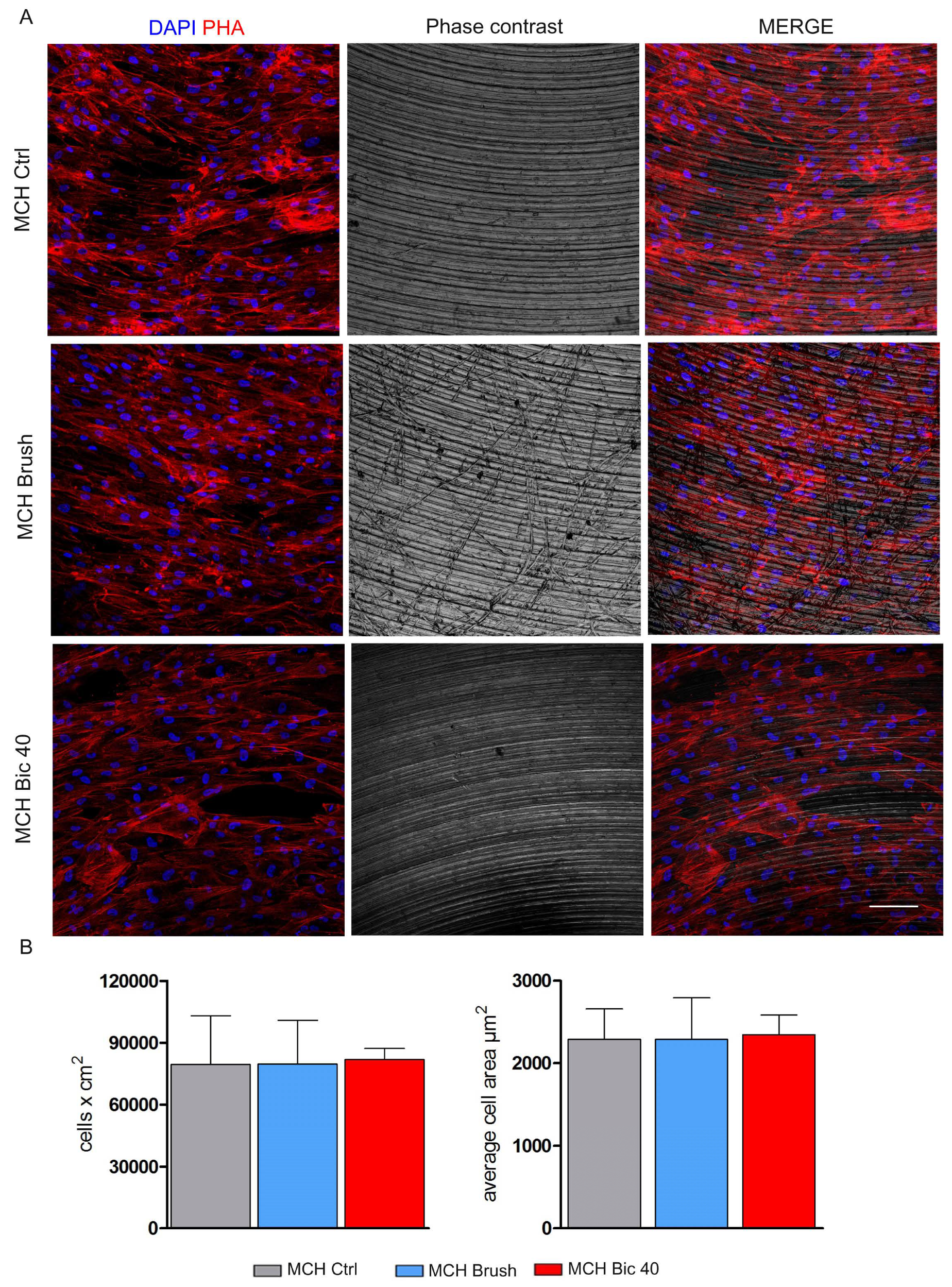
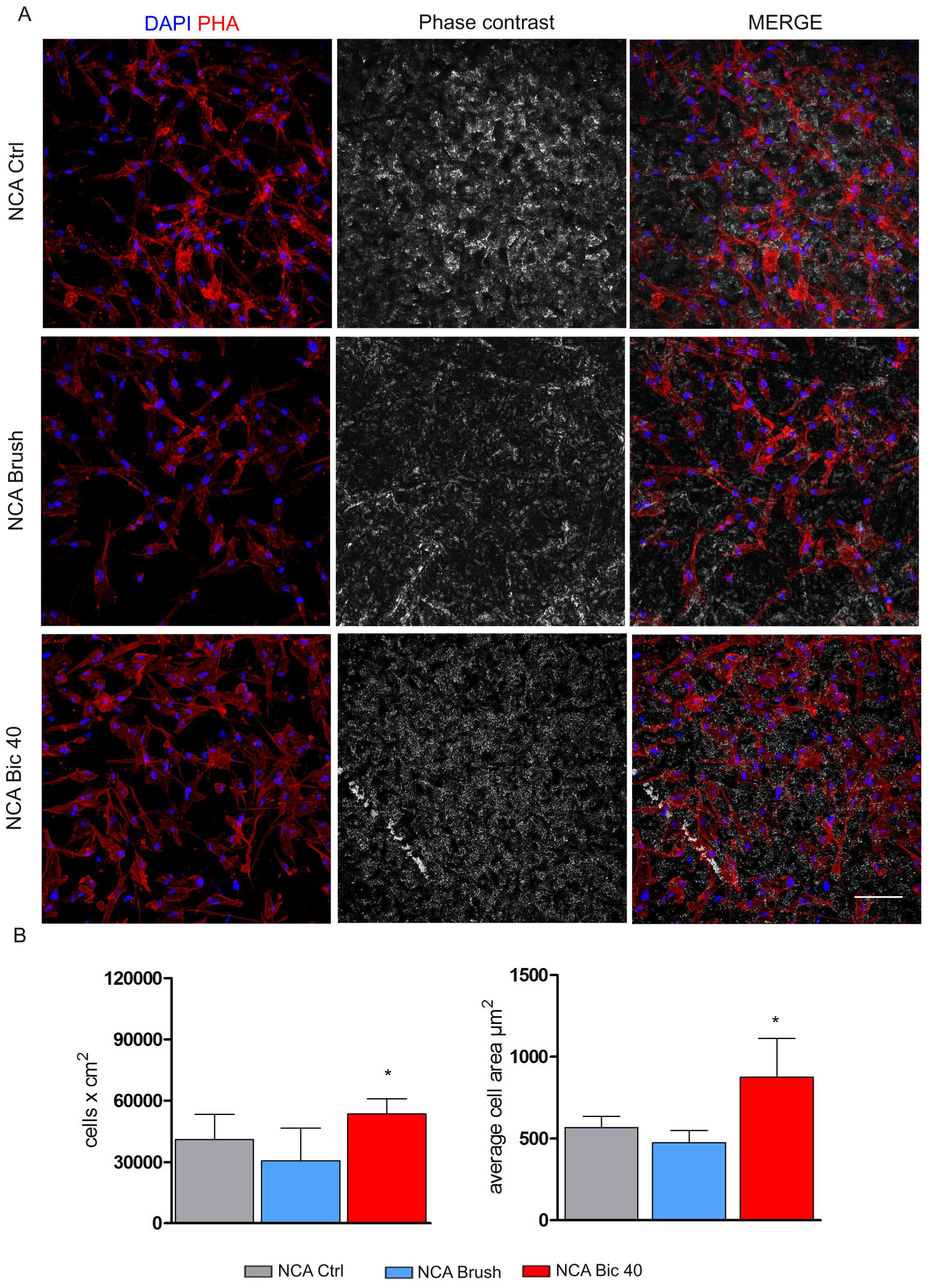
2.2. Stem Cells Morphology and Proliferation on Titanium Surfaces after Polishing Treatments
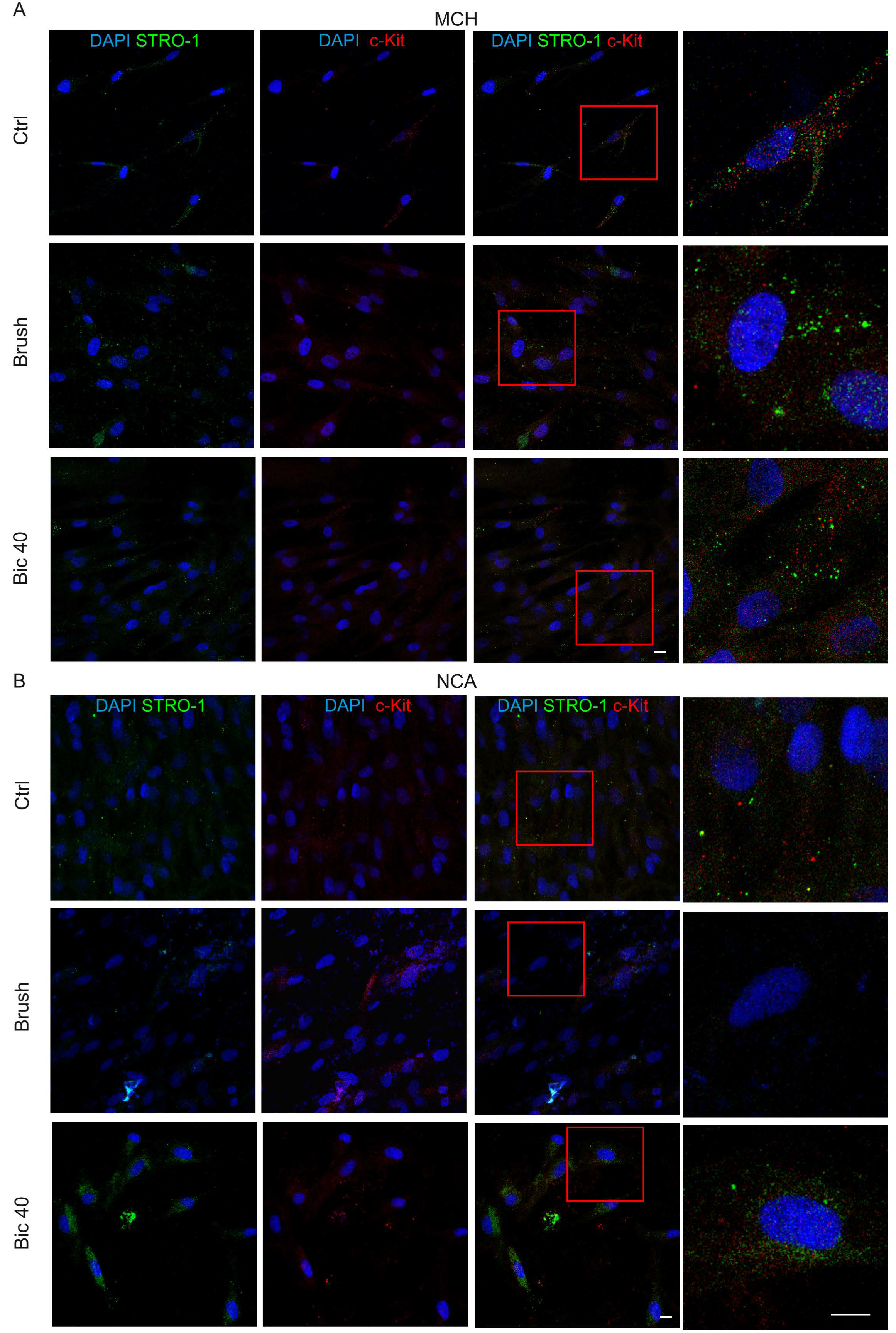
2.3. Expression of Stemness Markers
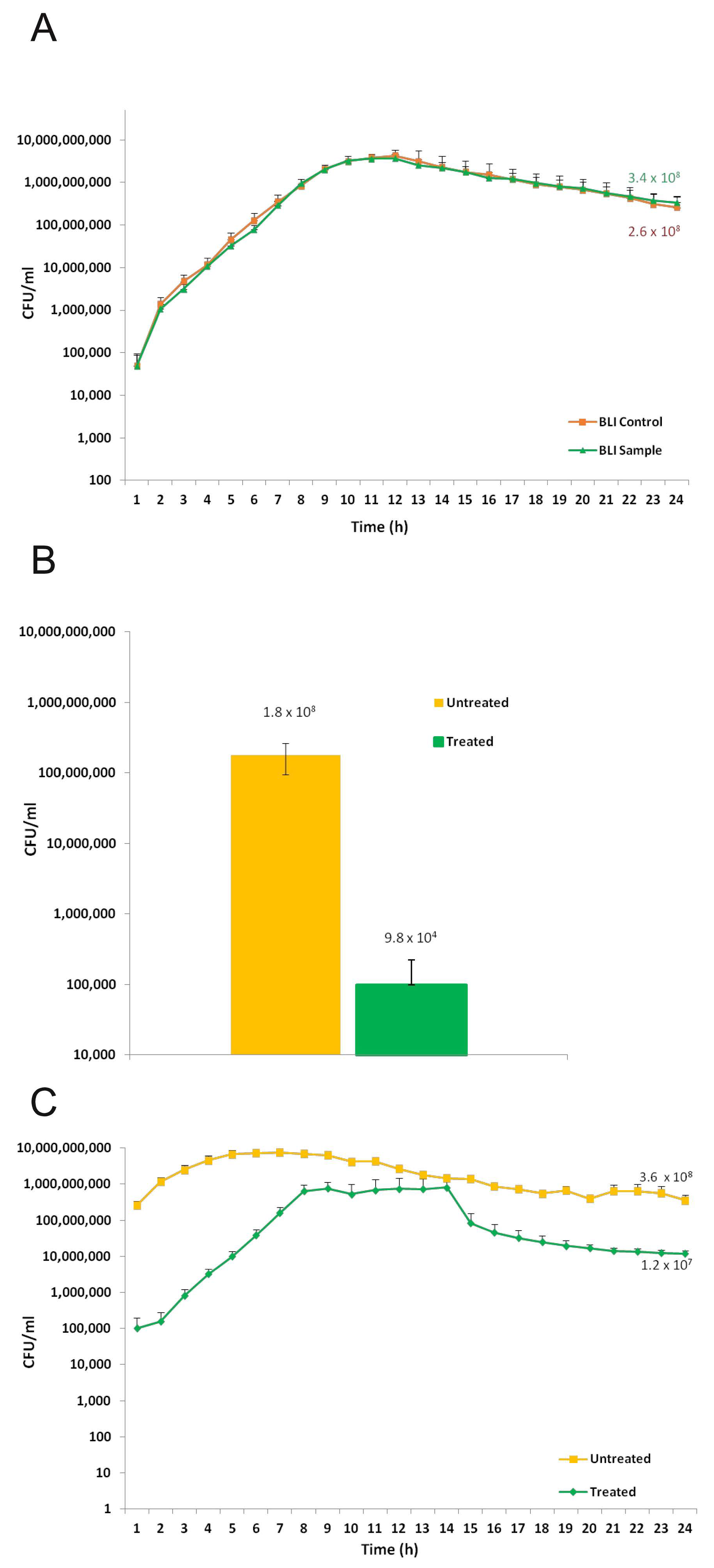
2.4. Microbial Biofilm Formation onto Titanium Disks
2.5. Microbial Biofilm Removal from Titanium Disks
2.6. Microbial Re-Growth on Treated and Untreated Titanium Disks
3. Discussion
4. Materials and Methods
4.1. Human DPSCs Isolation and Immune Selection
4.2. Titanium Surfaces Characterization
4.3. Cell Morphology and Proliferation
4.4. Evaluation of Stemness Markers in hDPSCs Cultured on Titanium Disks
4.5. Microbial Strain
4.6. Biofilm Formation onto Titanium Disks
4.7. Biofilm Re-Growth onto Treated and Untreated Titanium Disks
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Albrektsson, T.; Donos, N. Implant survival and complications. The Third EAO consensus conference 2012. Clin. Oral Implant. Res. 2012, 23, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J.; on behalf of Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef]
- Kumar, P.S. Systemic Risk Factors for the Development of Periimplant Diseases. Implant. Dent. 2019, 28, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, 158. [Google Scholar] [CrossRef] [PubMed]
- Dennison, D.K.; Huerzeler, M.B.; Quinones, C.; Caffesse, R.G. Contaminated Implant Surfaces: An In Vitro Comparison of Implant Surface Coating and Treatment Modalities for Decontamination. J. Periodontol. 1994, 65, 942–948. [Google Scholar] [CrossRef]
