BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine
Abstract
1. Introduction
2. Biological Features of BNP and NT-proBNP
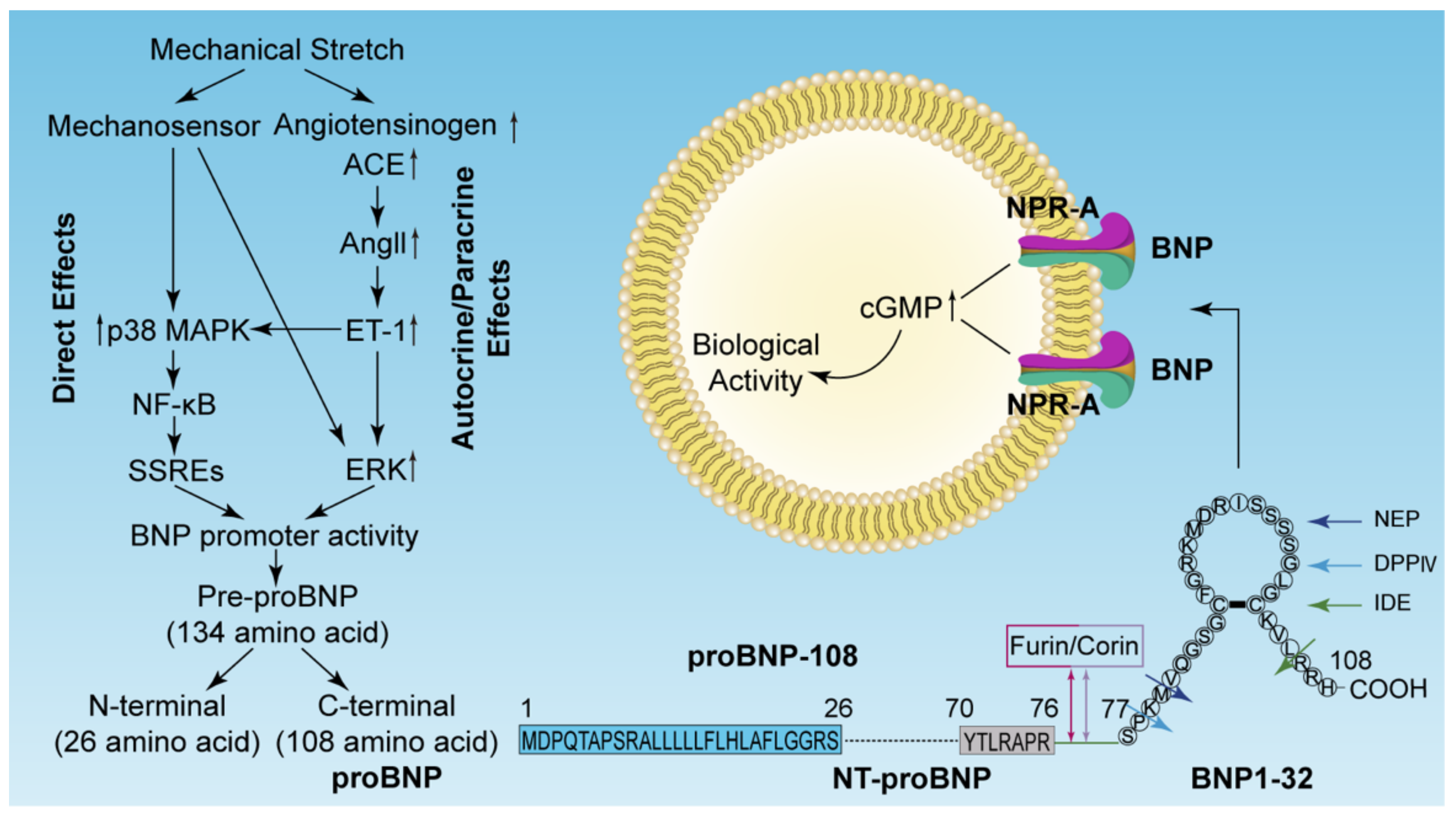
2.1. Structure, Synthesis, and Secretion of BNP and NT-proBNP
2.2. Receptors of Natriuretic Peptides
2.3. Degradation of BNP and NT-proBNP
3. Regulation of BNP Gene Expression
3.1. ET-Independent Pathway (Direct Effects)
3.2. ET-Dependent Pathway (Autocrine/Paracrine Effects)
3.3. Other Factors
4. BNP and NT-proBNP as Clinical Biomarkers for the Diagnosis of HF
4.1. Clinical Cutoffs of BNP and NT-proBNP
4.2. Diagnostic Role in a Failing Heart
4.3. Assessing the Severity and Prognosis of HF
4.4. Therapeutic Role in Cardiac Dysfunction
5. BNP and NT-proBNP as Postmortem Biomarkers to Evaluate Cardiac Function in Forensic Medicine
5.1. Forensic Significance of Functional Biomarkers
5.2. Pericardial Fluid in Postmortem Biochemistry
5.3. Postmortem BNP and NT-proBNP
5.4. Limitation of BNP and NT-proBNP in Forensic Medicine
6. Research and Application Prospects in Clinical and Forensic Medicine
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACCF | American College of Cardiology Foundation |
| AHA | American Heart Association |
| Ang | Angiotensin |
| ANP | Atrial natriuretic peptide |
| AMI | Acute myocardial infarction |
| AP | Activator protein |
| AT1R | Angiotensin II type 1 receptor |
| BNP | Brain natriuretic peptide |
| cGMP | Cyclic guanosine monophosphate |
| CK-MB | Creatine kinase MB |
| CNP | C-type natriuretic peptide |
| cTn | Cardiac troponin |
| DHF | Diastolic heart failure |
| DNA | Deoxyribonucleic acid |
| DPPIV | Dipeptidyl peptidase-IV |
| EF | Ejection fraction |
| ERK | Extracellular signal regulated kinase |
| ESC | European Society of Cardiology |
| ET | Endothelin |
| FDA | Food and Drug Administration |
| HF | Heart failure |
| HFnEF | Heart failure with normal ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| ICON | International Collaborative of NT-proBNP |
| IDE | Insulin degrading enzyme |
| LV | Left ventricle |
| LVEDD | Left ventricular end-diastolic dimension |
| LVEF | Left ventricular ejection fraction |
| NEP | Neutral endopeptidase |
| NF-κB | Nuclear factor kappa B |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| NPR | Natriuretic peptide receptor |
| NYHA | New York Heart Association |
| MAPK | Mitogen-activated protein kinase |
| RAAS | Renin–angiotensin–aldosterone system |
| rhBNP | Recombinant human brain natriuretic peptide |
| RNA | Ribonucleic acid |
| SHF | Systolic heart failure |
| SSREs | Shear stress-responsive elements |
References
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Stromberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Heart J. 2008, 29, 2388–2442. [Google Scholar] [CrossRef]
- Huffman, M.D.; Prabhakaran, D. Heart failure: epidemiology and prevention in India. Natl. Med. J. India 2010, 23, 283–288. [Google Scholar] [PubMed]
- Weiwei, C.; Runlin, G.; Lisheng, L.; Manlu, Z.; Wen, W.; Yongjun, W.; Zhaosu, W.; Huijun, L.; Zhe, Z.; Lixin, J.; et al. Outline of the report on cardiovascular diseases in China, 2014. Eur. Heart J. Suppl. 2016, 18, F2–F11. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Bloom, M.W.; Greenberg, B.; Jaarsma, T.; Januzzi, J.L.; Lam, C.S.P.; Maggioni, A.P.; Trochu, J.N.; Butler, J. Heart failure with reduced ejection fraction. Nat. Rev. Dis. Primers 2017, 3, 17058. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Cao, Z.P.; Xue, J.J.; Zhang, Y.; Tian, M.H.; Xiao, Y.; Jia, Y.Q.; Zhu, B.L. Differential expression of B-type natriuretic peptide between left and right ventricles, with particular regard to sudden cardiac death. Mol. Med. Rep. 2017, 16, 4763–4769. [Google Scholar] [CrossRef]
