The Secreted Protein MoHrip1 Is Necessary for the Virulence of Magnaporthe oryzae
Abstract
1. Introduction
2. Results
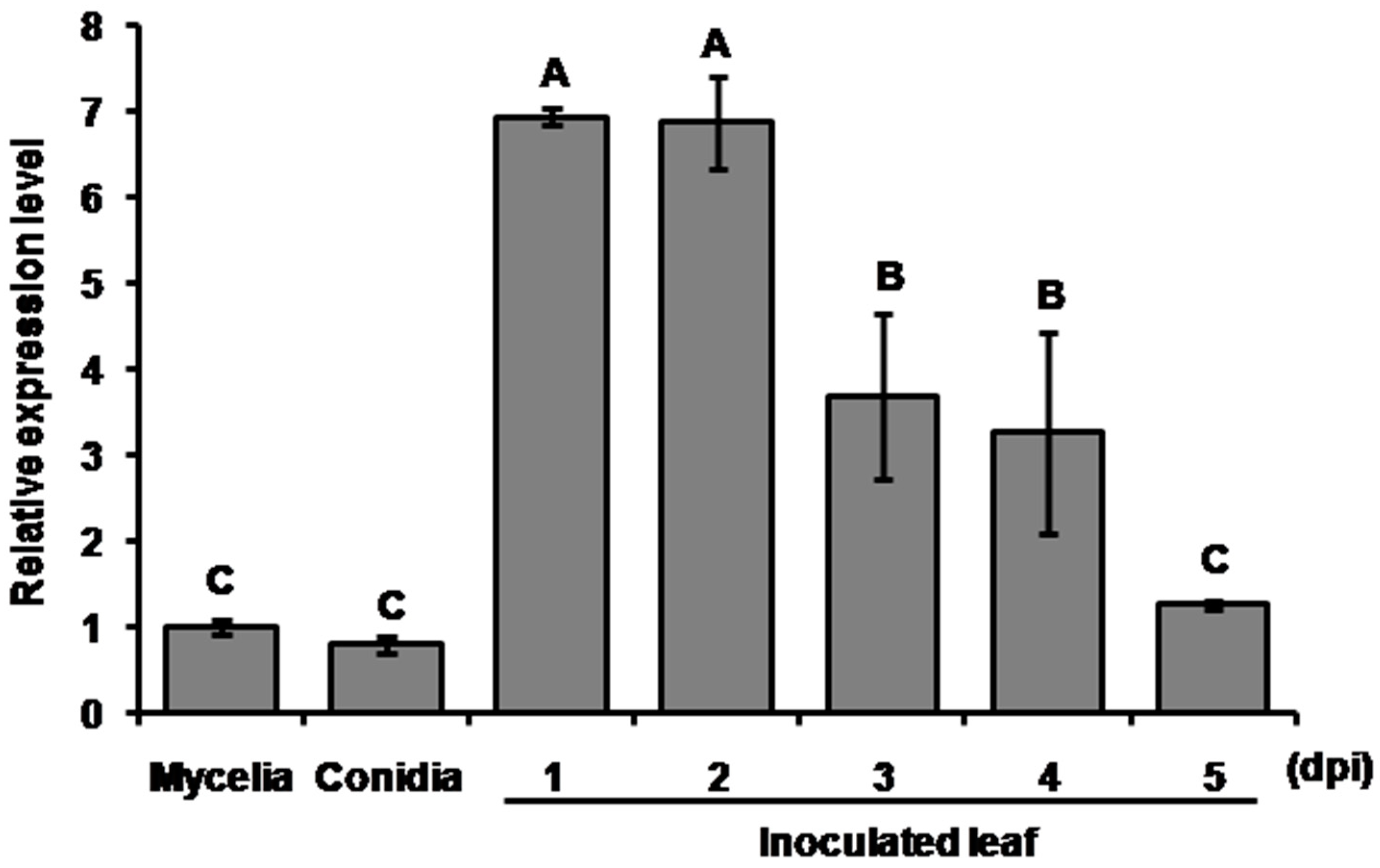
2.1. The Mohrip1 Is Highly Expressed During Fungal Penetration and Colonization
2.2. MoHrip1 Is Dispensable for Fungal Growth and Development In Vitro
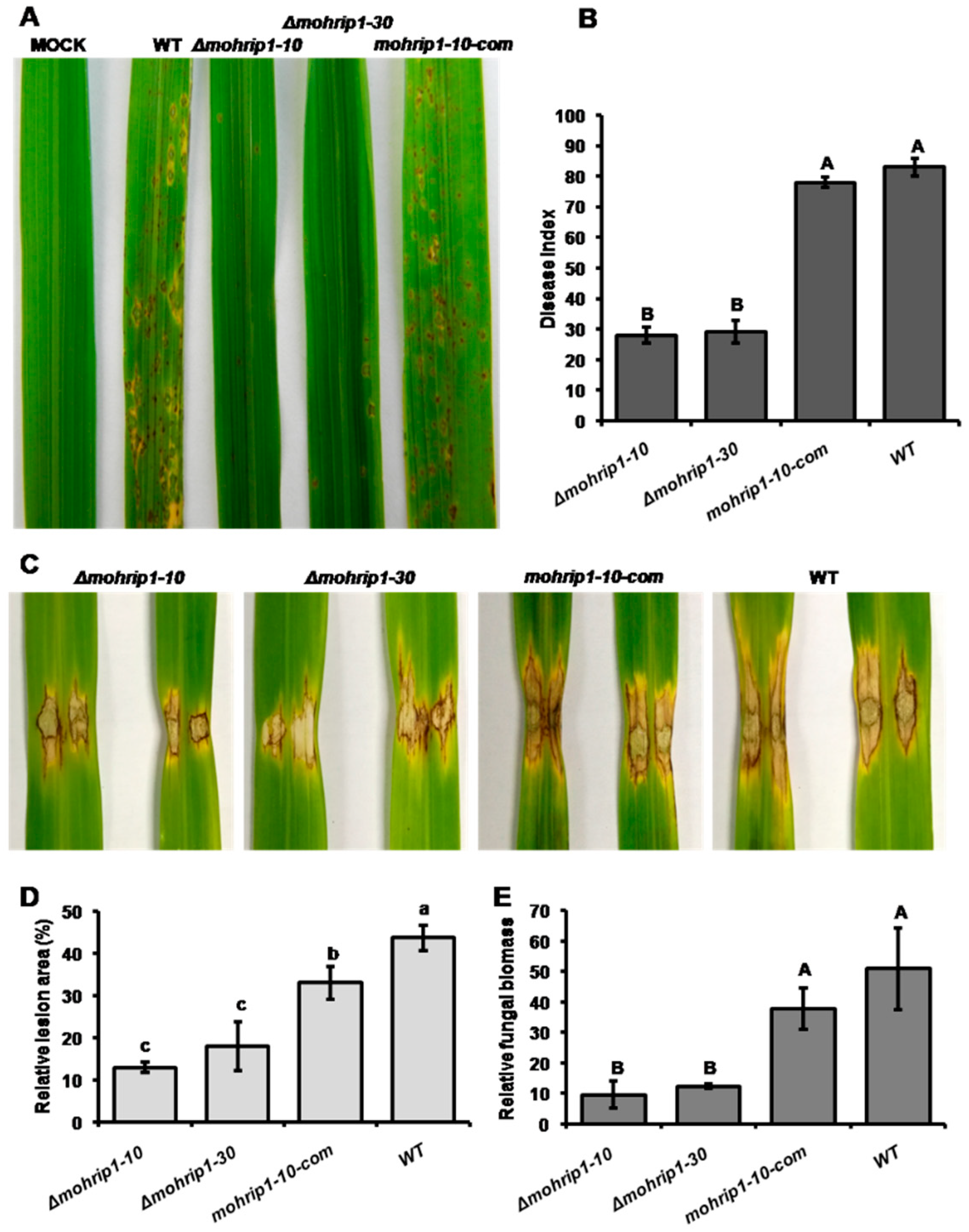
2.3. MoHrip1 Is Required for the Full Virulence of M. oryzae
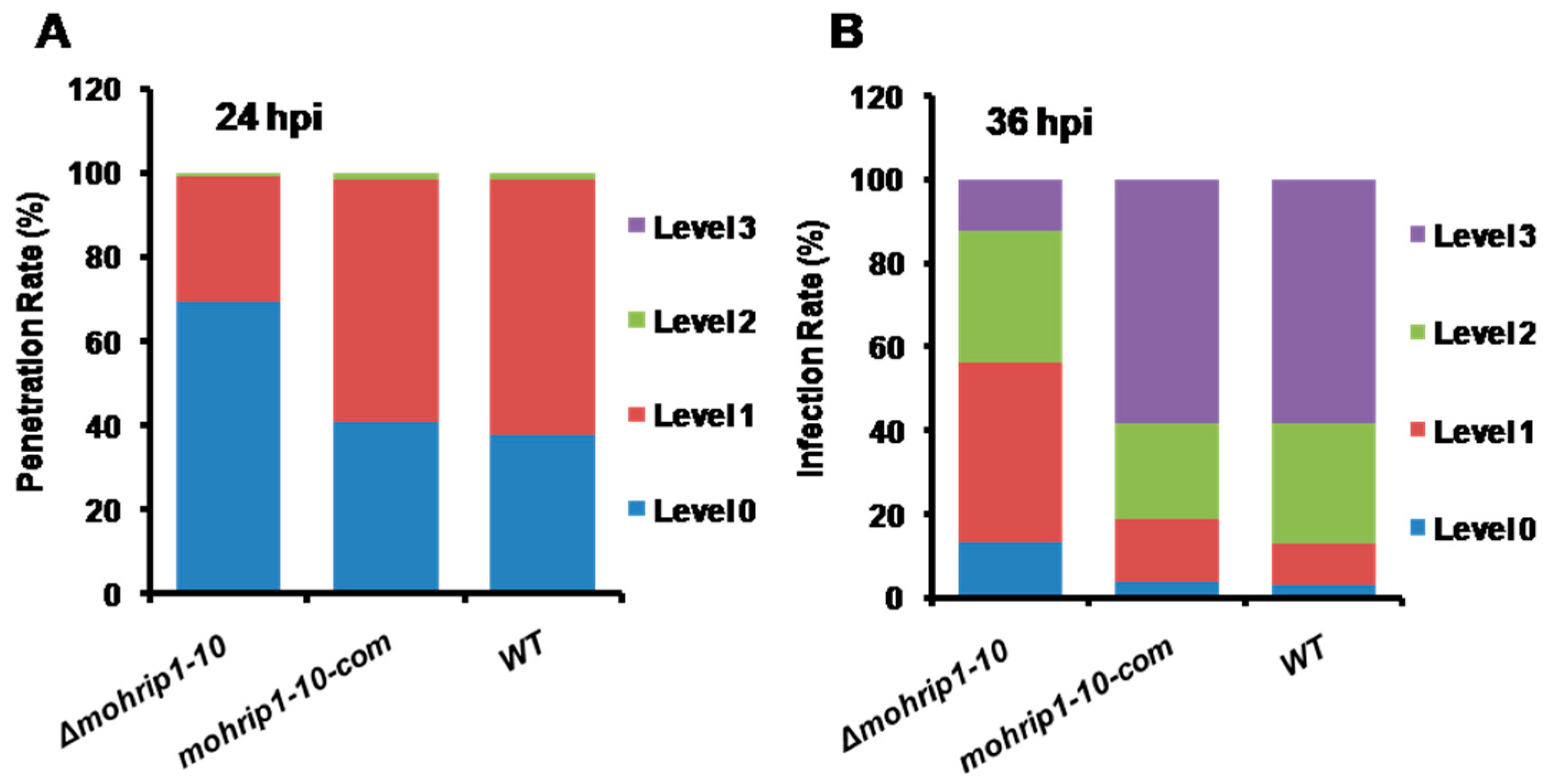
2.4. MoHrip1 Is Important for the Penetration and Proliferation of M. oryzae
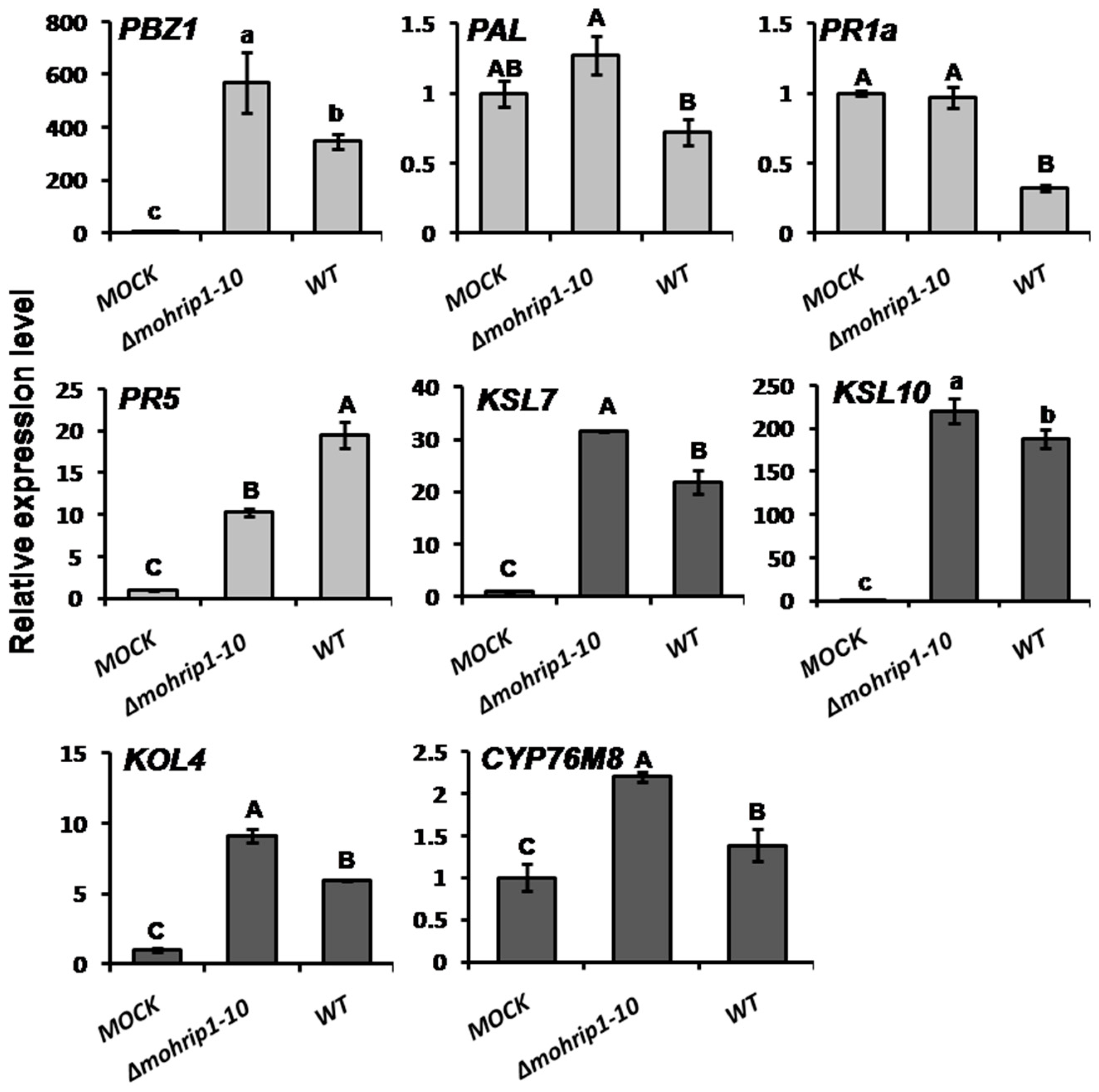
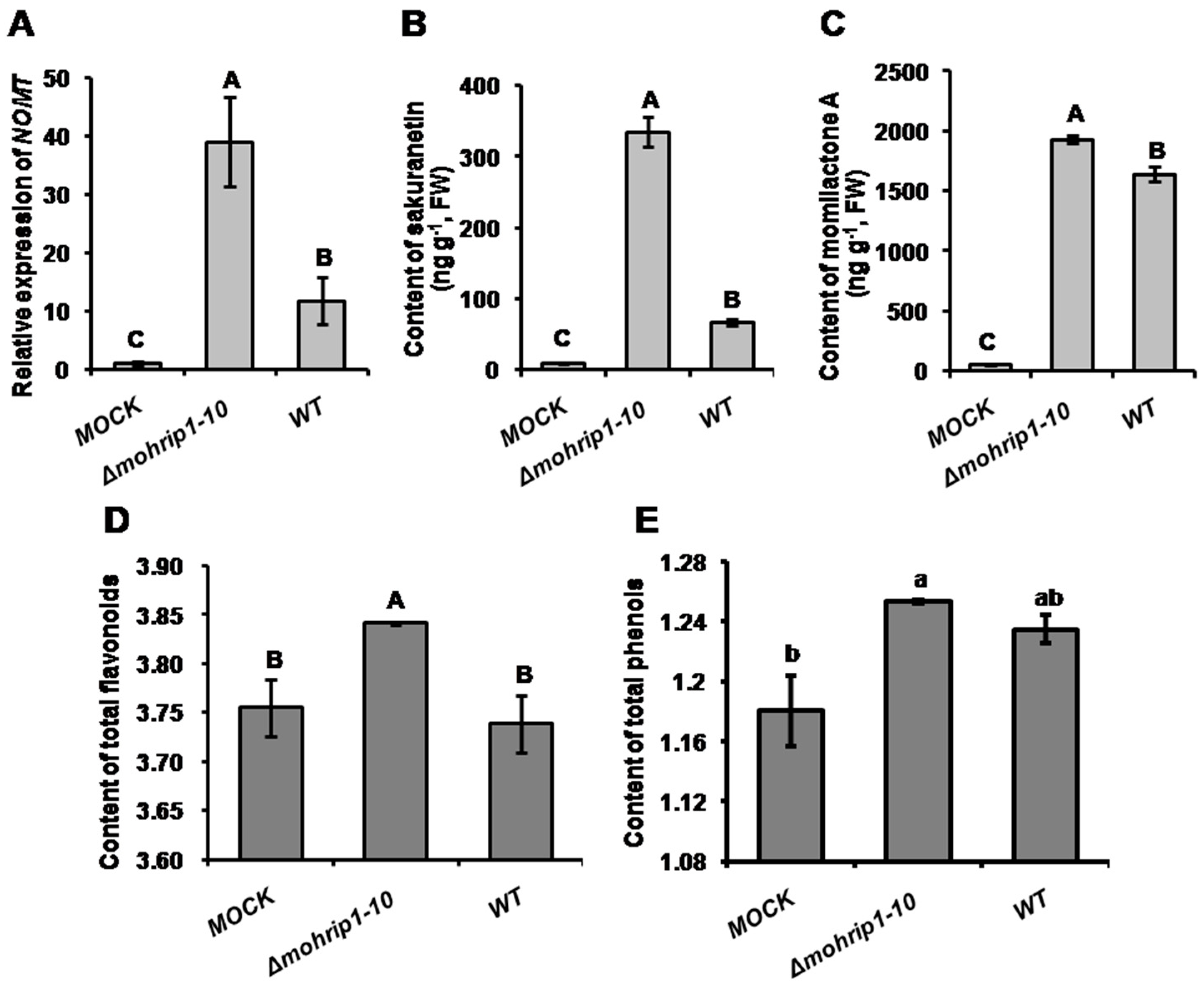
2.5. MoHrip1 Suppresses the Activation of Plant Immunity
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. QRT-PCR Analysis of Mohrip1 Expression
4.3. Vector Construction
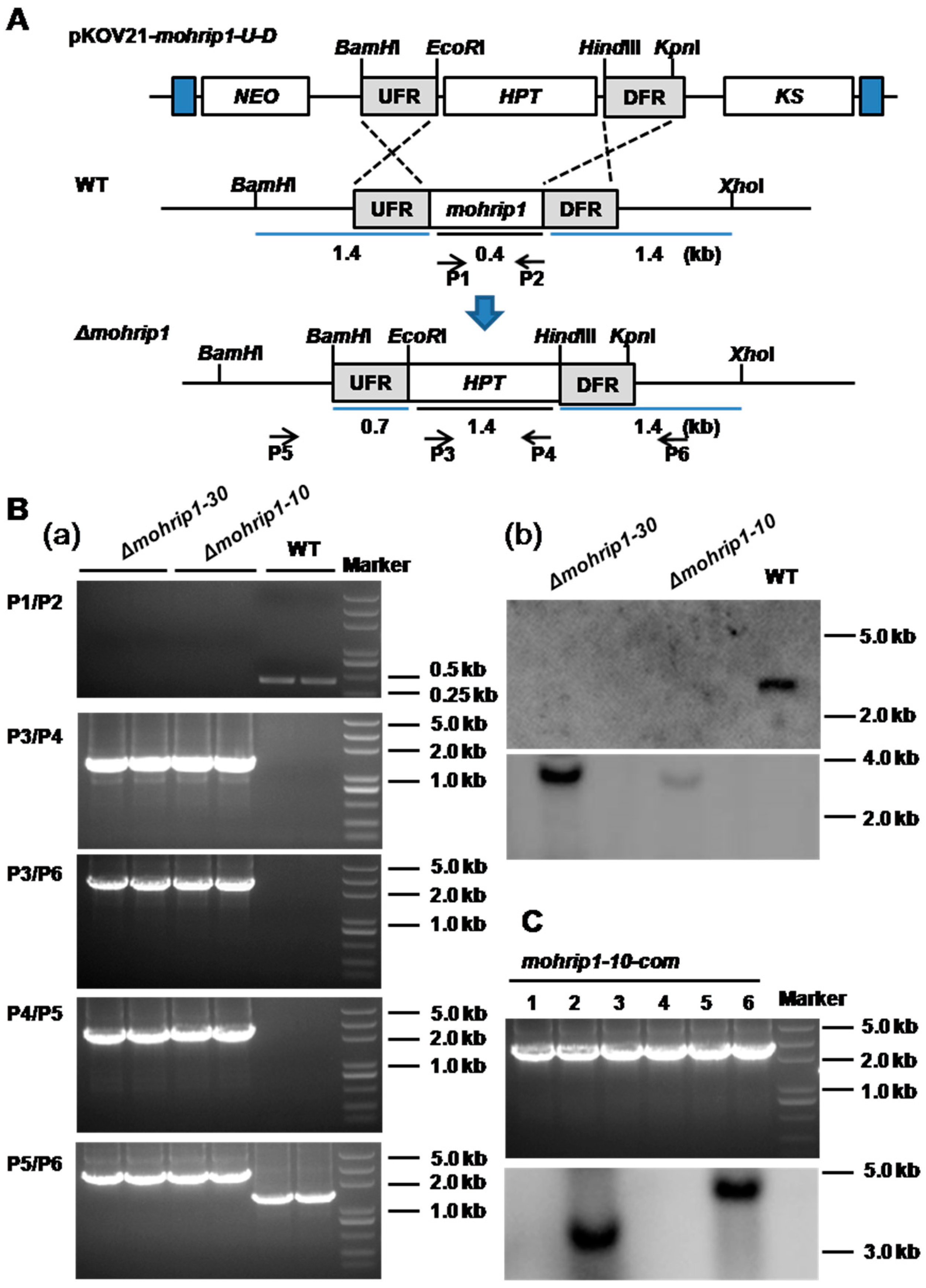
4.4. Fungal Transformation and Confirmation
4.5. Phenotypic Characterization
4.6. Pathogenicity Assays
4.7. Rice Leaf Sheath Infection Assays
4.8. Quantification of Defensive Compounds
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IH | invasive hyphae |
| BAS | biotrophy-associated secreted |
| MAX | M. oryzae Avrs and ToxB |
| HR | hypersensitive reaction |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| OA | oat agar medium |
| CM | complete medium |
| dpi | days post inoculation |
| hpi | hours post inoculation |
