Synthesis, Characterization and Adsorptive Performances of a Composite Material Based on Carbon and Iron Oxide Particles
Abstract
:1. Introduction
2. Results and Discussions
2.1. Synthesis of the Composite Material Based on Carbon and Fe3O4 Particles
2.2. Physical-Chemical Characterization of the Synthesized Composite Material
2.2.1. Thermal Gravimetric Analysis (TGA)
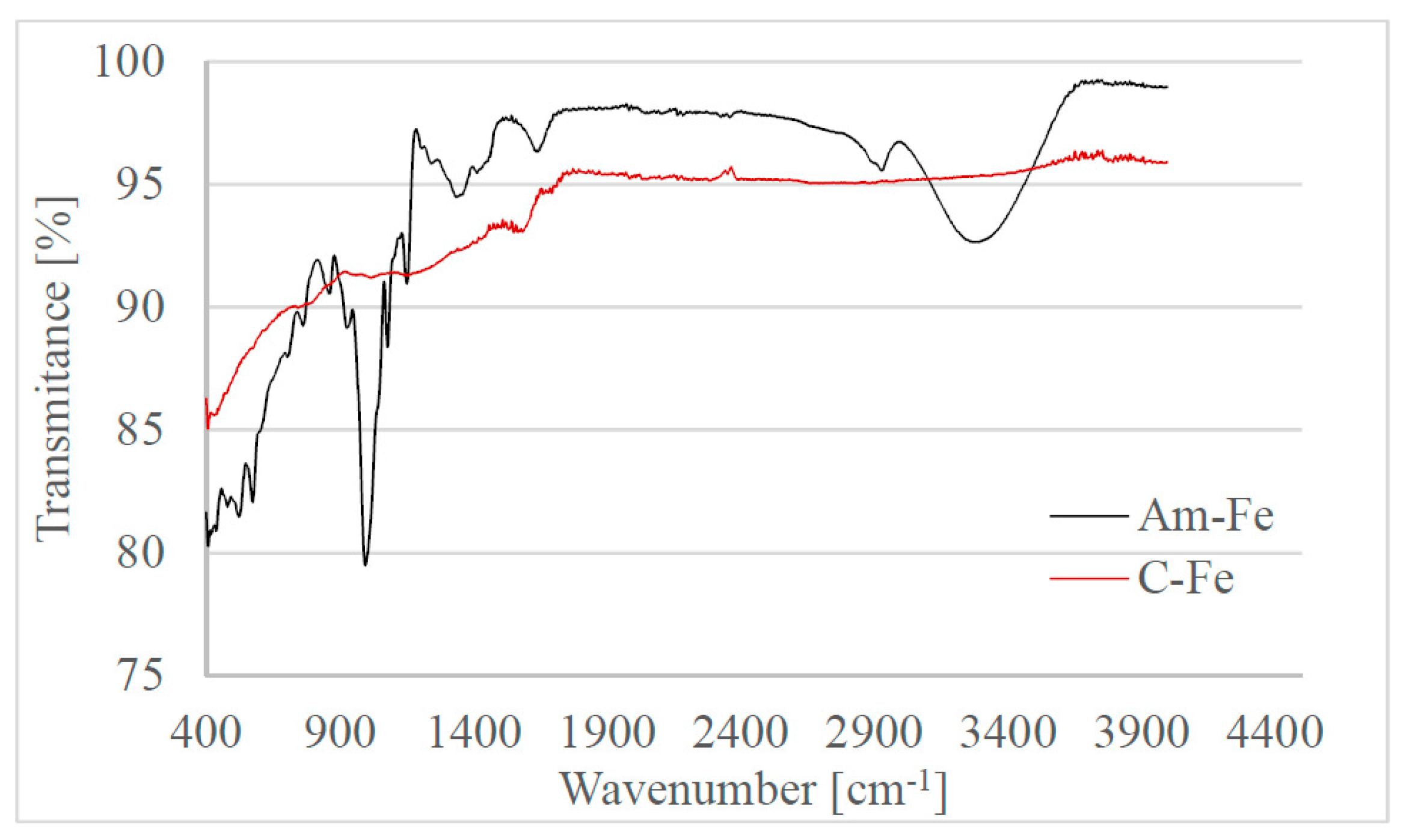
2.2.2. Fourier Transform Infra-Red (FT-IR) Spectroscopy Analysis
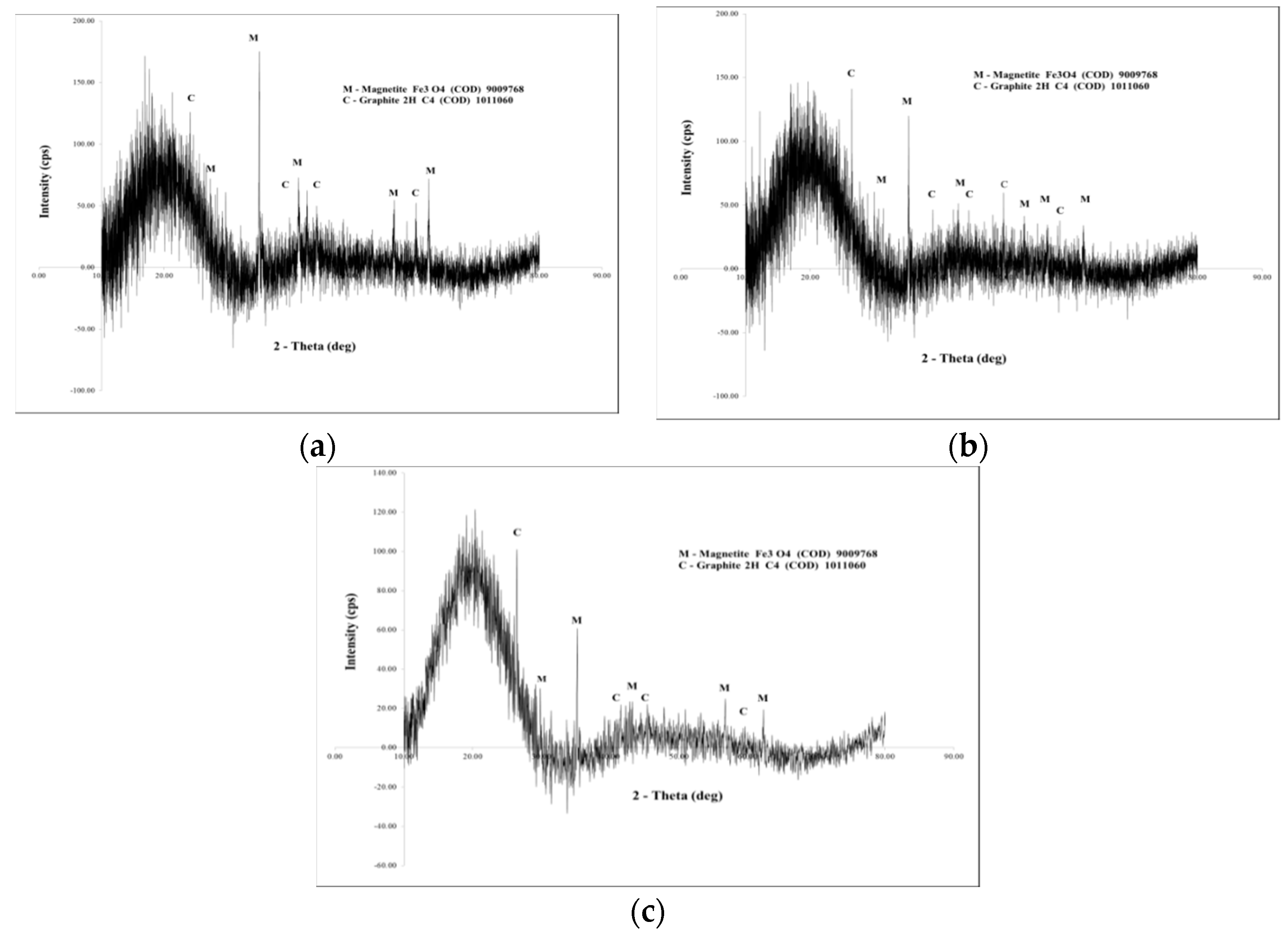
2.2.3. X-Ray Diffraction Analysis (DRX)
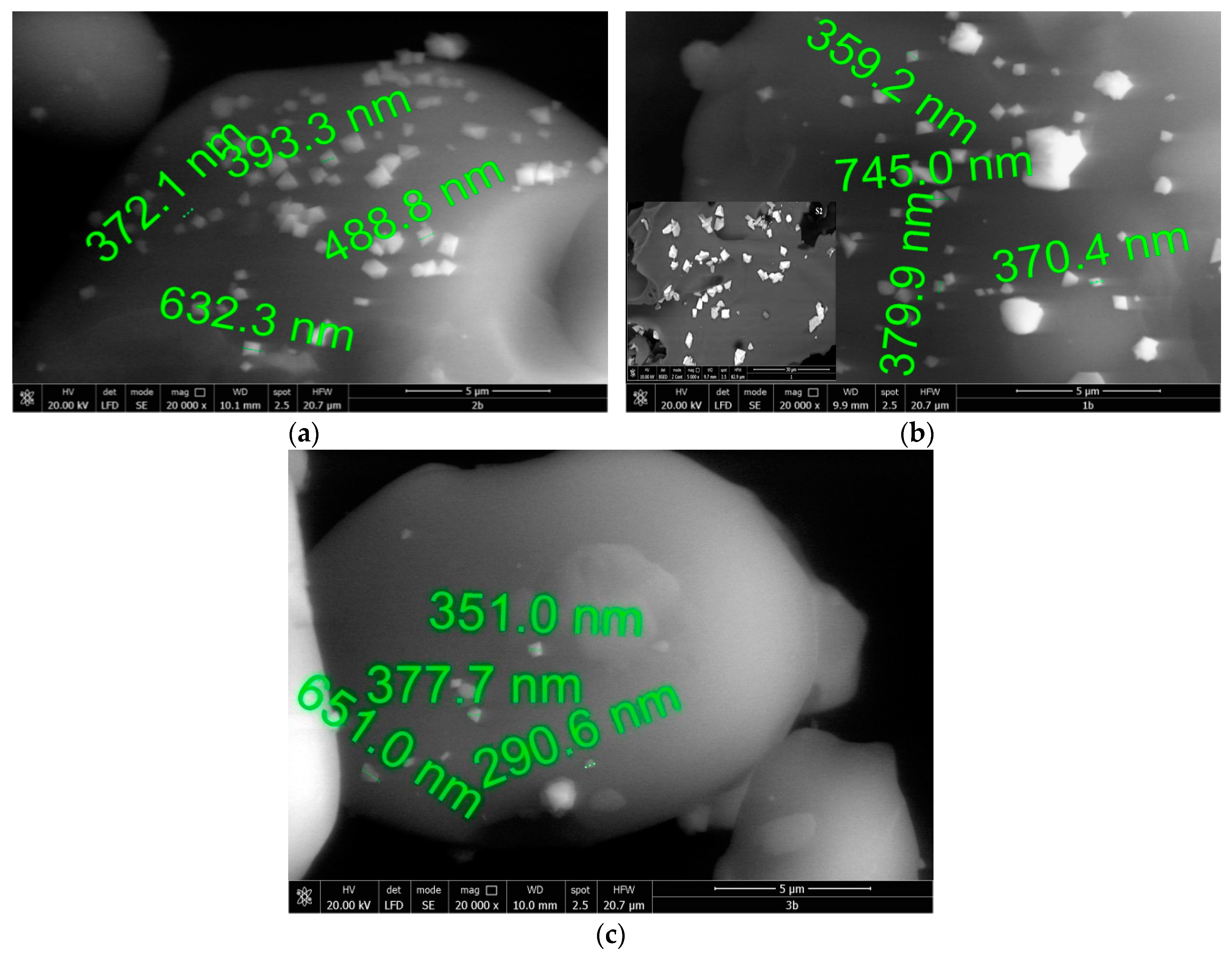
2.2.4. Scanning Electron Microscopy Analysis SEM
2.2.5. Sorption Performances of the Synthesized Material
3. Materials and Methods
3.1. Preparation and Characterization of Material
3.2. Sorption Performances of the Obtained Material
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, G.; Liu, Y.; Cai, J.L.; Chen, X.F.; Zhao, S.K.; Guo, Y.A.; Wei, S.J.; Li, X. Heterogeneous biomimetic catalysis using iron porphyrin for cyclohexane oxidation promoted by chitosan. Appl. Surf. Sci. 2017, 402, 436–443. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, Y.; Guo, S.; Chen, R. Facile template-free fabrication of iron manganese bimetal oxides nanospheres with excellent capability for heavy metals removal. J. Colloid Interface Sci. 2017, 486, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Voda, R.; Negrea, A.; Lupa, L.; Ciopec, M.; Negrea, P.; Davidescu Corneliu, M.; Butnariu, M. Nanocrystalline ferrites used as adsorbent in the treatment process of waste waters resulted from ink jet cartridges manufacturing. Open Chem. 2015, 13. [Google Scholar] [CrossRef]
- Davidescu, C.M.; Dumitru, R.; Negrea, A.; Lupa, L.; Ciopec, M.; Negrea, P. Arsenic removal through adsorption on cobalt nanoferrite. Rev. De Chim. 2015, 66, 1742–1746. [Google Scholar]
- Negrea, A.; Lupa, L.; Lazau, R.; Ciopec, M.; Pop, O.; Motoc, M. Adsorption properties of fe2o3 and fe2o3:Sio2 mixtures in the removal process of as(iii) from underground waters. Rev. De Chim. 2013, 64, 487–494. [Google Scholar]
- Ciopec, M.; Negrea, A.; Lupa, L.; Davidescu, C.M.; Negrea, P. Studies regarding as(v) adsorption from underground water by fe-xad8-dehpa impregnated resin. Equilibrium sorption and fixed-bed column tests. Molecules 2014, 19, 16082–16101. [Google Scholar] [CrossRef]
- Negrea, A.; Popa, A.; Ciopec, M.; Lupa, L.; Negrea, P.; Davidescu, C.M.; Motoc, M.; Minzatu, V. Phosphonium grafted styrene-divinylbenzene resins impregnated with iron(iii) and crown ethers for arsenic removal. Pure Appl. Chem. 2014, 86, 1729–1740. [Google Scholar] [CrossRef]