- Chen, J.H.; Michiue, T.; Ishikawa, T.; Maeda, H. Pathophysiology of sudden cardiac death as demonstrated by molecular pathology of natriuretic peptides in the myocardium. Forensic Sci. Int. 2012, 223, 342–348. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ishikawa, T.; Michiue, T.; Li, D.R.; Zhao, D.; Tanaka, S.; Kamikodai, Y.; Tsuda, K.; Okazaki, S.; Maeda, H. Postmortem pericardial natriuretic peptides as markers of cardiac function in medico-legal autopsies. Int. J. Legal Med. 2007, 121, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Michiue, T.; Ishikawa, T.; Maeda, H. Molecular pathology of natriuretic peptides in the myocardium with special regard to fatal intoxication, hypothermia, and hyperthermia. Int. J. Legal Med. 2012, 126, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Oldgren, J.; Siegbahn, A.; Granger, C.B.; Wallentin, L. Biomarkers in atrial fibrillation: A clinical review. Eur. Heart J. 2013, 34, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, R.; Bailey, S. A review on B-type natriuretic peptide monitoring: assays and biosensors. Heart Fail. Rev. 2016, 21, 567–578. [Google Scholar] [CrossRef]
- Maries, L.; Manitiu, I. Diagnostic and prognostic values of B-type natriuretic peptides (BNP) and N-terminal fragment brain natriuretic peptides (NT-pro-BNP). Cardiovasc. J. Afr. 2013, 24, 286–289. [Google Scholar] [CrossRef]
- Troughton, R.; Michael Felker, G.; Januzzi, J.L., Jr. Natriuretic peptide-guided heart failure management. Eur. Heart J. 2014, 35, 16–24. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; de Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L., Jr.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef]
- Cocco, G.; Jerie, P. Assessing the benefits of natriuretic peptides-guided therapy in chronic heart failure. Cardiol. J. 2015, 22, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Forte, M.; Marchitti, S.; Volpe, M. Molecular Implications of Natriuretic Peptides in the Protection from Hypertension and Target Organ Damage Development. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Michaud, K.; Augsburger, M.; Donze, N.; Sabatasso, S.; Faouzi, M.; Bollmann, M.; Mangin, P. Evaluation of postmortem measurement of NT-proBNP as a marker for cardiac function. Int. J. Legal Med. 2008, 122, 415–420. [Google Scholar] [CrossRef]
- Palmiere, C.; Tettamanti, C.; Bonsignore, A.; De Stefano, F.; Vanhaebost, J.; Rousseau, G.; Scarpelli, M.P.; Bardy, D. Cardiac troponins and NT-proBNP in the forensic setting: Overview of sampling site, postmortem interval, cardiopulmonary resuscitation, and review of the literature. Forensic Sci. Int. 2018, 282, 211–218. [Google Scholar] [CrossRef]
- Sabatasso, S.; Vaucher, P.; Augsburger, M.; Donze, N.; Mangin, P.; Michaud, K. Sensitivity and specificity of NT-proBNP to detect heart failure at post mortem examination. Int. J. Legal Med. 2011, 125, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Del Ry, S.; Cabiati, M.; Clerico, A. Natriuretic peptide system and the heart. Front. Horm. Res. 2014, 43, 134–143. [Google Scholar] [CrossRef]
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef]
- Clerico, A.; Recchia, F.A.; Passino, C.; Emdin, M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H17–H29. [Google Scholar] [CrossRef]
- De Lemos, J.A.; McGuire, D.K.; Drazner, M.H. B-type natriuretic peptide in cardiovascular disease. Lancet 2003, 362, 316–322. [Google Scholar] [CrossRef]
- Rodeheffer, R.J. Measuring plasma B-type natriuretic peptide in heart failure: good to go in 2004? J. Am. Coll. Cardiol. 2004, 44, 740–749. [Google Scholar] [CrossRef][Green Version]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar] [CrossRef]
- Grantham, J.A.; Borgeson, D.D.; Burnett, J.C., Jr. BNP: Pathophysiological and potential therapeutic roles in acute congestive heart failure. Am. J. Physiol. 1997, 272, R1077–R1083. [Google Scholar] [CrossRef]
- Cheung, B.M.; Kumana, C.R. Natriuretic peptides--relevance in cardiovascular disease. Jama 1998, 280, 1983–1984. [Google Scholar] [CrossRef]
- Daniels, L.B.; Maisel, A.S. Natriuretic peptides. J. Am. Coll. Cardiol. 2007, 50, 2357–2368. [Google Scholar] [CrossRef]