| HPT | hygromycin B resistance gene |
| PAMP | pathogen-associated molecular pattern |
| PR | pathogenesis-related |
| TLP | thaumatin-like protein |
| ANOVA | analysis of variance |
References
- Giraldo, M.C.; Valent, B. Filamentous plant pathogen effectors in action. Nat. Rev. Microbiol. 2013, 11, 800–814. [Google Scholar] [CrossRef] [PubMed]
- De Guillen, K.; Ortiz-Vallejo, D.; Gracy, J.; Fournier, E.; Kroj, T.; Padilla, A. Structure analysis uncovers a highly diverse but structurally conserved effector family in phytopathogenic fungi. PLoS Pathog. 2015, 11, e1005228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Luo, J.; Rossman, A.Y.; Aoki, T.; Chuma, I.; Crous, P.W.; Dean, R.; de Vries, R.P.; Donofrio, N.; Hyde, K.D.; et al. Generic names in Magnaporthales. IMA Fungus 2016, 7, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-H.; Ebbole, D.J.; Wang, Z.-H. The arms race between Magnaporthe oryzae and rice: Diversity and interaction of Avr and R genes. J. Integr. Agric. 2017, 16, 2746–2760. [Google Scholar] [CrossRef]
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K. The gemome sequence of the rice blast disease fungus Magnaporthe grisea. Nature 2005, 43, 4980–4986. [Google Scholar]
- Kankanala, P.; Czymmek, K.; Valent, B. Role for rice membrance dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 2007, 19, 706–724. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Talbot, N.J. Having a blast: Exploring the pathogenicity of Magnapothe grisea. Trends Microbiol. 1995, 3, 9–16. [Google Scholar] [CrossRef]
