Effects of Palm Oil Tocotrienol-Rich Fraction (TRF) and Carotenes in Ovalbumin (OVA)-Challenged Asthmatic Brown Norway Rats
Abstract
1. Introduction
2. Results
2.1. Clinical Presentation of Asthma upon OVA-Challenge in Experimental Rats
2.2. Effects of TRF and Palm Carotene on the Body Weight of OVA-Challenged Asthma Model Rats
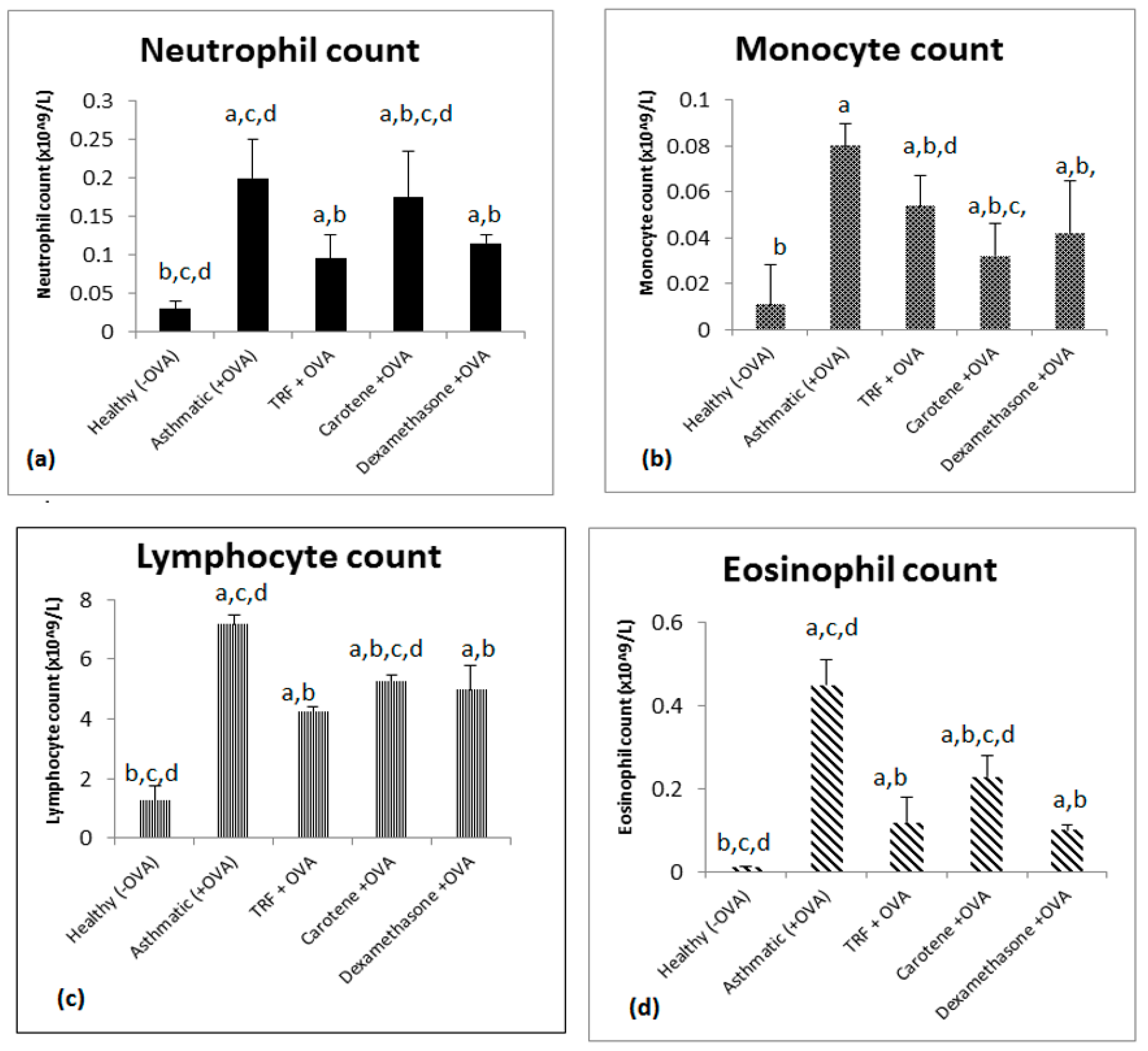
2.3. Effect of TRF and Palm Oil Carotene on Eosinophil, Lymphocyte, Neutrophil, and Monocyte Cell Morphology
2.4. Effect of Palm Oil TRF and Carotenes on White Blood Cell Count
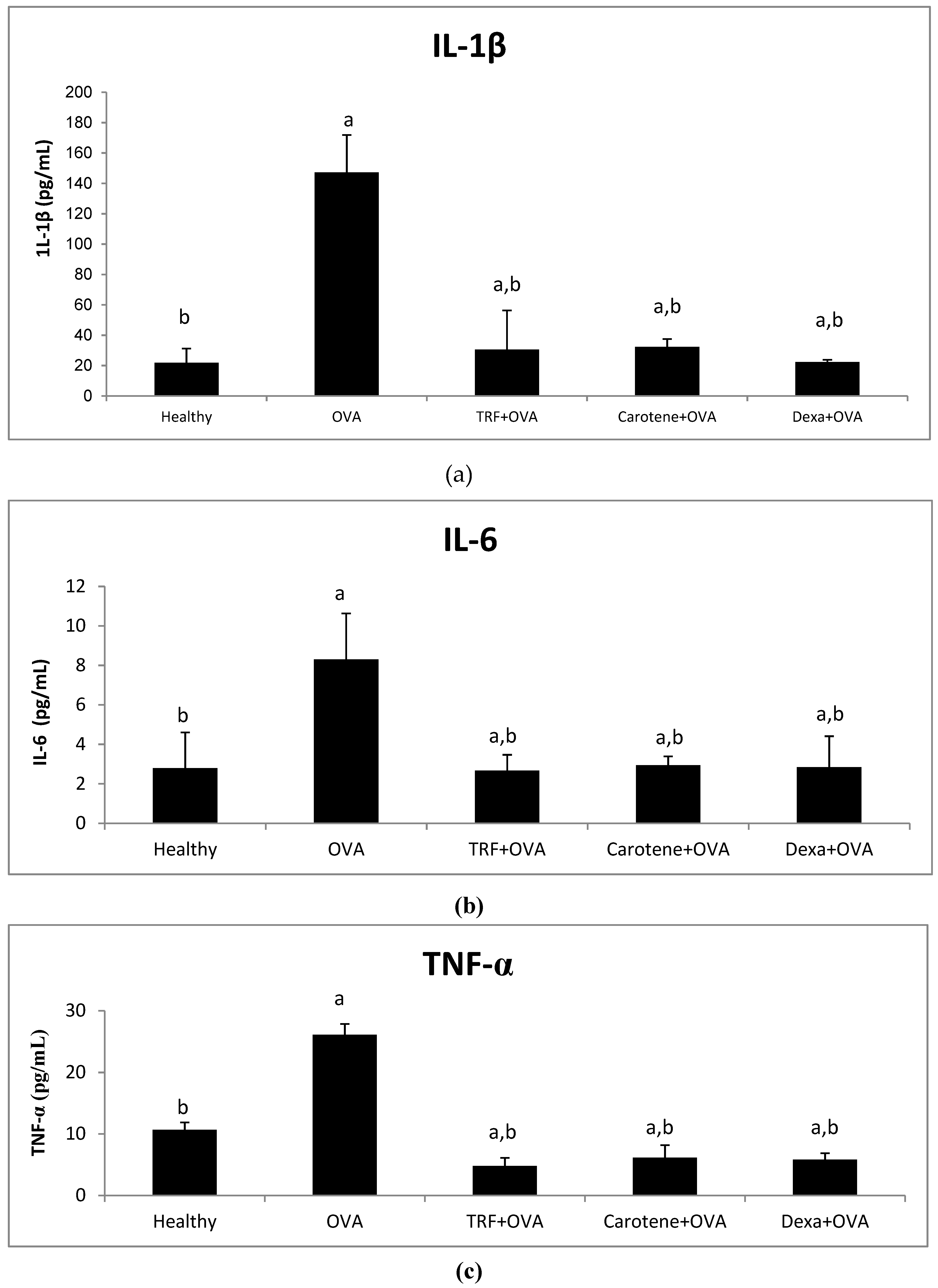
2.5. Effect of Palm Oil TRF and Carotenes on Proinflammatory Cytokines
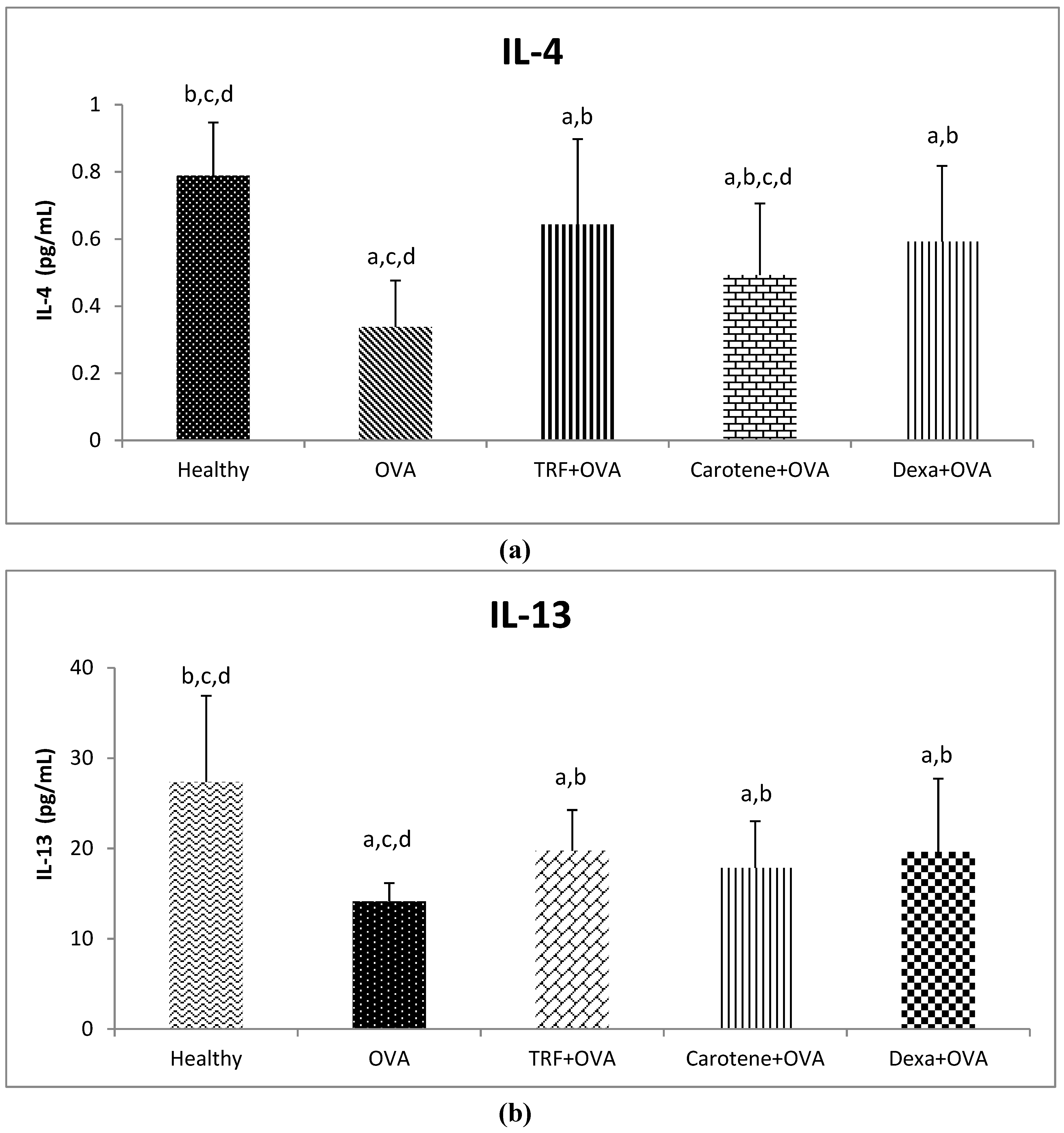
2.6. Effect of Palm Oil TRF and Carotenes on Anti-inflammatory Cytokines
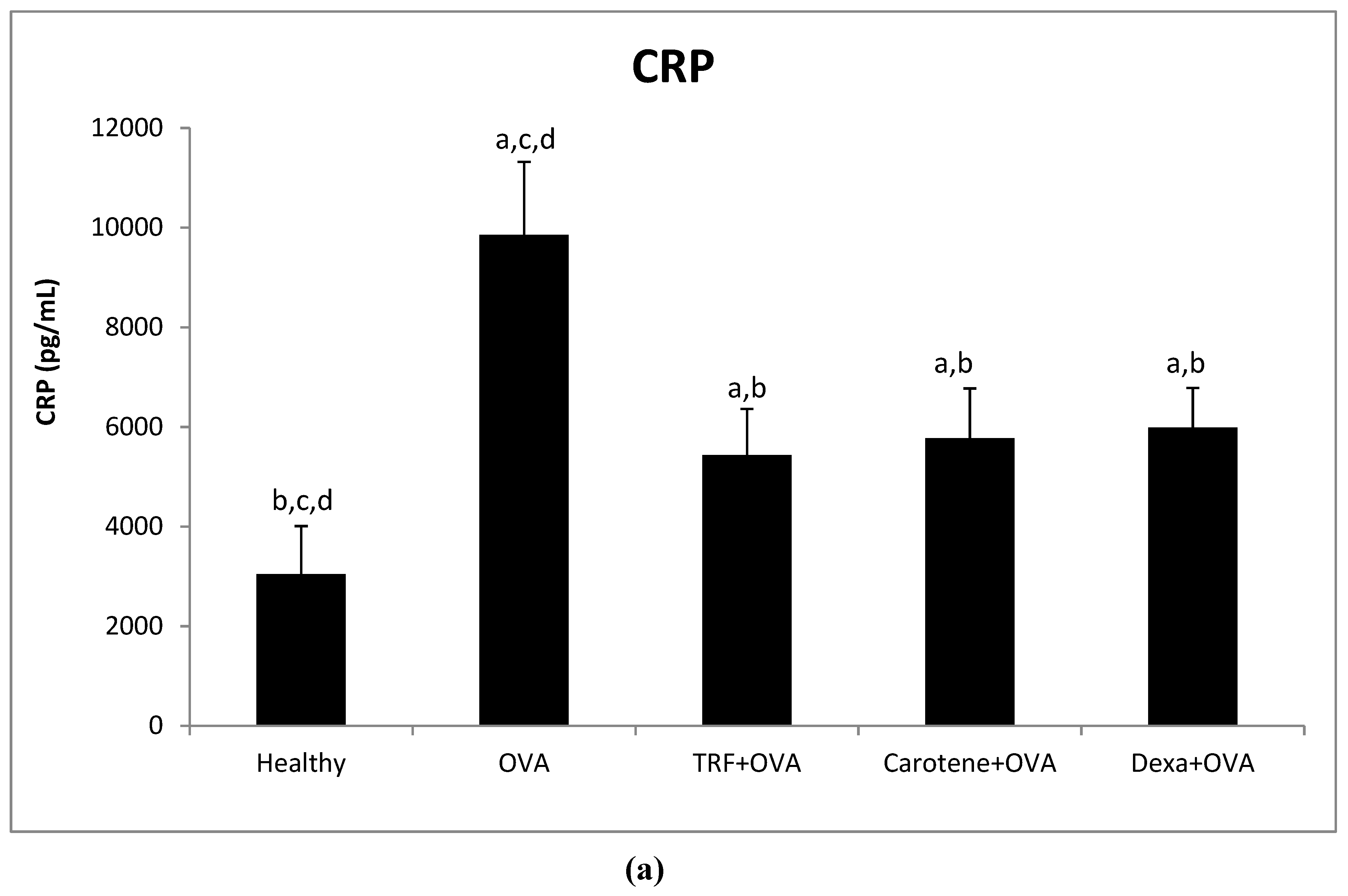
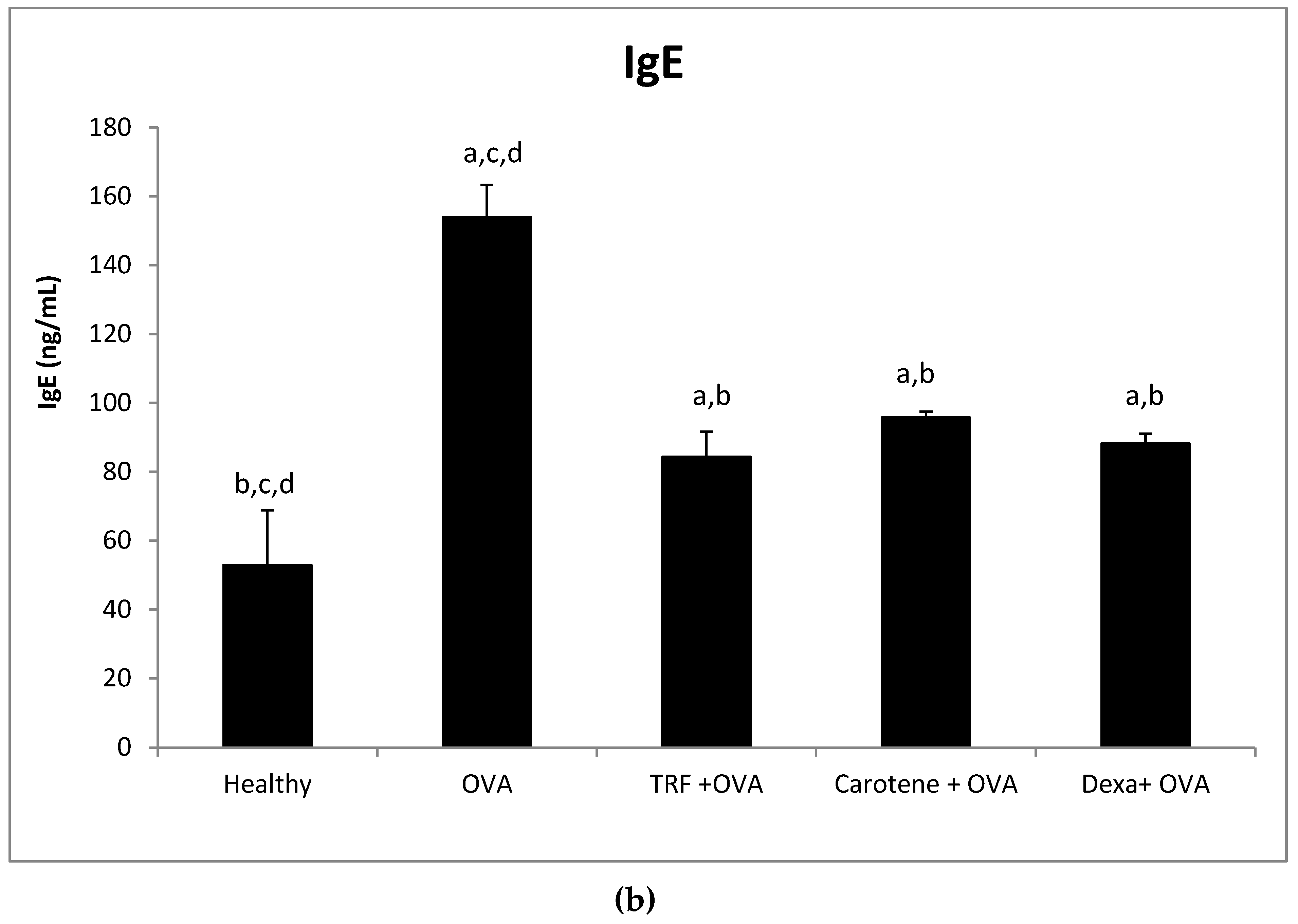
2.7. Effect of Palm Oil TRF and Carotenes on Plasma C-Reactive Protein (CRP) and IgE Levels
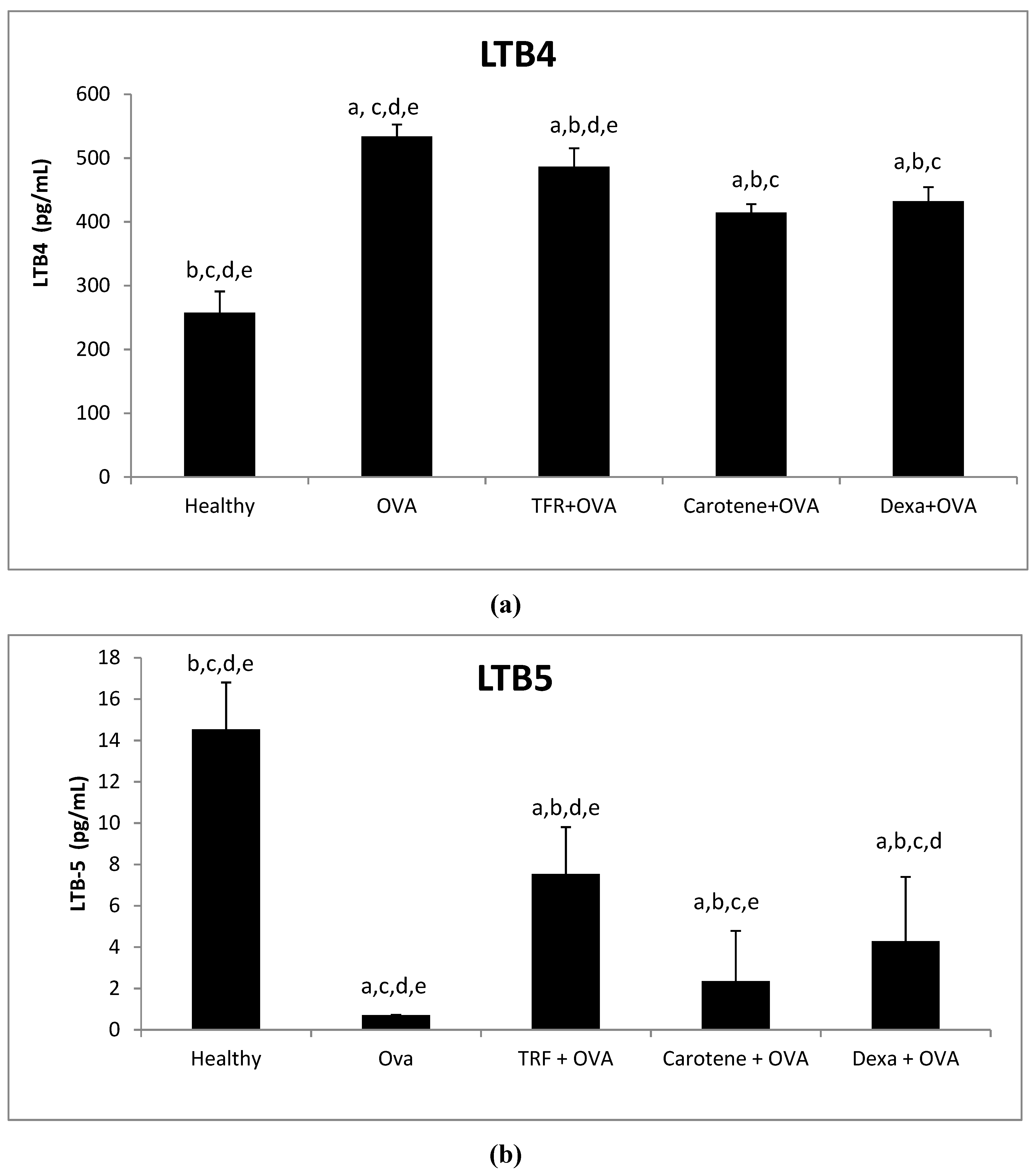
2.8. Effects of Palm Oil TRF and Carotenes on the Leukotrines LTB-4 and LTB-5
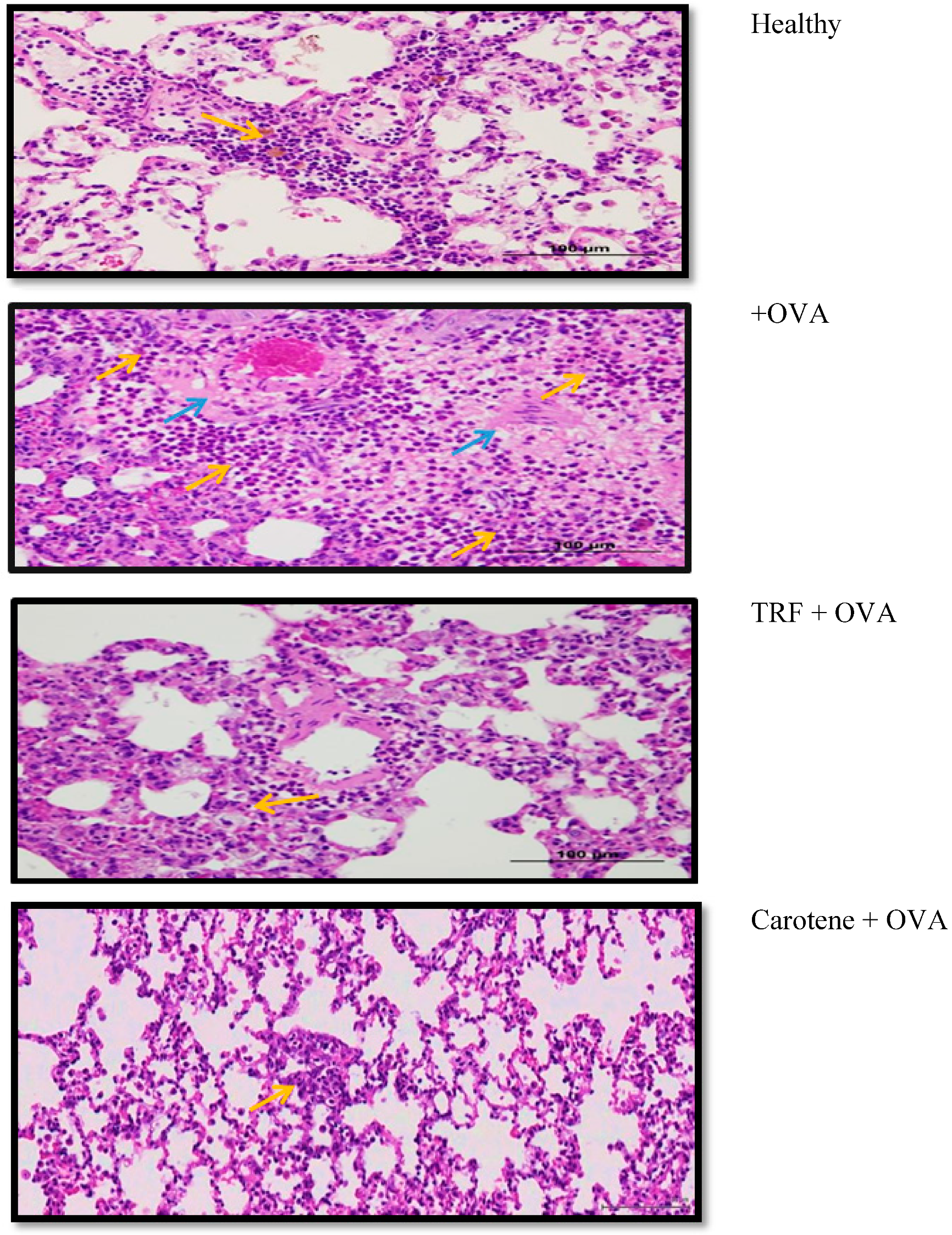
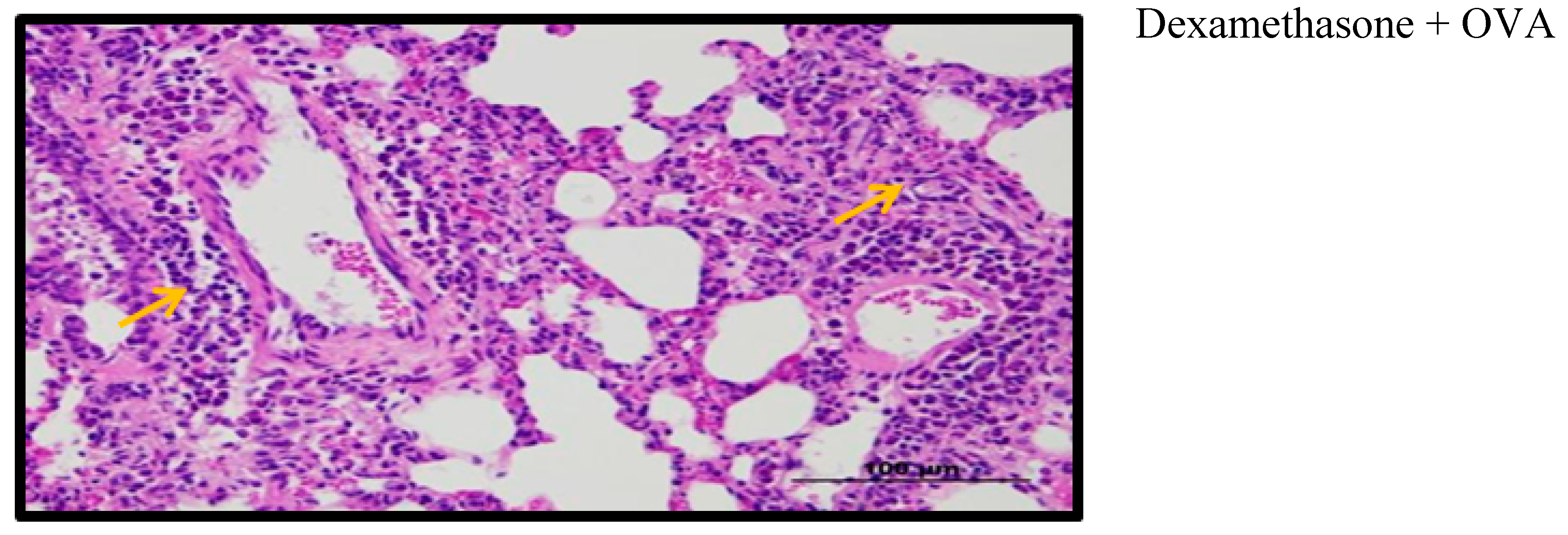
2.9. Effect of Palm Oil TRF and Carotenes on Lung Histology
3. Discussion
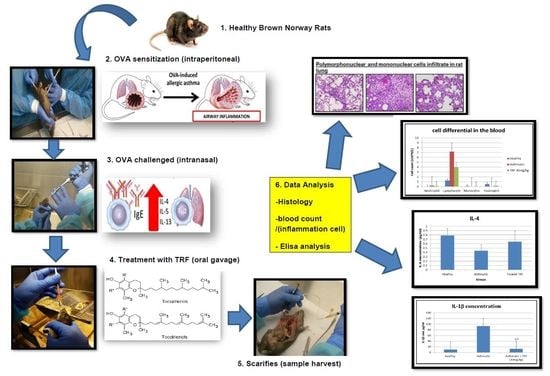
