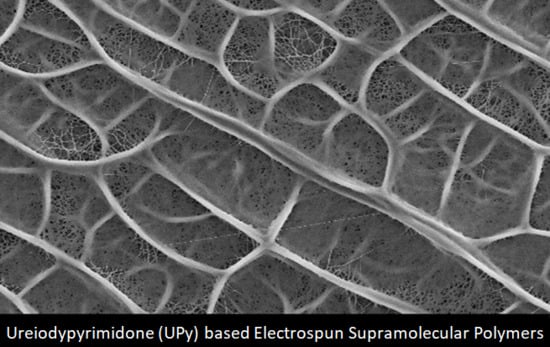
Graphene Nanoplatelet- and Hydroxyapatite-Doped Supramolecular Electrospun Fibers as Potential Materials for Tissue Engineering and Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Solution Preparation and Electrospinning
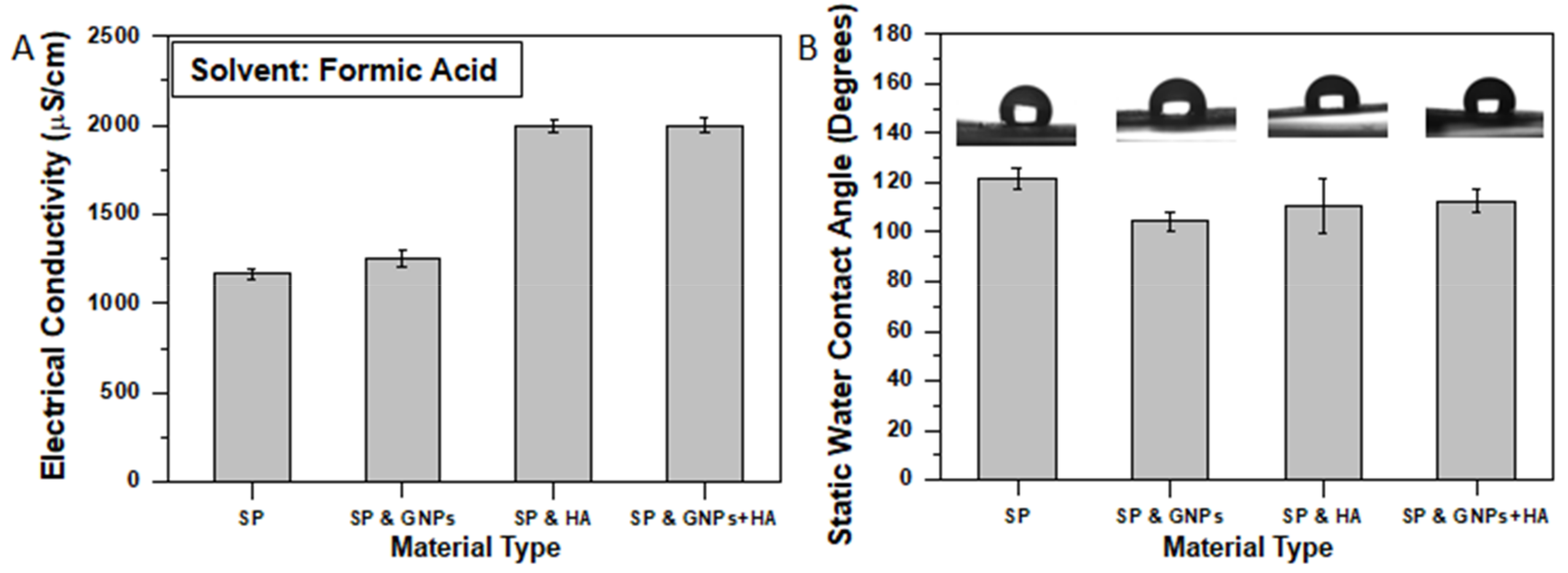
2.3. Electrical Conductivity
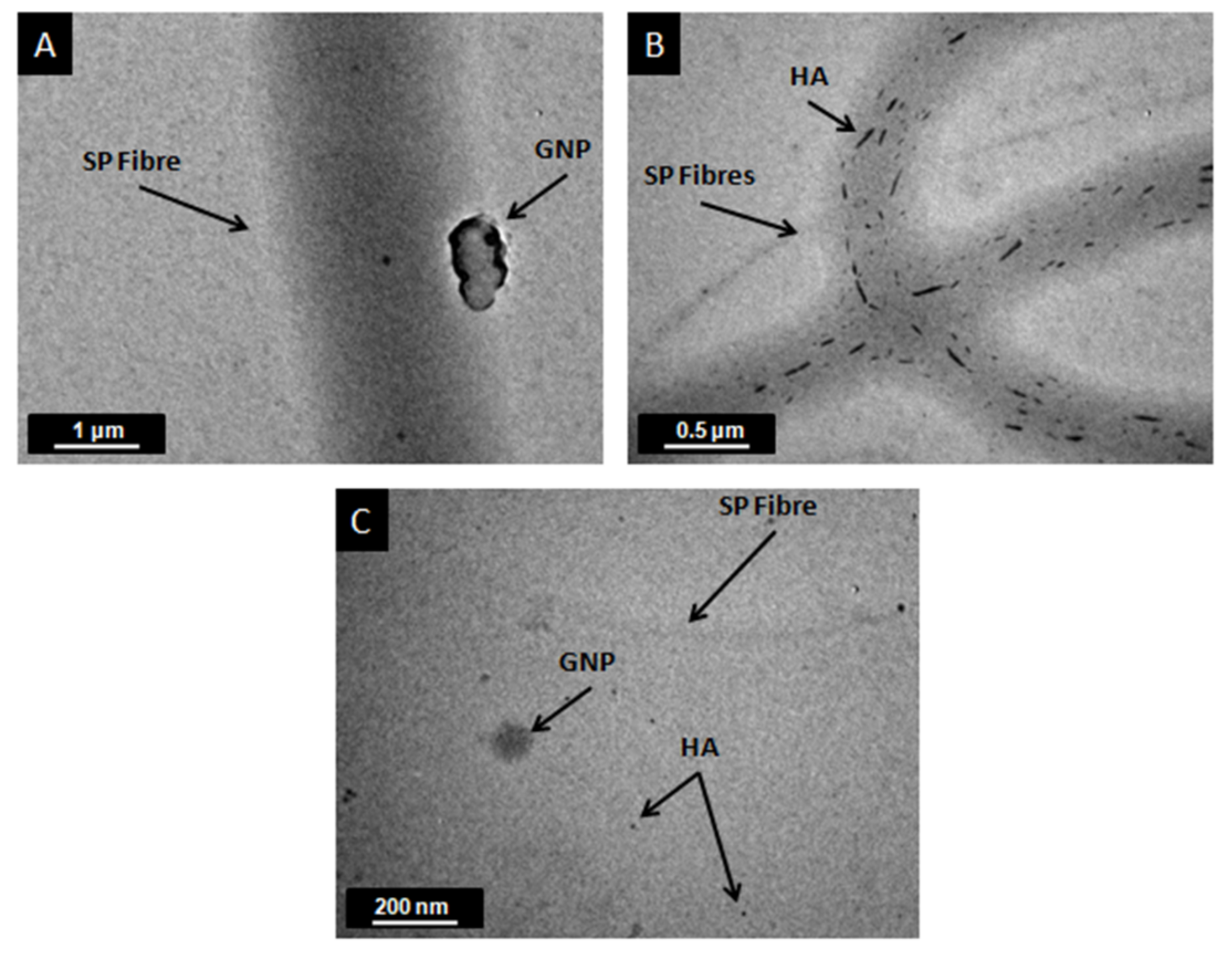
2.4. Scanning Electron Microscopy and Transmission Electron Microscopy
2.5. Porosimetry and Porosity
2.6. Static Water Contact Angle Assay
2.7. Tensile Tests
3. Results and Discussion
3.1. Structural and Morphological Analysis
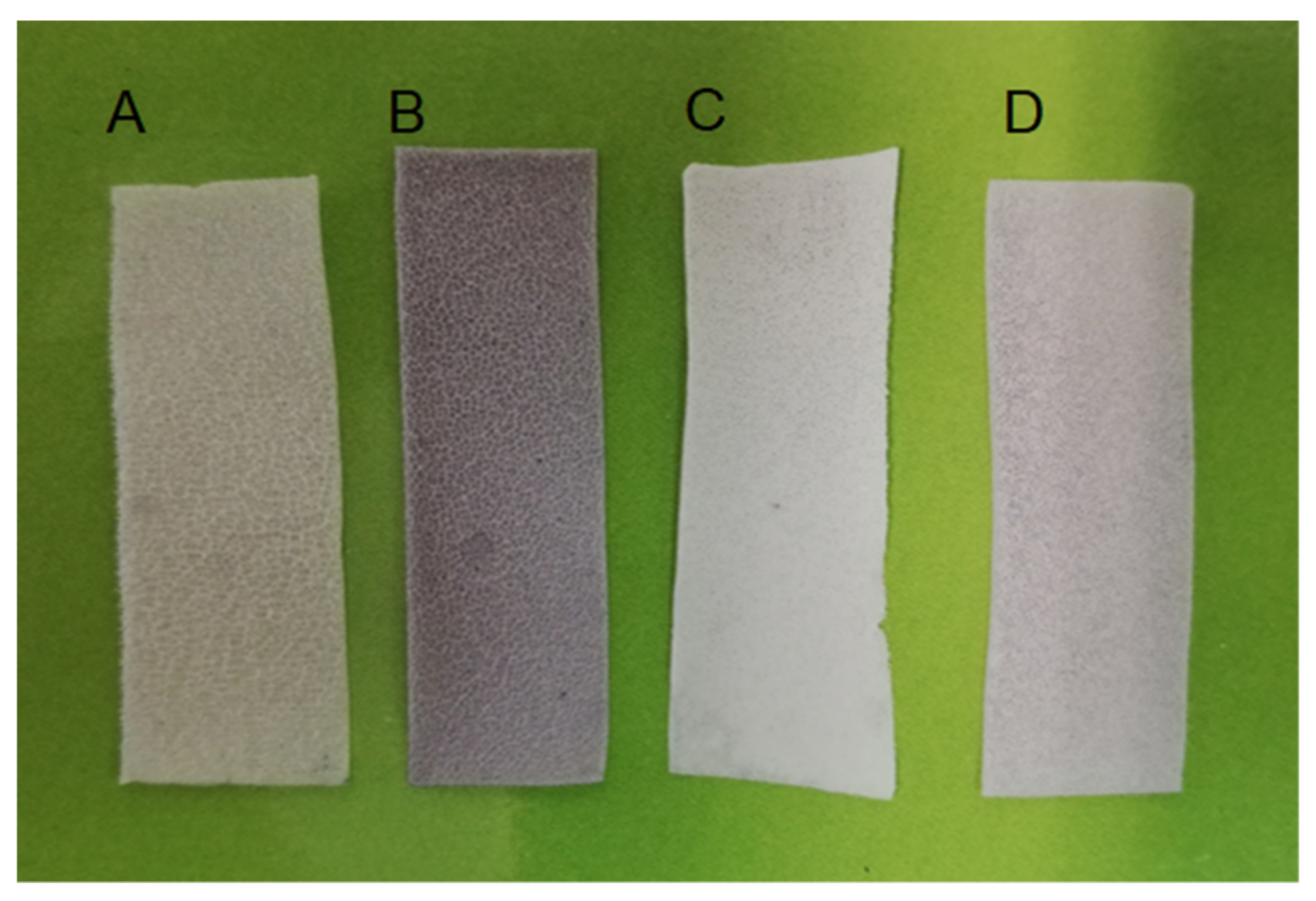
3.2. Physical Characterization
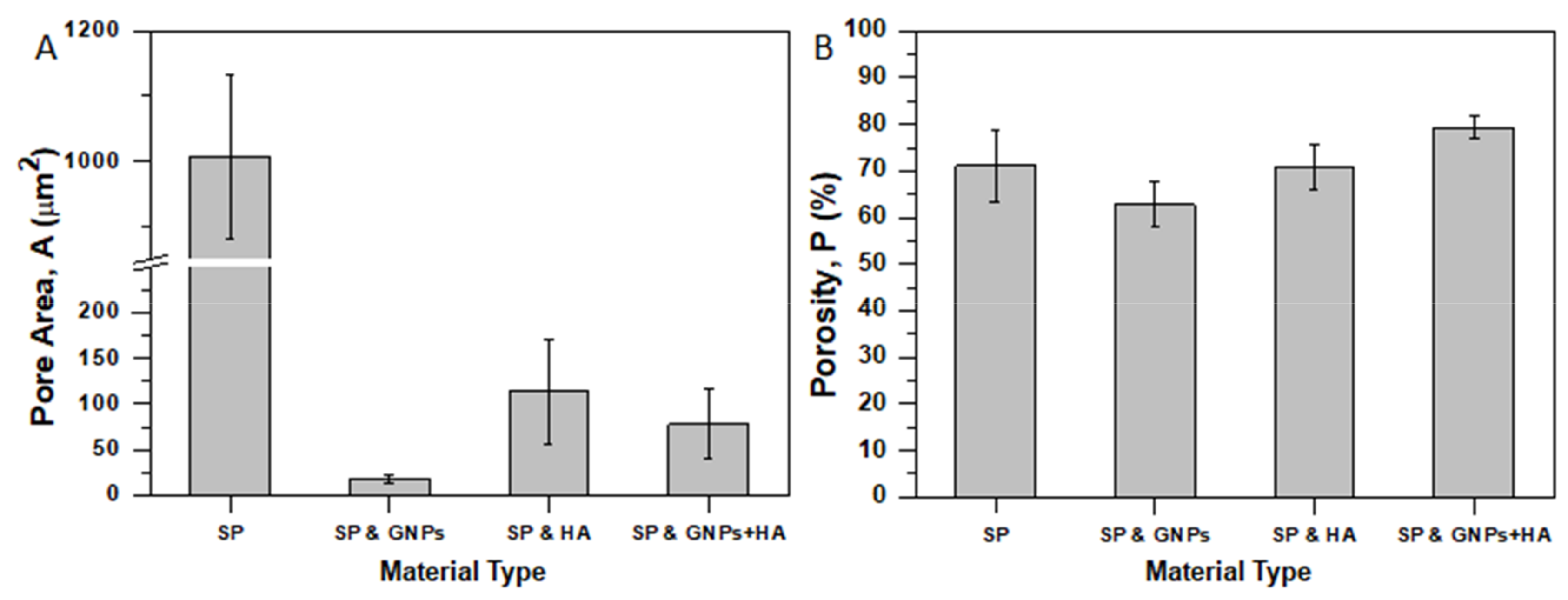
3.3. Porosimetry and Porosity
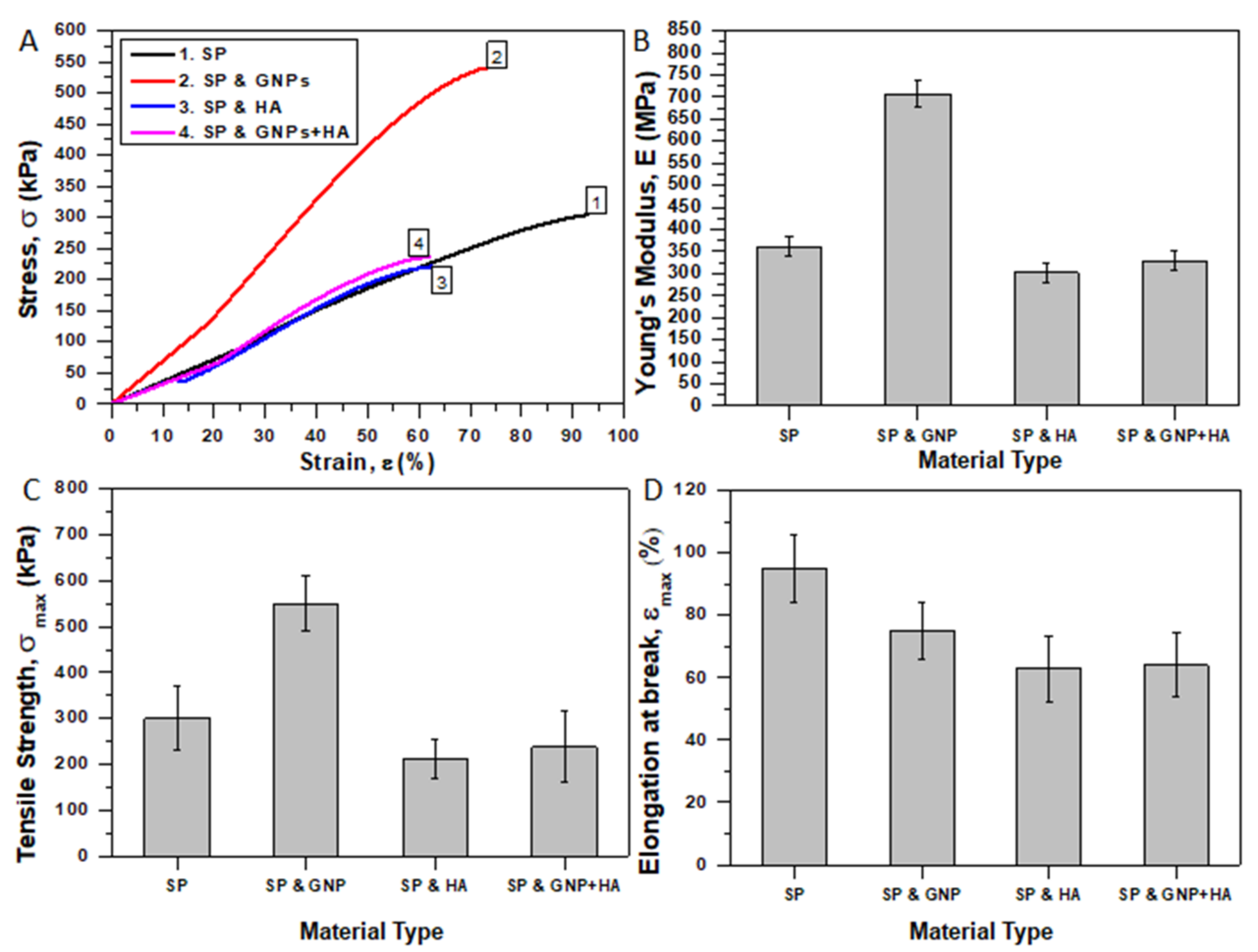
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SP | Supramolecular polymer |
| UPy | Ureiodypyrimidone |
| GNPs | Graphene nanoplatelets |
| HA | Hydroxyapatite |
| ECM | Extracellular matrices |
| PLGA | Poly(lactic-co-glycolicacid) |
| Tg | Glass transition temperature |
| FA | Formic acid |
| RT | Room temperature |
| SEM | Scanning electron microscopy |
| SE | Scanning electron |
| TEM | Transmission electron microscopy |
| A | Pore area |
| P | Porosity |
| M1 | Mass of a fully compacted sample |
| M2 | Mass of an electrospun sample (scaffold) |
| E | Young’s modulus |
| σmax | Ultimate tensile strength |
| εmax | Elongation at break |
| PMMA | Poly(methyl methacrylate) |
References