- Nishimura, T.; Mochizuki, S.; Ishii-Minami, N.; Fujisawa, Y.; Kawahara, Y.; Yoshida, Y.; Okada, K.; Ando, S.; Matsumura, H.; Terauchi, R.; et al. Magnaporthe oryzae glycine-rich secretion protein, Rbf1 critically participates in pathogenicity through the focal formation of the biotrophic interfacial complex. PLoS Pathog. 2016, 12, e1005921. [Google Scholar] [CrossRef]
- Park, C.H.; Chen, S.; Shirsekar, G.; Zhou, B.; Khang, C.H.; Songkumarn, P.; Afzal, A.J.; Ning, Y.; Wang, R.; Bellizzi, M.; et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell 2012, 24, 4748–4762. [Google Scholar] [CrossRef]
- Mosquera, G.; Giraldo, M.C.; Khang, C.H.; Coughlan, S.; Valent, B. Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as biotrophy-associated secreted proteins in rice blast disease. Plant Cell 2009, 21, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Varden, F.A.; De la Concepcion, J.C.; Maidment, J.H.; Banfield, M.J. Taking the stage: Effectors in the spotlight. Curr. Opin. Plant Biol. 2017, 38, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.C.; Dagdas, Y.F.; Gupta, Y.K.; Mentlak, T.A.; Yi, M.; Martinez-Rocha, A.L.; Saitoh, H.; Terauchi, R.; Talbot, N.J.; Valent, B. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Rodriguez-Moreno, L.; Ebert, M.K.; Bolton, M.D.; Thomma, B. Tools of the crook- infection strategies of fungal plant pathogens. Plant J. 2018, 93, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Ose, T.; Oikawa, A.; Nakamura, Y.; Maenaka, K.; Higuchi, Y.; Satoh, Y.; Fujiwara, S.; Demura, M.; Sone, T.; Kamiya, M. Solution structure of an avirulence protein, AVR-Pia, from Magnaporthe oryzae. J. Biomol. NMR 2015, 63, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Saitoh, H.; Fujisawa, S.; Kanzaki, H.; Matsumura, H.; Yoshida, K.; Tosa, Y.; Chuma, I.; Takano, Y.; Win, J.; et al. Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell 2009, 21, 1573–1591. [Google Scholar] [CrossRef] [PubMed]
- Miki, S.; Matsui, K.; Kito, H.; Otsuka, K.; Ashizawa, T.; Yasuda, N.; Fukiya, S.; Sato, J.; Hirayae, K.; Fujita, Y.; et al. Molecular cloning and characterization of the AVR-Pia locus from a Japanese field isolate of Magnaporthe oryzae. Mol. Plant Pathol. 2009, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Sornkom, W.; Miki, S.; Takeuchi, S.; Abe, A.; Asano, K.; Sone, T. Fluorescent reporter analysis revealed the timing and localization of AVR-Pia expression, an avirulence effector of Magnaporthe oryzae. Mol. Plant Pathol. 2017, 18, 1138–1149. [Google Scholar] [CrossRef]