- Nakagawa, O.; Ogawa, Y.; Itoh, H.; Suga, S.; Komatsu, Y.; Kishimoto, I.; Nishino, K.; Yoshimasa, T.; Nakao, K. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J. Clin. Invest. 1995, 96, 1280–1287. [Google Scholar] [CrossRef]
- Sudoh, T.; Maekawa, K.; Kojima, M.; Minamino, N.; Kangawa, K.; Matsuo, H. Cloning and sequence analysis of cDNA encoding a precursor for human brain natriuretic peptide. Biochem. Biophys. Res. Commun. 1989, 159, 1427–1434. [Google Scholar] [CrossRef]
- Hama, N.; Itoh, H.; Shirakami, G.; Nakagawa, O.; Suga, S.; Ogawa, Y.; Masuda, I.; Nakanishi, K.; Yoshimasa, T.; Hashimoto, Y.; et al. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation 1995, 92, 1558–1564. [Google Scholar] [CrossRef]
- Kerkela, R.; Ulvila, J.; Magga, J. Natriuretic Peptides in the Regulation of Cardiovascular Physiology and Metabolic Events. J. Am. Heart Assoc. 2015, 4, e002423. [Google Scholar] [CrossRef]
- Vanderheyden, M.; Bartunek, J.; Goethals, M. Brain and other natriuretic peptides: Molecular aspects. Eur. J. Heart Fail. 2004, 6, 261–268. [Google Scholar] [CrossRef]
- Yamanouchi, S.; Kudo, D.; Endo, T.; Kitano, Y.; Shinozawa, Y. Blood N-terminal proBNP as a potential indicator of cardiac preload in patients with high volume load. Tohoku J. Exp. Med. 2010, 221, 175–180. [Google Scholar] [CrossRef]
- Volpe, M.; Rubattu, S.; Burnett, J. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur. Heart J. 2014, 35, 419–425. [Google Scholar] [CrossRef]
- Cataliotti, A.; Boerrigter, G.; Costello-Boerrigter, L.C.; Schirger, J.A.; Tsuruda, T.; Heublein, D.M.; Chen, H.H.; Malatino, L.S.; Burnett, J.C., Jr. Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation 2004, 109, 1680–1685. [Google Scholar] [CrossRef]
- Diez, J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: implications for therapy. Eur. J. Heart Fail. 2017, 19, 167–176. [Google Scholar] [CrossRef]
- Potter, L.R. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011, 278, 1808–1817. [Google Scholar] [CrossRef]
- Fu, S.; Ping, P.; Wang, F.; Luo, L. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Hubers, S.A.; Brown, N.J. Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition. Circulation 2016, 133, 1115–1124. [Google Scholar] [CrossRef]
- Kobalava, Z.; Kotovskaya, Y.; Averkov, O.; Pavlikova, E.; Moiseev, V.; Albrecht, D.; Chandra, P.; Ayalasomayajula, S.; Prescott, M.F.; Pal, P. Pharmacodynamic and Pharmacokinetic Profiles of Sacubitril/Valsartan (LCZ696) in Patients with Heart Failure and Reduced Ejection Fraction. Cardiovasc. Ther. 2016, 34, 191–198. [Google Scholar] [CrossRef]
- Liang, F.; Lu, S.; Gardner, D.G. Endothelin-dependent and -independent components of strain-activated brain natriuretic peptide gene transcription require extracellular signal regulated kinase and p38 mitogen-activated protein kinase. Hypertension 2000, 35, 188–192. [Google Scholar] [CrossRef]
- Koivisto, E.; Kaikkonen, L.; Tokola, H.; Pikkarainen, S.; Aro, J.; Pennanen, H.; Karvonen, T.; Rysa, J.; Kerkela, R.; Ruskoaho, H. Distinct regulation of B-type natriuretic peptide transcription by p38 MAPK isoforms. Mol. Cell Endocrinol. 2011, 338, 18–27. [Google Scholar] [CrossRef]
- Piuhola, J.; Szokodi, I.; Ruskoaho, H. Endothelin-1 and angiotensin II contribute to BNP but not c-fos gene expression response to elevated load in isolated mice hearts. Biochim. Biophys. Acta 2007, 1772, 338–344. [Google Scholar] [CrossRef]
- Pikkarainen, S.; Tokola, H.; Kerkela, R.; Majalahti-Palviainen, T.; Vuolteenaho, O.; Ruskoaho, H. Endothelin-1-specific activation of B-type natriuretic peptide gene via p38 mitogen-activated protein kinase and nuclear ETS factors. J. Biol. Chem. 2003, 278, 3969–3975. [Google Scholar] [CrossRef]
- Majalahti, T.; Suo-Palosaari, M.; Sarman, B.; Hautala, N.; Pikkarainen, S.; Tokola, H.; Vuolteenaho, O.; Wang, J.; Paradis, P.; Nemer, M.; et al. Cardiac BNP gene activation by angiotensin II in vivo. Mol. Cell Endocrinol. 2007, 273, 59–67. [Google Scholar] [CrossRef]