- Szentivanyi, A.; Zernetsch, H.; Menzel, H.; Glasmacher, B. A review of developments in electrospinning technology: New opportunities for the design of artificial tissue structures. Int. J. Artif. Organs 2011, 34, 986–997. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Akangah, P.; Lingaiah, S.; Shivakumar, K. Effect of Nylon-66 nano-fiber interleaving on impact damage resistance of epoxy/carbon fiber composite laminates. Compos. Struct. 2010, 92, 1432–1439. [Google Scholar] [CrossRef]
- Repanas, A.; Kotrotsos, A.; Kostopoulos, V.; Glasmacher, B. MWCNT-doped Nylon electrospun fibers as materials for increasing damage tolerance of CFRPs in structural applications. Int. J. Innov. Sci. Eng. Technol. 2016, 3, 272–277. [Google Scholar]
- Kim, S.E.; Wang, J.; Jordan, A.M.; Korley, L.T.; Baer, E.; Pokorski, J.K. Surface Modification of Melt Extruded Poly(ε-caprolactone) Nanofibers: Toward a New Scalable Biomaterial Scaffold. ACS Macro Lett. 2014, 3, 585–589. [Google Scholar] [CrossRef]
- Kim, O. Rapid Prototyping of Electrically Small Spherical Wire Antennas. IEEE Trans. Antennas Propag. 2014, 62, 3839–3842. [Google Scholar] [CrossRef]
- Zhai, P.; Chen, X.B.; Schreyer, D.J. PLGA/alginate composite microspheres for hydrophilic protein delivery. Mat. Sci. Eng. C-Mater. 2015, 56, 251–259. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Sutera, F.; Botta, L. Development of polymeric functionally graded scaffolds: A brief review. J. Appl. Biomater. Funct. Mater. 