4. Materials and Methods
4.1. Animals and Diets
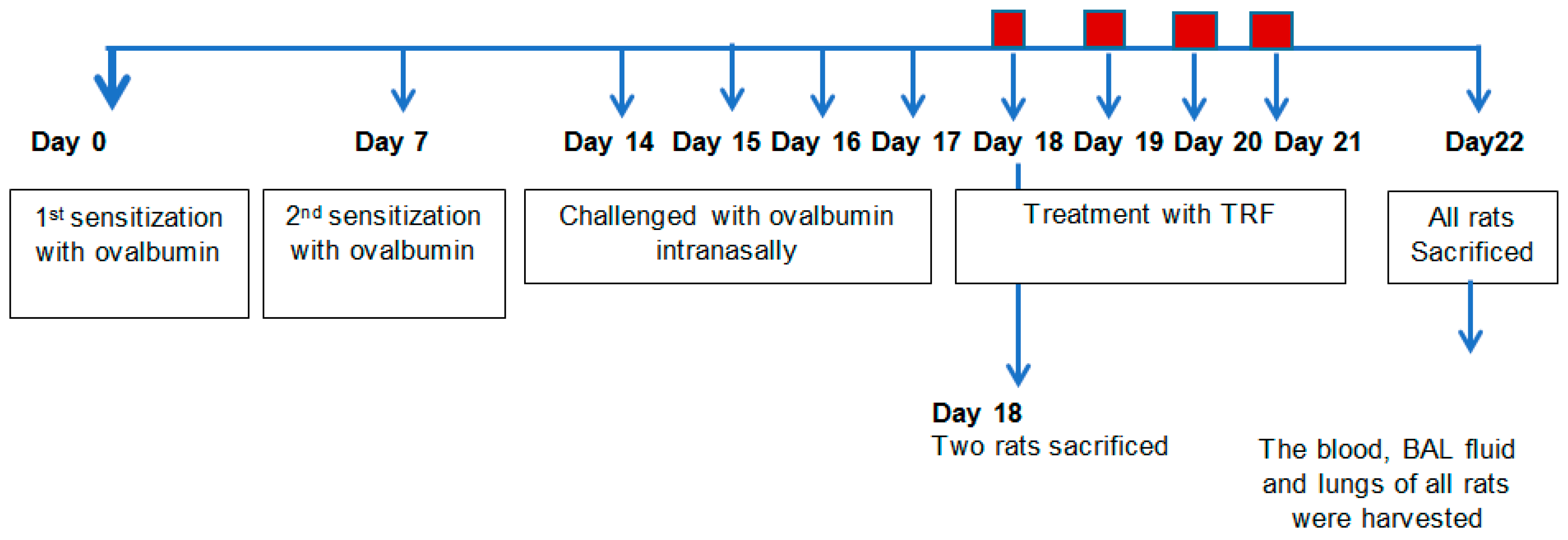
4.2. Sensitization and Allergen Challenge
4.3. Collection of Plasma, BAL, and Tissue Harvest
4.4. Necropsy and Tissue Preparation
4.5. Hematology
4.6. Cytokine, CRP, IgE, LTD4, and LTD5 Concentration Level in Serum and BAL Fluid
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ni, Z.; Tang, J.; Cai, Z.; Yang, W.; Zhang, L.; Chen, Q.; Zhang, L.; Wang, X. A new pathway of glucocorticoid action for asthma treatment through the regulation of PTEN expression. Respir. Res. 2011, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.C.; Chaudhuri, R.; Spears, M. Emerging therapies for severe asthma. BMC Med. 2011, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Sirois, J.; Bissonnette, E.Y. Alveolar macrophages of allergic resistant and susceptible strains of rats show distinct cytokine profiles. Clin. Exp. Immunol. 2001, 126, 9–15. [Google Scholar] [CrossRef] [PubMed]
- World Life Expectancy. Asthma in Malaysia. Available online: http://www.worldlifeexpectancy.com/malaysia-asthma (accessed on 23 February 2017).