- Cheng, T.H.; Cheng, P.Y.; Shih, N.L.; Chen, I.B.; Wang, D.L.; Chen, J.J. Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J. Am. Coll. Cardiol. 2003, 42, 1845–1854. [Google Scholar] [CrossRef]
- Freeman, B.D.; Machado, F.S.; Tanowitz, H.B.; Desruisseaux, M.S. Endothelin-1 and its role in the pathogenesis of infectious diseases. Life Sci. 2014, 118, 110–119. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, P.H.; Zhang, X.B.; Xu, C.G.; Wang, W. Pathophysiological functions of adrenomedullin and natriuretic peptides in patients with primary aldosteronism. Endocrine 2015, 48, 661–668. [Google Scholar] [CrossRef]
- Liang, F.; Kapoun, A.M.; Lam, A.; Damm, D.L.; Quan, D.; O’Connell, M.; Protter, A.A. B-Type natriuretic peptide inhibited angiotensin II-stimulated cholesterol biosynthesis, cholesterol transfer, and steroidogenesis in primary human adrenocortical cells. Endocrinology 2007, 148, 3722–3729. [Google Scholar] [CrossRef] [PubMed]
- Queisser, N.; Schupp, N. Aldosterone, oxidative stress, and NF-kappaB activation in hypertension-related cardiovascular and renal diseases. Free Radic. Biol. Med. 2012, 53, 314–327. [Google Scholar] [CrossRef]
- Azibani, F.; Fazal, L.; Chatziantoniou, C.; Samuel, J.L.; Delcayre, C. Aldosterone mediates cardiac fibrosis in the setting of hypertension. Curr. Hypertens. Rep. 2013, 15, 395–400. [Google Scholar] [CrossRef]
- Selvaraj, S.; Klein, I.; Danzi, S.; Akhter, N.; Bonow, R.O.; Shah, S.J. Association of serum triiodothyronine with B-type natriuretic peptide and severe left ventricular diastolic dysfunction in heart failure with preserved ejection fraction. Am. J. Cardiol. 2012, 110, 234–239. [Google Scholar] [CrossRef]
- Liang, F.; Webb, P.; Marimuthu, A.; Zhang, S.; Gardner, D.G. Triiodothyronine increases brain natriuretic peptide (BNP) gene transcription and amplifies endothelin-dependent BNP gene transcription and hypertrophy in neonatal rat ventricular myocytes. J. Biol. Chem. 2003, 278, 15073–15083. [Google Scholar] [CrossRef]
- Sergeeva, I.A.; Christoffels, V.M. Regulation of expression of atrial and brain natriuretic peptide, biomarkers for heart development and disease. Biochim. Biophys. Acta 2013, 1832, 2403–2413. [Google Scholar] [CrossRef]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef]
- Katz, A.M.; Rolett, E.L. Heart failure: when form fails to follow function. Eur. Heart J. 2016, 37, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Sun, Y.P.; Wei, C.P.; Ma, S.C.; Zhang, Y.F.; Qiao, L.Y.; Li, D.H.; Shan, R.B. Effect of Carvedilol on Serum Heart-type Fatty Acid-binding Protein, Brain Natriuretic Peptide, and Cardiac Function in Patients With Chronic Heart Failure. J. Cardiovasc. Pharmacol. 2015, 65, 480–484. [Google Scholar] [CrossRef]
- Dini, F.L.; Gabutti, A.; Passino, C.; Fontanive, P.; Emdin, M.; De Tommasi, S.M. Atrial fibrillation and amino-terminal pro-brain natriuretic peptide as independent predictors of prognosis in systolic heart failure. Int. J. Cardiol. 2010, 140, 344–350. [Google Scholar] [CrossRef]
- Shao, M.; Huang, C.; Li, Z.; Yang, H.; Feng, Q. Effects of glutamine and valsartan on the brain natriuretic peptide and N-terminal pro-B-type natriuretic peptide of patients with chronic heart failure. Pak. J. Med. Sci. 2015, 31, 82–86. [Google Scholar] [CrossRef]
- Khanam, S.S.; Son, J.W.; Lee, J.W.; Youn, Y.J.; Yoon, J.; Lee, S.H.; Kim, J.Y.; Ahn, S.G.; Ahn, M.S.; Yoo, B.S. Prognostic value of short-term follow-up BNP in hospitalized patients with heart failure. BMC Cardiovasc. Disord. 2017, 17, 215. [Google Scholar] [CrossRef]
- Chang, K.W.; Hsu, J.C.; Toomu, A.; Fox, S.; Maisel, A.S. Clinical Applications of Biomarkers in Atrial Fibrillation. Am. J. Med. 2017, 130, 1351–1357. [Google Scholar] [CrossRef]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur. Heart J. 2006, 27, 330–337. [Google Scholar] [CrossRef]