2017, 15, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Maio, A.; Sutera, F.; Mistretta, M.C.; La Mantia, F.P. A Facile and Eco-friendly Route to Fabricate Poly(Lactic Acid) Scaffolds with Graded Pore Size. J. Vis. Exp. 2016, 116, 54595. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Rigogliuso, S.; Ghersi, G. Melt Processed PCL/PEG Scaffold with Discrete Pore Size Gradient for Selective Cellular Infiltration. Macromol. Mater. Eng. 2016, 301, 182–190. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Zhao, K.; Wu, Z.; Wang, Y.; Tan, Q. Fabrication of PLGA/HA (core)-collagen/amoxicillin (shell) nanofiber membranes through coaxial electrospinning for guided tissue regeneration. Compos. Sci. Technol. 2016, 125, 100–107. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, Y.; Zhang, P.B.; Chen, X.S. Methylsulfonylmethane-loaded electrospun poly(lactideco-glycolide) mats for cartilage tissue engineering. RSC Adv. 2015, 5, 96725–96732. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Sutera, A.; Botta, L.; Fontana, R.M.; Gallo, G. Plasma modified PLA electrospun membranes for actinorhod in production intensification in Streptomyces coelicolor immobilized-cell cultivations. Colloids Surf. B Biointerfaces 2017, 157, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Corona-Gomez, J.; Chen, X.; Yang, Q. Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review. J. Funct. Biomater. 2016, 7, 18. [Google Scholar] [CrossRef]
- Fu, C.; Bai, H.; Zhu, J.; Niu, Z.; Wang, Y.; Li, J.; Yang, X.; Bai, Y. Enhanced cell proliferation and osteogenic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS ONE 2017, 12, 1–20. [Google Scholar] [CrossRef]