- Ortiz, D.; de Guillen, K.; Cesari, S.; Chalvon, V.; Gracy, J.; Padilla, A.; Kroj, T. Recognition of the Magnaporthe oryzae effector AVR-Pia by the decoy domain of the rice NLR immune receptor RGA5. Plant Cell 2017, 29, 156–168. [Google Scholar] [CrossRef]
- Cesari, S.; Thilliez, G.; Ribot, C.; Chalvon, V.; Michel, C.; Jauneau, A.; Rivas, S.; Alaux, L.; Kanzaki, H.; Okuyama, Y.; et al. The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell 2013, 25, 1463–1481. [Google Scholar] [CrossRef]
- Kang, S.; Sweigard, J.A.; Valent, B. The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Mol. Plant Microbe Interact. 1995, 8, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, L.; Wang, Y.; Wang, C.; Yan, J.; Liu, Y.; Wang, C.; Li, C. Overexpression of BAS1 in rice blast fungus can promote blast fungus growth, sporulation and virulence in planta. Saudi J. Biol. Sci. 2017, 24, 1884–1893. [Google Scholar] [CrossRef]
- Saitoh, H.; Fujisawa, S.; Mitsuoka, C.; Ito, A.; Hirabuchi, A.; Ikeda, K.; Irieda, H.; Yoshino, K.; Yoshida, K.; Matsumura, H.; et al. Large-scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens. PLoS Pathog. 2012, 8, e1002711. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, H.; Qiu, D.; Guo, L.; Yang, X.; Shi, H.; Zhou, T.; Zhao, J. Purification and characterization of a novel hypersensitive response-inducing elicitor from Magnaporthe oryzae that triggers defense response in rice. PLoS ONE 2012, 