- Mishra, R.K.; Beatty, A.L.; Jaganath, R.; Regan, M.; Wu, A.H.; Whooley, M.A. B-type natriuretic peptides for the prediction of cardiovascular events in patients with stable coronary heart disease: The Heart and Soul Study. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef]
- Radwan, H.; Selem, A.; Ghazal, K. Reply to: N-terminal pro brain natriuretic peptide in coronary artery disease. J. Saudi. Heart Assoc. 2015, 27, 225. [Google Scholar] [CrossRef][Green Version]
- Radwan, H.; Selem, A.; Ghazal, K. Value of N-terminal pro brain natriuretic peptide in predicting prognosis and severity of coronary artery disease in acute coronary syndrome. J. Saudi. Heart Assoc. 2014, 26, 192–198. [Google Scholar] [CrossRef][Green Version]
- Gill, D.; Seidler, T.; Troughton, R.W.; Yandle, T.G.; Frampton, C.M.; Richards, M.; Lainchbury, J.G.; Nicholls, G. Vigorous response in plasma N-terminal pro-brain natriuretic peptide (NT-BNP) to acute myocardial infarction. Clin. Sci. 2004, 106, 135–139. [Google Scholar] [CrossRef]
- Tesic, M.; Seferovic, J.; Trifunovic, D.; Djordjevic-Dikic, A.; Giga, V.; Jovanovic, I.; Petrovic, O.; Marinkovic, J.; Stankovic, S.; Stepanovic, J.; et al. N-terminal pro-brain natriuretic peptide is related with coronary flow velocity reserve and diastolic dysfunction in patients with asymmetric hypertrophic cardiomyopathy. J. Cardiol. 2017, 70, 323–328. [Google Scholar] [CrossRef]
- Amorim, S.; Campelo, M.; Moura, B.; Martins, E.; Rodrigues, J.; Barroso, I.; Faria, M.; Guimaraes, T.; Macedo, F.; Silva-Cardoso, J.; et al. The role of biomarkers in dilated cardiomyopathy: Assessment of clinical severity and reverse remodeling. Rev. Port. Cardiol. 2017, 36, 709–716. [Google Scholar] [CrossRef]
- Geske, J.B.; McKie, P.M.; Ommen, S.R.; Sorajja, P. B-type natriuretic peptide and survival in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2013, 61, 2456–2460. [Google Scholar] [CrossRef]
- Cao, Z.P.; Zhang, Y.; Mi, L.; Luo, X.Y.; Tian, M.H.; Zhu, B.L. The Expression of B-Type Natriuretic Peptide After CaCl2-Induced Arrhythmias in Rats. Am. J. Forensic Med. Pathol. 2016, 37, 133–140. [Google Scholar] [CrossRef]
- Randhawa, M.S.; Dhillon, A.S.; Taylor, H.C.; Sun, Z.; Desai, M.Y. Diagnostic utility of cardiac biomarkers in discriminating Takotsubo cardiomyopathy from acute myocardial infarction. J. Card. Fail. 2014, 20, 2–8. [Google Scholar] [CrossRef]
- Grewal, J.; McKelvie, R.; Lonn, E.; Tait, P.; Carlsson, J.; Gianni, M.; Jarnert, C.; Persson, H. BNP and NT-proBNP predict echocardiographic severity of diastolic dysfunction. Eur. J. Heart Fail. 2008, 10, 252–259. [Google Scholar] [CrossRef]
- Wieczorek, S.J.; Wu, A.H.; Christenson, R.; Krishnaswamy, P.; Gottlieb, S.; Rosano, T.; Hager, D.; Gardetto, N.; Chiu, A.; Bailly, K.R.; et al. A rapid B-type natriuretic peptide assay accurately diagnoses left ventricular dysfunction and heart failure: a multicenter evaluation. Am. Heart J. 2002, 144, 834–839. [Google Scholar] [CrossRef]
- Tapanainen, J.M.; Lindgren, K.S.; Makikallio, T.H.; Vuolteenaho, O.; Leppaluoto, J.; Huikuri, H.V. Natriuretic peptides as predictors of non-sudden and sudden cardiac death after acute myocardial infarction in the beta-blocking era. J. Am. Coll. Cardiol. 2004, 43, 757–763. [Google Scholar] [CrossRef]
- Gueant Rodriguez, R.M.; Spada, R.; Pooya, S.; Jeannesson, E.; Moreno Garcia, M.A.; Anello, G.; Bosco, P.; Elia, M.; Romano, A.; Alberto, J.M.; et al. Homocysteine predicts increased NT-pro-BNP through impaired fatty acid oxidation. Int. J. Cardiol. 2013, 167, 768–775. [Google Scholar] [CrossRef]
- Elkayam, U.; Akhter, M.W.; Singh, H.; Khan, S.; Usman, A. Comparison of effects on left ventricular filling pressure of intravenous nesiritide and high-dose nitroglycerin in patients with decompensated heart failure. Am. J. Cardiol. 2004, 93, 237–240. [Google Scholar] [CrossRef]
- Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: A randomized controlled trial. Jama 2002, 287, 1531–1540.