- Nejati, E.; Mirzadeh, H.; Zandi, M. Synthesis and characterization of nano-hydroxyapatite rods/poly(L-lactide acid) composite scaffolds for bone tissue engineering. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1589–1596. [Google Scholar] [CrossRef]
- Tae-Hyung, K.; Take, L.; Waleed, A.E.; Jeong-Woo, C. Graphene-Based Materials for Stem Cell Applications. Materials 2015, 8, 8674–8690. [Google Scholar]
- Dubey, N.; Bentini, R.; Islam, I.; Cao, T.; Castro Neto, A.H.; Rosa, V. Graphene: A Versatile Carbon-Based Material for Bone Tissue Engineering. Stem Cells Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F. Properties-morphology relationships in electrospun mats based on polylactic acid and graphene nanoplatelets. Compos. Part A Appl. Sci. Manuf. 2018, 108, 23–29. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F. Effect of graphene and fabrication technique on the release kinetics of carvacrol from polylactic acid. Compos. Sci. Technol. 2019, 169, 60–69. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F.; Botta, L. Nanocarbons in Electrospun Polymeric Nanomats for Tissue Engineering: A Review. Polymers 2017, 9, 76. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, J.H.; Kim, K.-S.; Hwangbo, Y.; Yoon, J.-H.; Lee, E.-K.; Ryu, J.; Lee, H.-J.; Cho, S.; Lee, S.-M. Synthesis of CVD-graphene on rapidly heated copper foils. Nanoscale 2014, 6, 4728–4734. [Google Scholar] [CrossRef]