- Harada, T.; Yamasaki, A.; Fukushima, T.; Hashimoto, K.; Takata, M.; Kodani, M.; Okazaki, R.; Takeda, K.; Watanabe, M.; Kurai, J.; et al. Causes of death in patients with asthma and asthma–chronic obstructive pulmonary disease overlap syndrome. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 595–602. [Google Scholar]
- Peh, H.Y.; Ho, W.E.; Cheng, C.; Chan, T.K.; Seow, A.C.; Lim, A.Y.; Fong, C.W.; Seng, K.Y.; Ong, C.N.; Wong, W.F. Vitamin E isoform γ-tocotrienol downregulates house dust mite–induced asthma. J. Immunol. 2015, 195, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Lemanske, R.F., Jr. Asthma. N. Engl. J. Med. 2001, 344, 350–362. [Google Scholar] [CrossRef]
- Stevenson, D.D.; Szczeklik, A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J. Allergy Clin. Immunol. 2006, 118, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Agrawal, D.K. Key mediators in the immunopathogenesis of allergic asthma. Int. Immunopharmacol. 2014, 23, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.P. Recent advances in the management of asthma using leukotriene modifiers. Am. J. Respir. Med. 2003, 2, 139–156. [Google Scholar] [CrossRef]
- Grimble, R.F. Anti-oxidant modulation in immune function. In Nutrigenomics; Rimbach, G., Fuchs, J., Packer, L., Eds.; Taylor & Francis: New York, NY, USA, 2005; pp. 97–122. [Google Scholar]