- Colucci, W.S.; Elkayam, U.; Horton, D.P.; Abraham, W.T.; Bourge, R.C.; Johnson, A.D.; Wagoner, L.E.; Givertz, M.M.; Liang, C.S.; Neibaur, M.; et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N. Engl. J. Med. 2000, 343, 246–253. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z. Effect of recombinant human brain natriuretic peptide (rhBNP) versus nitroglycerin in patients with heart failure: A systematic review and meta-analysis. Medicine 2016, 95, e4757. [Google Scholar] [CrossRef]
- Issa, V.S.; Dinardi, L.F.; Pereira, T.V.; de Almeida, L.K.; Barbosa, T.S.; Benvenutti, L.A.; Ayub-Ferreira, S.M.; Bocchi, E.A. Diagnostic discrepancies in clinical practice: An autopsy study in patients with heart failure. Medicine 2017, 96, e5978. [Google Scholar] [CrossRef]
- Mendis, S.; Thygesen, K.; Kuulasmaa, K.; Giampaoli, S.; Mahonen, M.; Ngu Blackett, K.; Lisheng, L. World Health Organization definition of myocardial infarction: 2008–09 revision. Int. J. Epidemiol. 2011, 40, 139–146. [Google Scholar] [CrossRef]
- Lawler, W. The negative coroner’s necropsy: A personal approach and consideration of difficulties. J. Clin. Pathol 1990, 43, 977–980. [Google Scholar] [CrossRef]
- Campuzano, O.; Allegue, C.; Partemi, S.; Iglesias, A.; Oliva, A.; Brugada, R. Negative autopsy and sudden cardiac death. Int. J. Legal Med. 2014, 128, 599–606. [Google Scholar] [CrossRef]
- Maeda, H.; Ishikawa, T.; Michiue, T. Forensic biochemistry for functional investigation of death: Concept and practical application. Leg. Med. 2011, 13, 55–67. [Google Scholar] [CrossRef]
- Woydt, L.; Bernhard, M.; Kirsten, H.; Burkhardt, R.; Hammer, N.; Gries, A.; Dressler, J.; Ondruschka, B. Intra-individual alterations of serum markers routinely used in forensic pathology depending on increasing post-mortem interval. Sci. Rep. 2018, 8, 12811. [Google Scholar] [CrossRef]
- Maeda, H.; Zhu, B.L.; Ishikawa, T.; Quan, L.; Michiue, T. Significance of postmortem biochemistry in determining the cause of death. Leg. Med. 2009, 11, S46–S49. [Google Scholar] [CrossRef]
- Madea, B.; Musshoff, F. Postmortem biochemistry. Forensic Sci. Int. 2007, 165, 165–171. [Google Scholar] [CrossRef]
- Vogiatzidis, K.; Zarogiannis, S.G.; Aidonidis, I.; Solenov, E.I.; Molyvdas, P.A.; Gourgoulianis, K.I.; Hatzoglou, C. Physiology of pericardial fluid production and drainage. Front. Physiol. 2015, 6, 62. [Google Scholar] [CrossRef]
- Mao, R.M.; Zheng, P.P.; Zhu, C.R.; Zhu, B.L. The analysis of pericardial fluid in forensic practice. Fa Yi Xue Za Zhi 2010, 26, 202–205. [Google Scholar]
- Palmiere, C.; Grabherr, S. Biochemical investigations performed in pericardial fluid in forensic cases that underwent postmortem angiography. Forensic Sci. Int. 2019, 297, e11–e13. [Google Scholar] [CrossRef]
- Comment, L.; Reggiani Bonetti, L.; Mangin, P.; Palmiere, C. Measurement of beta-tryptase in postmortem serum, pericardial fluid, urine and vitreous humor in the forensic setting. Forensic Sci. Int. 2014, 240, 29–34. [Google Scholar] [CrossRef]
- Mizutani, T.; Yoshimoto, T.; Ishii, A. Pericardial fluid is suitable as an alternative specimen for the measurement of beta-hydroxybutyrate within 96 h after death. Leg. Med. 2018, 33, 53–54. [Google Scholar] [CrossRef]
- Chen, J.H.; Michiue, T.; Inamori-Kawamoto, O.; Ikeda, S.; Ishikawa, T.; Maeda, H. Comprehensive investigation of postmortem glucose levels in blood and body fluids with regard to the cause of death in forensic autopsy cases. Leg. Med. 2015, 17, 475–482. [Google Scholar] [CrossRef]
- Chen, J.H.; Inamori-Kawamoto, O.; Michiue, T.; Ikeda, S.; Ishikawa, T.; Maeda, H. Cardiac biomarkers in blood, and pericardial and cerebrospinal fluids of forensic autopsy cases: A reassessment with special regard to postmortem interval. Leg. Med. 2015, 17, 343–350. [Google Scholar] [CrossRef]