- Gkermpoura, S.S.; Papadimitriou, K.D.; Skountzos, E.N.; Polyzos, I.; Pastore Carbone, M.-G.; Kotrotsos, A.; Mavrantzas, V.G.; Galiotis, C.; Tsitsilianis, C. 3-Arm star pyrene-functional PMMAs for efficient exfoliation of graphite in chloroform: Fabrication of graphene-reinforced fibrous veils. Nanoscale 2019, 11, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Mc Kee, M.G.; Layman, J.M.; Cashion, M.P.; Long, T.E. Phospholipid Nonwoven Electrospun Membranes. Science 2006, 311, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Bittner, A.M.; Loscher, S.; Malinowski, N.; Kern, K. Electrospinning of diphenylalanine nanotubes. Adv. Mater. 2008, 20, 2332–2336. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospinning of polymer-free nanofibers from cyclodextrin inclusion complexes. Langmuir 2011, 27, 6218–6226. [Google Scholar] [CrossRef]
- Yan, X.Z.; Zhou, M.; Chen, J.Z.; Chi, X.D.; Dong, S.Y.; Zhang, M.M.; Ding, X.; Yu, Y.H.; Shao, S.; Huang, F.H. Supramolecular polymer nanofibers via electrospinning of a heteroditopic monomer. Chem. Commun. 2011, 47, 7086–7088. [Google Scholar] [CrossRef]
- Hermida-Merino, D.; Belal, M.; Greenland, B.W.; Woodward, P.; Slark, A.T.; Davis, F.J.; Mitchell, G.R.; Hamley, I.W.; Hayes, W. Electrospun supramolecular polymer fibres. Eur. Polym. J. 2012, 48, 1249–1255. [Google Scholar] [CrossRef]
- Tayi, A.S.; Pashuck, T.E.; Newcomb, C.J.; McClendon, M.T.; Stupp, S.I. Electrospinning Bioactive Supramolecular polymers from water. Biomacromolecules 2014, 15, 1323–1327. [Google Scholar] [CrossRef]