7, e37654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, Q.; Zi, Q.; Lv, S.; Qiu, D.; Zeng, H. Enhanced disease resistance and drought tolerance in transgenic rice plants overexpressing protein elicitors from Magnaporthe oryzae. PLoS ONE 2017, 12, e0175734. [Google Scholar] [CrossRef] [PubMed]
- Valent, B.; Farrall, L.; Chumley, F.G. Magnaporthe grisea genes for pathogenicity and virulence through a series of backcrosses. Genetics 1991, 127, 87–101. [Google Scholar]
- Liu, Q.; Ning, Y.; Zhang, Y.; Yu, N.; Zhao, C.; Zhan, X.; Wu, W.; Chen, D.; Wei, X.; Wang, G.L.; et al. OsCUL3a negatively regulates cell death and immunity by degrading OsNPR1 in Rice. Plant Cell 2017, 29, 345–359. [Google Scholar] [CrossRef]
- Sun, D.; Cao, H.; Shi, Y.; Huang, P.; Dong, B.; Liu, X.; Lin, F.; Lu, J. The regulatory factor X protein MoRfx1 is required for development and pathogenicity in the rice blast fungus Magnaporthe oryzae. Mol. Plant Pathol. 2017, 18, 1075–1088. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, K.T.; Lee, Y.H. SUMOylation is required for fungal development and pathogenicity in the rice blast fungus Magnaporthe oryzae. Mol. Plant Pathol. 2018, 19, 2134–2148. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Wang, Z.; Zhang, L.; Zhuang, H.; Zeng, H. Apoplastic effector MoHrip2 is required for the full virulence of rice blast fungus Magnaporthe oryzae. Unpublished work.