- Hazlewood, L.C.; Wood, L.G.; Hansbro, P.M.; Foster, P.S. Dietary lycopene supplementation suppresses Th2 responses and lung eosinophilia in a mouse model of allergic asthma. J. Nutr. Biochem. 2011, 22, 95–100. [Google Scholar] [CrossRef]
- Kolleck, I.; Sinha, P.; Rüstow, B. Vitamin E as an antioxidant of the lung: Mechanisms of vitamin E delivery to alveolar type II cells. Am. J. Respir. Crit. Care Med. 2002, 166, S62–S66. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, L. Update on the role of alternatively activated macrophages in asthma. J. Asthma Allergy 2016, 9, 101–107. [Google Scholar] [CrossRef]
- Ghaffari, J.; Ashrafi, H.; Ranjbar, A.R.; Nazari, Z. Vitamin E in children with asthma: A review. J. Pediatr. Rev. 2014, 2, 57–65. [Google Scholar]
- Choo, Y.M.; Ma, A.N.; Basiron, Y. Red palm oil: A potential source of dietary carotenes. Malays. Oil Sci. Technol. 1993, 2, 54–55. [Google Scholar]
- Yap, S.C.; Choo, Y.M.; Ooi, C.K.; Ong, A.S.; Goh, S.H. Quantitative analysis of carotenes in the oil from different palm species. J. Oil Palm Res. 1991, 3, 369–378. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Wang, Y.; Park, N.Y.; Jang, Y.; Ma, A.; Jiang, Q. Vitamin E γ-tocotrienol inhibits cytokine-stimulated NF-κB activation by induction of anti-inflammatory A20 via stress adaptive response due to modulation of sphingolipids. J. Immunol. 2015, 195, 126–133. [Google Scholar] [CrossRef]