- Toma, S.; Lasserre, J.F.; Taïeb, J.; Brecx, M.C. Evaluation of an air-abrasive device with amino acid glycine-powder during surgical treatment of peri-implantitis. Quintessence Int 2014, 45, 209–219. [Google Scholar]
- Sahrmann, P.; Ronay, V.; Hofer, D.; Attin, T.; Jung, R.E.; Schmidlin, P.R. In vitro cleaning potential of three different implant debridement methods. Clin. Oral Implant. Res. 2015, 26, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Ferrari, D.; Popovski, K.; Hartig, B.; Becker, J. Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Tastepe, C.S.; Liu, Y.; Visscher, C.M.; Wismeijer, D. Cleaning and modification of intraorally contaminated titanium disks with calcium phosphate powder abrasive treatment. Clin. Oral Implant. Res. 2013, 24, 1238–1246. [Google Scholar]
- Wei, M.C.; Tran, C.; Meredith, N.; Walsh, L.J. Effectiveness of implant surface debridement using particle beams at differing air pressures. Clin. Exp. Dent. Res. 2017, 3, 148–153. [Google Scholar] [CrossRef]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. Influence of mechanical instruments on the biocompatibility of titanium dental implants surfaces: A systematic review. Clin. Oral Implant. Res. 2015, 26, 841–850. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Feller, L.; Jadwat, Y.; Khammissa, R.A.G.; Meyerov, R.; Schechter, I.; Lemmer, J. Cellular Responses Evoked by Different Surface Characteristics of Intraosseous Titanium Implants. Biomed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Conserva, E.; Pisciotta, A.; Borghi, F.; Nasi, M.; Pecorini, S.; Bertoni, L.; de Pol, A.; Consolo, U.; Carnevale, G. Titanium Surface Properties Influence the Biological Activity and FasL Expression of Craniofacial Stromal Cells. Stem Cells Int. 2019, 2019, 4670560. [Google Scholar] [CrossRef]
- John, G.; Becker, J.; Schwarz, F. Rotating titanium brush for plaque removal from rough titanium surfaces—An in vitro study. Clin. Oral Implant. Res. 2014, 25, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Duddeck, D.; Karapetian, V.; Grandoch, A. Time-saving debridment of implants with rotating titanium brushes. Implants 2012, 3, 20–22. [Google Scholar]
- Park, J.-B.; Jeon, Y.; Ko, Y. Effects of titanium brush on machined and sand-blasted/acid-etched titanium disc using confocal microscopy and contact profilometry. Clin. Oral Implant. Res. 2015, 26, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: A systematic review. Clin. Oral Implant. Res. 2014, 25, 1149–1160. [Google Scholar] [CrossRef]
- Tastepe, C.S.; Van Waas, R.; Liu, Y.; Wismeijer, D. Air powder abrasive treatment as an implant surface cleaning method: A literature review. Int. J. Oral Maxillofac. Implant. 2012, 27, 1461–1473. [Google Scholar]
- Nemer Vieira, L.F.; Lopes de Chaves e Mello Dias, E.C.; Cardoso, E.S.; Machado, S.J.; Pereira da Silva, C.; Vidigal, G.M. Effectiveness of implant surface decontamination using a high-pressure sodium bicarbonate protocol: An in vitro study. Implant Dent. 2012, 21, 390–393. [Google Scholar] [CrossRef]