- Tysseling, V.M.; Sahni, V.; Pashuck, E.T.; Birch, D.; Hebert, A.; Czeisler, C.; Stupp, S.I.; Kessler, J.A.J. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. Neurosci. Res. 2010, 88, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
- Rajangam, K.; Behanna, H.A.; Hui, M.J.; Han, X.; Hulvat, J.F.; Lomasney, J.W.; Stupp, S.I. Heparin Binding Nanostructures to Promote Growth of Blood Vessels. Nano Lett. 2006, 6, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Newcomb, C.J.; Bringas, P., Jr.; Stupp, S.I.; Snead, M.L. Biological synthesis of tooth enamel instructed by an artificial matrix. Biomaterials 2010, 31, 9202–9211. [Google Scholar] [CrossRef]
- Mata, A.; Geng, Y.B.; Henrikson, K.J.; Aparicio, C.; Stock, S.R.; Satcher, R.L.; Stupp, S.I. Bone Regeneration Mediated by Biomimetic Mineralization of a Nanofiber Matrix. Biomaterials 2010, 31, 6004–6012. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar] [CrossRef]
- Bosman, A.W.; Rint, P.; Meijer, E.W. Supramolecular polymers at work. Mater. Today 2004, 7, 34–39. [Google Scholar] [CrossRef]
- De Greef, T.F.A.; Meijer, E.W. Supramolecular polymers. Nature 2008, 453, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.C.; Velichko, Y.S.; de la Cruz, M.M.O.; Stupp, S.I. Supramolecular self-assembly codes for functional structures. Philos. Trans. R. Soc. A 2007, 365, 1417–1433. [Google Scholar] [CrossRef] [PubMed]
- Stendahl, J.C.; Li, L.; Zubarev, E.R.; Chen, Y.; Stupp, S.I. Toughening of polymers by self-assembling molecules. Adv. Mater. 2002, 14, 1540–1543. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W. Supramolecular polymers. MRS Bull. 2000, 25, 49–53. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulie-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Richard-Lacroix, M.; Pellerin, C. Electrospinning of supramolecular polymer complexes. Sci. China 2013, 56, 24–32. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Monemian, S.; Korley, L.T.J. Exploring the role of supramolecular associations in mechanical toughening of interpenetrating polymer networks. Macromolecules 2015, 48, 7146–7155. [Google Scholar] [CrossRef]
- Kostopoulos, V.; Kotrotsos, A.; Tsantzalis, S.; Tsokanas, P.; Loutas, T.; Bosman, A.W. Toughening and healing of continuous fibre reinforced composites by supramolecular polymers. Compos. Sci. Technol. 2016, 128, 84–93. [Google Scholar] [CrossRef]
- Kostopoulos, V.; Kotrotsos, A.; Baltopoulos, A.; Tsantzalis, S.; Tsokanas, P.; Loutas, T.; Bosman, A.W. Mode II fracture toughening and healing of composites using supramolecular polymer interlayers. Express Polym. Lett. 2016, 10, 914–926. [Google Scholar] [CrossRef]
- Kostopoulos, V.; Kotrotsos, A. Self-healing of Structural Composites Containing Dendrimers as Healing Agent. In Dendrimers-Fundamentals and Applications; Simonescu, C.M., Ed.; Intech Open Science: London, UK, 2018; Chapter 6; pp. 101–132. [Google Scholar]
- Tyagi, P.; Catledge, S.A.; Stanishevsky, A.; Thomas, V.; Vohra, Y.K. Nanomechanical properties of electrospun composite scaffolds based on polycaprolactone and hydroxyapatite. J. Nanosci. Nanotechol. 2009, 9, 4839–4845. [Google Scholar] [CrossRef]