- Wilkinson, M.; Hart, A.; Milan, S.J.; Sugumar, K. Vitamins C and E for asthma and exercise-induced bronchoconstriction. Cochrane Database Syst. Rev. 2014, 17, CD010749. [Google Scholar] [CrossRef] [PubMed]
- Wiser, J.; Alexis, N.E.; Jiang, Q.; Wu, W.; Robinette, C.; Roubey, R.; Peden, D.B. In vivo γ-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic. Biol. Med. 2008, 45, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Siddig, A.S.; Ismail, A.A.; Mohammed, B.A. The role of medications misused for body weight gain on some fertility hormones concentration of female rabbits. J. Food Sci. Nutr. 2018, 1, 13–16. [Google Scholar]
- Kuang, Z.; Wilson, J.J.; Luo, S.; Zhu, S.W.; Huang, R.P. Deciphering asthma biomarkers with protein profiling technology. Int. J. Inflamm. 2015, 2015, 630637. [Google Scholar] [CrossRef]
- Fahy, J.V. Eosinophilic and neutrophilic inflammation in asthma: Insights from clinical studies. Proc. Am. Thorac. Soc. 2009, 6, 256–259. [Google Scholar] [CrossRef]
- Wagner, J.G.; Harkema, J.R.; Jiang, Q.; Illek, B.; Ames, B.N.; Peden, D.B. γ-Tocopherol attenuates ozone-induced exacerbation of allergic rhinosinusitis in rats. Toxicol. Pathol. 2009, 37, 481–491. [Google Scholar] [CrossRef]
- Shen, C.L.; Klein, A.; Chin, K.Y.; Mo, H.; Tsai, P.; Yang, R.S.; Chyu, M.C.; Ima-Nirwana, S. Tocotrienols for bone health: A translational approach. Ann. N. Y. Acad. Sci. 2017, 1401, 150–165. [Google Scholar] [CrossRef]
- Hernandez, M.L.; Wagner, J.G.; Kala, A.; Mills, K.; Wells, H.B.; Alexis, N.E.; Lay, J.C.; Jiang, Q.; Zhang, H.; Zhou, H.; et al. Vitamin E, γ-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic. Biol. Med. 2013, 60, 56–62. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, X. Natural forms of vitamin E inhibited leukotriene B4 generation and 5-lipoxygenase translocation in Ca2+ ionophore-activated human HL-60 cells. FASEB J. 2008, 22, 1100–1101. [Google Scholar]
- Taubes, G. Does inflammation cut to the heart of the matter? Science 2002, 296, 242–246. [Google Scholar] [CrossRef]
- Michihara, A.; Ogawa, S.; Kamizaki, Y.; Akasaki, K. Effect of delta-tocotrienol on melanin content and enzymes for melanin synthesis in mouse melanoma cells. Biol. Pharm. Bull. 2010, 33, 1471–1476. [Google Scholar] [CrossRef]