- Kim, S.G.; Wang, Y.; Lee, K.H.; Park, Z.Y.; Park, J.; Wu, J.; Kwon, S.J.; Lee, Y.H.; Agrawal, G.K.; Rakwal, R.; et al. In-depth insight into in vivo apoplastic secretome of rice-Magnaporthe oryzae interaction. J. Proteom. 2013, 78, 58–71. [Google Scholar] [CrossRef]
- Bialas, A.; Zess, E.K.; De la Concepcion, J.C.; Franceschetti, M.; Pennington, H.G.; Yoshida, K.; Upson, J.L.; Chanclud, E.; Wu, C.H.; Langner, T.; et al. Lessons in effector and NLR biology of plant-microbe systems. Mol. Plant Microbe Interact. 2018, 31, 34–45. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Z.; Tang, W.; Cai, X.; Gao, C.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. The thioredoxin MoTrx2 protein mediates reactive oxygen species (ROS) balance and controls pathogenicity as a target of the transcription factor MoAP1 in Magnaporthe oryzae. Mol. Plant Pathol. 2017, 18, 1199–1209. [Google Scholar] [CrossRef]
- Cho, M.H.; Lee, S.W. Phenolic phytoalexins in rice: Biological functions and biosynthesis. Int. J. Mol. Sci. 2015, 16, 29120–29133. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Lin, F.; Hasegawa, M.; Okada, K.; Nojiri, H.; Yamane, H. Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J. Biol. Chem. 2012, 287, 19315–19325. [Google Scholar] [CrossRef]
- Gaulin, E. Effector-mediated communication of filamentous plant pathogens with their hosts. Adv. Bot. Res. 2017, 82, 161–185. [Google Scholar]
- Sharpee, W.C.; Dean, R.A. Form and function of fungal and oomycete effectors. Fungal Biol. Rev. 2016, 30, 62–73. [Google Scholar] [CrossRef]
- Fang, A.; Han, Y.; Zhang, N.; Zhang, M.; Liu, L.; Li, S.; Lu, F.; Sun, W. Identification and characterization of plant cell death-inducing secreted proteins from Ustilaginoidea virens. Mol. Plant Microbe Interact. 2016, 29, 405–416. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Z.; Yang, X.; Guo, L.; Qiu, D.; Zeng, H. Transcriptional profiling of rice treated with MoHrip1 reveal the function of protein elicitor in enhancement of disease resistance and plant growth. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef]
- Jeong, J.S.; Mitchell, T.K.; Dean, R.A. The Magnaporthe grisea snodprot1 homolog, MSP1, is required for virulence. FEMS Microbiol. Lett. 2007, 273, 157–165. [Google Scholar] [CrossRef]
- Wei, Z.M.; Laby, R.J.; Zumoff, C.H.; Bauer, D.W.; He, S.Y.; Colimer, A.; Beer, S.V. Harpin, Elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 1992, 257, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Song, T.; Zhu, L.; Ye, W.; Wang, Y.; Shao, Y.; Dong, S.; Zhang, Z.; Dou, D.; Zheng, X.; et al. A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell 2015, 27, 2057–2072. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.; Nurnberger, T.; Joosten, M.H. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef]
- Liu, J.J.; Sturrock, R.; Ekramoddoullah, A.K. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Rout, E.; Nanda, S.; Joshi, R.K. Molecular characterization and heterologous expression of a pathogen induced PR5 gene from garlic (Allium sativum L.) conferring enhanced resistance to necrotrophic fungi. Eur. J. Plant Pathol. 2015, 144, 345–360. [Google Scholar] [CrossRef]
- Yasmin, N.; Saleem, M.; Naz, M.; Gul, R.; Rehman, H.M. Molecular characterization, structural modeling, and evaluation of antimicrobial activity of Basrai thaumatin-like protein against fungal infection. Biomed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Misra, R.C.; Sandeep; Kamthan, M.; Kumar, S.; Ghosh, S. A thaumatin-like protein of Ocimum basilicum confers tolerance to fungal pathogen and abiotic stress in transgenic Arabidopsis. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Brito, N.; Gonzalez, C. The Botrytis cinerea elicitor protein BcIEB1 interacts with the tobacco PR5-family protein osmotin and protects the fungus against its antifungal activity. New Phytol. 2017, 215, 397–410. [Google Scholar] [CrossRef]
- Gomez-Casado, C.; Murua-Garcia, A.; Garrido-Arandia, M.; Gonzalez-Melendi, P.; Sanchez-Monge, R.; Barber, D.; Pacios, L.F.; Diaz-Perales, A. Alt a 1 from Alternaria interacts with PR5 thaumatin-like proteins. FEBS Lett. 2014, 588, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Zhang, L.; Shi, W.; Zhuang, H.; Zeng, H. Crystal structure analysis and partner identification of Magnaporthe oryzae effector MoHrip1 in rice. Unpublished work.