- Gray, J.A. Kinetics of the dissolution of human dental enamel in acid. J. Dent. Res. 1962, 41, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, B.; Shastry, V.V.; Ramamurty, U.; Ravishankar, N. Effect of calcium deficiency on the mechanical properties of hydroxyapatite crystals. Acta Mater. 2010, 58, 4841–4848. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostopoulos, V.; Kotrotsos, A.; Fouriki, K. Graphene Nanoplatelet- and Hydroxyapatite-Doped Supramolecular Electrospun Fibers as Potential Materials for Tissue Engineering and Cell Culture. Int. J. Mol. Sci. 2019, 20, 1674. https://doi.org/10.3390/ijms20071674
Kostopoulos V, Kotrotsos A, Fouriki K. Graphene Nanoplatelet- and Hydroxyapatite-Doped Supramolecular Electrospun Fibers as Potential Materials for Tissue Engineering and Cell Culture. International Journal of Molecular Sciences. 2019; 20(7):1674. https://doi.org/10.3390/ijms20071674
Chicago/Turabian StyleKostopoulos, Vassilis, Athanasios Kotrotsos, and Kalliopi Fouriki. 2019. "Graphene Nanoplatelet- and Hydroxyapatite-Doped Supramolecular Electrospun Fibers as Potential Materials for Tissue Engineering and Cell Culture" International Journal of Molecular Sciences 20, no. 7: 1674. https://doi.org/10.3390/ijms20071674
APA StyleKostopoulos, V., Kotrotsos, A., & Fouriki, K. (2019). Graphene Nanoplatelet- and Hydroxyapatite-Doped Supramolecular Electrospun Fibers as Potential Materials for Tissue Engineering and Cell Culture. International Journal of Molecular Sciences, 20(7), 1674. https://doi.org/10.3390/ijms20071674