- Pisciotta, A.; Bertoni, L.; Riccio, M.; Mapelli, J.; Bigiani, A.; La Noce, M.; Orciani, M.; De Pol, A.; Carnevale, G. Use of a 3D Floating Sphere Culture System to Maintain the Neural Crest-Related Properties of Human Dental Pulp Stem Cells. Front. Physiol. 2018, 9, 547. [Google Scholar] [CrossRef]
- Pisciotta, A.; Carnevale, G.; Meloni, S.; Riccio, M.; De Biasi, S.; Gibellini, L.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human Dental pulp stem cells (hDPSCs): Isolation, enrichment and comparative differentiation of two sub-populations. Bmc Dev. Boil. 2015, 15, 14. [Google Scholar] [CrossRef]
- Bianchi, M.; Pisciotta, A.; Bertoni, L.; Berni, M.; Gambardella, A.; Visani, A.; Russo, A.; De Pol, A.; Carnevale, G. Osteogenic Differentiation of hDPSCs on Biogenic Bone Apatite Thin Films. Stem Cells Int. 2017, 2017, 1–10. [Google Scholar]
- Heitz-Mayfield, L.J.A.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontology 2010, 53, 167–181. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Conserva, E.; Generali, L.; Bandieri, A.; Cavani, F.; Borghi, F.; Consolo, U. Plaque accumulation on titanium disks with different surface treatments: An in vivo investigation. Odontology 2018, 106, 145–153. [Google Scholar] [CrossRef]
- Anselme, K.; Ponche, A.; Bigerelle, M. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: Biological aspects. Proc. Inst. Mech. Eng. H 2010, 224, 1487–1507. [Google Scholar] [CrossRef]