- Ishikawa, T.; Quan, L.; Michiue, T.; Kawamoto, O.; Wang, Q.; Chen, J.H.; Zhu, B.L.; Maeda, H. Postmortem catecholamine levels in pericardial and cerebrospinal fluids with regard to the cause of death in medicolegal autopsy. Forensic Sci. Int. 2013, 228, 52–60. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Soufras, G.; Koutsogiannis, N.; Hahalis, G. Specific IgE levels in pericardial and cerebrospinal fluids in forensic casework: The presence of additional molecules for sudden cardiac death diagnosis. Forensic Sci. Int. 2018, 282, 79. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ishikawa, T.; Michiue, T.; Li, D.R.; Zhao, D.; Kamikodai, Y.; Tsuda, K.; Okazaki, S.; Maeda, H. Postmortem cardiac troponin T levels in the blood and pericardial fluid. Part 2: Analysis for application in the diagnosis of sudden cardiac death with regard to pathology. Leg. Med. 2006, 8, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.G.; Seferian, K.R. Biochemistry of the human B-type natriuretic peptide precursor and molecular aspects of its processing. Clin. Chim. Acta 2011, 412, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Yasue, H.; Yoshimura, M.; Sumida, H.; Kikuta, K.; Kugiyama, K.; Jougasaki, M.; Ogawa, H.; Okumura, K.; Mukoyama, M.; Nakao, K. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994, 90, 195–203. [Google Scholar] [CrossRef]
- Omland, T.; Aakvaag, A.; Bonarjee, V.V.; Caidahl, K.; Lie, R.T.; Nilsen, D.W.; Sundsfjord, J.A.; Dickstein, K. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation 1996, 93, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Pfister, R.; Scholz, M.; Wielckens, K.; Erdmann, E.; Schneider, C.A. Use of NT-proBNP in routine testing and comparison to BNP. Eur. J. Heart Fail. 2004, 6, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Kragelund, C.; Gronning, B.; Kober, L.; Hildebrandt, P.; Steffensen, R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N. Engl. J. Med. 2005, 352, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Nowatzke, W.L.; Cole, T.G. Stability of N-terminal pro-brain natriuretic peptide after storage frozen for one year and after multiple freeze-thaw cycles. Clin. Chem. 2003, 49, 1560–1562. [Google Scholar] [CrossRef]
- Wu, A.H.; Packer, M.; Smith, A.; Bijou, R.; Fink, D.; Mair, J.; Wallentin, L.; Johnston, N.; Feldcamp, C.S.; Haverstick, D.M.; et al. Analytical and clinical evaluation of the Bayer ADVIA Centaur automated B-type natriuretic peptide assay in patients with heart failure: A multisite study. Clin. Chem. 2004, 50, 867–873. [Google Scholar] [CrossRef]
- Koseoglu, M.; Hur, A.; Atay, A.; Cuhadar, S. Effects of hemolysis interferences on routine biochemistry parameters. Biochem. Med. 2011, 21, 79–85. [Google Scholar] [CrossRef]
- Nishiumi, S.; Shima, K.; Azuma, T.; Yoshida, M. Evaluation of a novel system for analyzing hydrophilic blood metabolites. J. Biosci. Bioeng. 2017, 123, 754–759. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, M.; Chen, C.; Gong, W.; Yin, Z.; Li, H.; Fan, J.; Zhang, X.A.; Wang, D.W.; Zuo, H. MiR-124 aggravates failing hearts by suppressing CD151-facilitated angiogenesis in heart. Oncotarget 2018, 9, 14382–14396. [Google Scholar] [CrossRef]
- Bao, Q.; Chen, L.; Li, J.; Zhao, M.; Wu, S.; Wu, W.; Liu, X. Role of microRNA-124 in cardiomyocyte hypertrophy inducedby angiotensin II. Cell. Mol. Biol. 2017, 63, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Murach, K.A.; McCarthy, J.J. MicroRNAs, heart failure, and aging: Potential interactions with skeletal muscle. Heart Fail. Rev. 2017, 22, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Gupta, S.K.; O’Connell, E.; Thum, S.; Glezeva, N.; Fendrich, J.; Gallagher, J.; Ledwidge, M.; Grote-Levi, L.; McDonald, K.; et al. MicroRNA signatures differentiate preserved from reduced ejection fraction heart failure. Eur. J. Heart Fail. 2015, 17, 405–415. [Google Scholar] [CrossRef]