- Fujita, M.; Ueki, S.; Ito, W.; Chiba, T.; Takeda, M.; Saito, N.; Kayaba, H.; Chihara, J. C-reactive protein levels in the serum of asthmatic patients. Ann. Allergy Asthma Immunol. 2007, 99, 48–53. [Google Scholar] [CrossRef]
- Lim, Y.; Vasu, V.T.; Valacchi, G.; Leonard, S.; Aung, H.H.; Schock, B.C.; Kenyon, N.J.; Li, C.S.; Traber, M.G.; Cross, C.E. Severe vitamin E deficiency modulates airway allergic inflammatory responses in the murine asthma model. Free Radic. Res. 2008, 42, 387–396. [Google Scholar] [CrossRef]
- Zein, J.G.; Erzurum, S.C. Asthma is different in women. Curr. Allergy Asthma Rep. 2015, 15, 28. [Google Scholar] [CrossRef]
- Raju, K.R.; Kumar, M.N.; Gupta, S.; Naga, S.T.; Shankar, J.K.; Murthy, V.; Madhunapanthula, S.R.; Mulukutla, S.; Ambhore, N.S.; Tummala, S.; et al. 5-Aminosalicylic acid attenuates allergen-induced airway inflammation and oxidative stress in asthma. Pulm. Pharmacol. Ther. 2014, 29, 209–216. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L., Jr.; Valanis, B.; Williams, J.H., Jr.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Campbell, P.L.; McCluskey, J.; Yeo, J.P.; Toh, B.H. Electroporation of antibodies into mammalian cells. Methods Mol. Biol. 1995, 48, 83–92. [Google Scholar]