- Wang, W.; Li, Y.; Dang, P.; Zhao, S.; Lai, D.; Zhou, L. Rice secondary metabolites: Structures, roles, biosynthesis, and metabolic regulation. Molecules 2018, 23, 3098. [Google Scholar] [CrossRef]
- Shi, Z.; Leung, H. Genetic analysis of sporulation in Magnaporthe grisea by chemical and insertional mutagenesis. Mol. Plant Microbe Interact. 1995, 8, 949–959. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, S.; Sun, Y.; Li, M.; Dong, Y.; Wang, X.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoTup1 is required for growth, conidiogenesis and pathogenicity of Magnaporthe oryzae. Mol. Plant Pathol. 2015, 16, 799–810. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, W.; Xu, X.W.; Li, Z.G.; Yin, C.F.; Peng, J.B.; Pan, S.; Chen, X.L.; Zhao, W.S.; Zhang, Y.; et al. Glutamate synthase MoGlt1-mediated glutamate homeostasis is important for autophagy, virulence and conidiation in the rice blast fungus. Mol. Plant Pathol. 2018, 19, 564–578. [Google Scholar] [CrossRef]
- Zhou, X.; Li, G.; Xu, J.R. Efficient approaches for generating GFP fusion and epitope-tagging constructs in filamentous fungi. In Fungal Genomics: Methods and Protocols, Methods in Molecular Biology; Xu, J.R., Bluhm, B.H., Eds.; Springer Science+Business Media, LLC: Berlin/Heidelberg, Germany, 2011; Volume 16, pp. 199–212. [Google Scholar]
- Guo, M.; Chen, Y.; Du, Y.; Dong, Y.; Guo, W.; Zhai, S.; Zhang, H.; Dong, S.; Zhang, Z.; Wang, Y.; et al. The bZIP transcription factor MoAP1 mediates the oxidative stress response and is critical for pathogenicity of the rice blast fungus Magnaporthe oryzae. PLoS Pathog. 2011, 7, e1001302. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Zhang, H.; Zhou, C.; Qi, Z.; Zheng, X.; Wang, P.; Zhang, Z. The actin-regulating kinase homologue MoArk1 plays a pleiotropic function in Magnaporthe oryzae. Mol. Plant Pathol. 2013, 14, 470–482. [Google Scholar] [CrossRef]
- Koga, H.; Dohi, K.; Nakayachi, O.; Mori, M. A novel inoculation method of Magnaporthe grisea for cytological observation of the infection process using intact leaf sheaths of rice plants. Physiol. Mol. Plant Pathol. 2004, 64, 67–72. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Y.; Yang, S.; Li, T.; Chen, P.; Li, Q. Study on efficacy and mechanism of tobacco anti-virus agent applied in the field. J. Yunnan Agric. Univ. 2001, 16, 9–12. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, H.-Z.; Zhang, L.; Zhuang, H.-Q.; Shi, W.-J.; Yang, X.-F.; Qiu, D.-W.; Zeng, H.-M. The Secreted Protein MoHrip1 Is Necessary for the Virulence of Magnaporthe oryzae. Int. J. Mol. Sci. 2019, 20, 1643. https://doi.org/10.3390/ijms20071643
Nie H-Z, Zhang L, Zhuang H-Q, Shi W-J, Yang X-F, Qiu D-W, Zeng H-M. The Secreted Protein MoHrip1 Is Necessary for the Virulence of Magnaporthe oryzae. International Journal of Molecular Sciences. 2019; 20(7):1643. https://doi.org/10.3390/ijms20071643
Chicago/Turabian StyleNie, Hai-Zhen, Lin Zhang, Hui-Qian Zhuang, Wen-Jiong Shi, Xiu-Fen Yang, De-Wen Qiu, and Hong-Mei Zeng. 2019. "The Secreted Protein MoHrip1 Is Necessary for the Virulence of Magnaporthe oryzae" International Journal of Molecular Sciences 20, no. 7: 1643. https://doi.org/10.3390/ijms20071643
APA StyleNie, H.-Z., Zhang, L., Zhuang, H.-Q., Shi, W.-J., Yang, X.-F., Qiu, D.-W., & Zeng, H.-M. (2019). The Secreted Protein MoHrip1 Is Necessary for the Virulence of Magnaporthe oryzae. International Journal of Molecular Sciences, 20(7), 1643. https://doi.org/10.3390/ijms20071643