- Zhang, X.; Sha, M.; Yao, Y.; Da, J.; Jing, D. Increased B-type-natriuretic peptide promotes myocardial cell apoptosis via the B-type-natriuretic peptide/long non-coding RNA LSINCT5/caspase-1/interleukin 1beta signaling pathway. Mol. Med. Rep. 2015, 12, 6761–6767. [Google Scholar] [CrossRef]
- Bi, S.; Wang, C.; Jin, Y.; Lv, Z.; Xing, X.; Lu, Q. Correlation between serum exosome derived miR-208a and acute coronary syndrome. Int. J. Clin. Exp. Med. 2015, 8, 4275–4280. [Google Scholar]
- Staals, R.H.; Pruijn, G.J. The human exosome and disease. Adv. Exp. Med. Biol. 2011, 702, 132–142. [Google Scholar] [CrossRef]
- Ye, W.; Tang, X.; Yang, Z.; Liu, C.; Zhang, X.; Jin, J.; Lyu, J. Plasma-derived exosomes contribute to inflammation via the TLR9-NF-kappaB pathway in chronic heart failure patients. Mol. Immunol. 2017, 87, 114–121. [Google Scholar] [CrossRef]
- Yang, V.K.; Loughran, K.A.; Meola, D.M.; Juhr, C.M.; Thane, K.E.; Davis, A.M.; Hoffman, A.M. Circulating exosome microRNA associated with heart failure secondary to myxomatous mitral valve disease in a naturally occurring canine model. J. Extracell. Vesicles 2017, 6, 1350088. [Google Scholar] [CrossRef]
- Wendt, S.; Goetzenich, A.; Goettsch, C.; Stoppe, C.; Bleilevens, C.; Kraemer, S.; Benstoem, C. Evaluation of the cardioprotective potential of extracellular vesicles-a systematic review and meta-analysis. Sci. Rep. 2018, 8, 15702. [Google Scholar] [CrossRef]
- Gartz, M.; Strande, J.L. Examining the Paracrine Effects of Exosomes in Cardiovascular Disease and Repair. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Poe, A.J.; Knowlton, A.A. Exosomes as agents of change in the cardiovascular system. J. Mol. Cell. Cardiol. 2017, 111, 40–50. [Google Scholar] [CrossRef]
- Sahoo, S.; Mathiyalagan, P.; Hajjar, R.J. Pericardial Fluid Exosomes: A New Material to Treat Cardiovascular Disease. Mol. Ther. 2017, 25, 568–569. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, C.; Besnier, M.; Shantikumar, S.; Shearn, A.I.; Rajakaruna, C.; Laftah, A.; Sessa, F.; Spinetti, G.; Petretto, E.; Angelini, G.D.; et al. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and Promotes therapeutic angiogenesis. Mol. Ther. 2017, 25, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Kuosmanen, S.M.; Hartikainen, J.; Hippelainen, M.; Kokki, H.; Levonen, A.L.; Tavi, P. MicroRNA profiling of pericardial fluid samples from patients with heart failure. PLoS ONE 2015, 10, e0119646. [Google Scholar] [CrossRef] [PubMed]
- Foglio, E.; Puddighinu, G.; Fasanaro, P.; D’Arcangelo, D.; Perrone, G.A.; Mocini, D.; Campanella, C.; Coppola, L.; Logozzi, M.; Azzarito, T.; et al. Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following MI through epicardial activation, enhanced arteriogenesis and reduced apoptosis. Int. J. Cardiol. 2015, 197, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Ishikawa, T.; Michiue, T. Forensic molecular pathology: Its impacts on routine work, education and training. Leg. Med. 2014, 16, 61–69. [Google Scholar] [CrossRef]
- Maeda, H.; Zhu, B.L.; Ishikawa, T.; Michiue, T. Forensic molecular pathology of violent deaths. Forensic Sci. Int. 2010, 203, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ishikawa, T.; Quan, L.; Michiue, T.; Zhu, B.L.; Maeda, H. Postmortem quantitative mRNA analyses of death investigation in forensic pathology: An overview and prospects. Leg. Med. 2009, 11, S43–S45. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. https://doi.org/10.3390/ijms20081820
Cao Z, Jia Y, Zhu B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. International Journal of Molecular Sciences. 2019; 20(8):1820. https://doi.org/10.3390/ijms20081820
Chicago/Turabian StyleCao, Zhipeng, Yuqing Jia, and Baoli Zhu. 2019. "BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine" International Journal of Molecular Sciences 20, no. 8: 1820. https://doi.org/10.3390/ijms20081820
APA StyleCao, Z., Jia, Y., & Zhu, B. (2019). BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. International Journal of Molecular Sciences, 20(8), 1820. https://doi.org/10.3390/ijms20081820