- He, Z.; Ong, C.H.; Halper, J.; Bateman, A. Progranulin is a mediator of the wound response. Nat. Med. 2003, 9, 225–229. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainal, Z.; Abdul Rahim, A.; Khaza’ai, H.; Chang, S.K. Effects of Palm Oil Tocotrienol-Rich Fraction (TRF) and Carotenes in Ovalbumin (OVA)-Challenged Asthmatic Brown Norway Rats. Int. J. Mol. Sci. 2019, 20, 1764. https://doi.org/10.3390/ijms20071764
Zainal Z, Abdul Rahim A, Khaza’ai H, Chang SK. Effects of Palm Oil Tocotrienol-Rich Fraction (TRF) and Carotenes in Ovalbumin (OVA)-Challenged Asthmatic Brown Norway Rats. International Journal of Molecular Sciences. 2019; 20(7):1764. https://doi.org/10.3390/ijms20071764
Chicago/Turabian StyleZainal, Zaida, Afiqah Abdul Rahim, Huzwah Khaza’ai, and Sui Kiat Chang. 2019. "Effects of Palm Oil Tocotrienol-Rich Fraction (TRF) and Carotenes in Ovalbumin (OVA)-Challenged Asthmatic Brown Norway Rats" International Journal of Molecular Sciences 20, no. 7: 1764. https://doi.org/10.3390/ijms20071764
APA StyleZainal, Z., Abdul Rahim, A., Khaza’ai, H., & Chang, S. K. (2019). Effects of Palm Oil Tocotrienol-Rich Fraction (TRF) and Carotenes in Ovalbumin (OVA)-Challenged Asthmatic Brown Norway Rats. International Journal of Molecular Sciences, 20(7), 1764. https://doi.org/10.3390/ijms20071764