- Pericolini, E.; Colombari, B.; Ferretti, G.; Iseppi, R.; Ardizzoni, A.; Girardis, M.; Sala, A.; Peppoloni, S.; Blasi, E. Real-time monitoring of Pseudomonas aeruginosa biofilm formation on endotracheal tubes in vitro. Bmc Microbiol. 2018, 18, 84. [Google Scholar] [CrossRef]
- Pagano, S.; Chieruzzi, M.; Balloni, S.; Lombardo, G.; Torre, L.; Bodo, M.; Cianetti, S.; Marinucci, L. Biological, thermal and mechanical characterization of modified glass ionomer cements: The role of nanohydroxyapatite, ciprofloxacin and zinc l-carnosine. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 76–8516. [Google Scholar] [CrossRef]
- Carnevale, G.; Riccio, M.; Pisciotta, A.; Beretti, F.; Maraldi, T.; Zavatti, M.; Cavallini, G.M.; La Sala, G.B.; Ferrari, A.; De Pol, A. In vitro differentiation into insulin-producing β-cells of stem cells isolated from human amniotic fluid and dental pulp. Dig. Liver Dis. 2013, 45, 669–676. [Google Scholar]
- Carnevale, G.; Pisciotta, A.; Riccio, M.; Bertoni, L.; De Biasi, S.; Gibellini, L.; Zordani, A.; Cavallini, GM.; La Sala, GB.; Bruzzesi, G.; et al. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e774–e785. [Google Scholar] [CrossRef]
- Choi, K.-H.; Schweizer, H.P. mini-Tn7 insertion in bacteria with single attTn7 sites: Example Pseudomonas aeruginosa. Nat. Protoc. 2006, 1, 153–161. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conserva, E.; Pisciotta, A.; Bertoni, L.; Bertani, G.; Meto, A.; Colombari, B.; Blasi, E.; Bellini, P.; de Pol, A.; Consolo, U.; et al. Evaluation of Biological Response of STRO-1/c-Kit Enriched Human Dental Pulp Stem Cells to Titanium Surfaces Treated with Two Different Cleaning Systems. Int. J. Mol. Sci. 2019, 20, 1868. https://doi.org/10.3390/ijms20081868
Conserva E, Pisciotta A, Bertoni L, Bertani G, Meto A, Colombari B, Blasi E, Bellini P, de Pol A, Consolo U, et al. Evaluation of Biological Response of STRO-1/c-Kit Enriched Human Dental Pulp Stem Cells to Titanium Surfaces Treated with Two Different Cleaning Systems. International Journal of Molecular Sciences. 2019; 20(8):1868. https://doi.org/10.3390/ijms20081868
Chicago/Turabian StyleConserva, Enrico, Alessandra Pisciotta, Laura Bertoni, Giulia Bertani, Aida Meto, Bruna Colombari, Elisabetta Blasi, Pierantonio Bellini, Anto de Pol, Ugo Consolo, and et al. 2019. "Evaluation of Biological Response of STRO-1/c-Kit Enriched Human Dental Pulp Stem Cells to Titanium Surfaces Treated with Two Different Cleaning Systems" International Journal of Molecular Sciences 20, no. 8: 1868. https://doi.org/10.3390/ijms20081868
APA StyleConserva, E., Pisciotta, A., Bertoni, L., Bertani, G., Meto, A., Colombari, B., Blasi, E., Bellini, P., de Pol, A., Consolo, U., & Carnevale, G. (2019). Evaluation of Biological Response of STRO-1/c-Kit Enriched Human Dental Pulp Stem Cells to Titanium Surfaces Treated with Two Different Cleaning Systems. International Journal of Molecular Sciences, 20(8), 1868. https://doi.org/10.3390/ijms20081868