- Negrea, A.; Ciopec, M.; Negrea, P.; Lupa, L.; Popa, A.; Davidescu, C.M.; Ilia, G. Separation of as-v from aqueous solutions using chelating polymers containing fe-iii-loaded phosphorus groups. Open Chem. 2015, 13, 105–112. [Google Scholar]
- Gabor, A.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Lupa, L. Behaviour of silica and florisil as solid supports in the removal process of as(v) from aqueous solutions. J. Anal. Methods Chem. 2015, 10, 562780. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.M. Fundamentals of the theory of adsorption in micropores of carbon adsorbents: Characteristics of their adsorption properties and microporous structures. Carbon 1989, 27, 457–467. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2 ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Cao, Y.; Wang, K.; Wang, X.; Gu, Z.; Ambrico, T.; Gibbons, W.; Fan, Q.; Talukder, A.-A. Preparation of active carbons from corn stalk for butanol vapor adsorption. J. Energy Chem. 2017, 26, 35–41. [Google Scholar] [CrossRef]
- Muñiz, G.; Fierro, V.; Celzard, A.; Furdin, G.; Gonzalez-Sánchez, G.; Ballinas, M.L. Synthesis, characterization and performance in arsenic removal of iron-doped activated carbons prepared by impregnation with fe(iii) and fe(ii). J. Hazard. Mater. 2009, 165, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Rajaković, L.V. The sorption of arsenic onto activated carbon impregnated with metallic silver and copper. Sep. Sci. Technol. 1992, 27, 1423–1433. [Google Scholar] [CrossRef]
- Onganer, Y.; Temur, Ç. Adsorption dynamics of fe(iii) from aqueous solutions onto activated carbon. J. Colloid Interface Sci. 1998, 205, 241–244. [Google Scholar] [CrossRef]
- Chang, X.; Chen, D.; Jiao, X. Starch-derived carbon aerogels with high-performance for sorption of cationic dyes. Polymer 2010, 51, 3801–3807. [Google Scholar] [CrossRef]
- Khlestkin, V.K.; Peltek, S.E.; Kolchanov, N.A. Review of direct chemical and biochemical transformations of starch. Carbohydr. Polym. 2018, 181, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Budarin, V.; Clark, J.H.; Hardy, J.J.E.; Luque, R.; Milkowski, K.; Tavener, S.J.; Wilson, A.J. Starbons: New starch-derived mesoporous carbonaceous materials with tunable properties. Angew. Chem. Int. Ed. 2006, 45, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, P.S.; Budarin, V.; White, R.J.; Gun’ko, V.M.; Luque, R.; Clark, J.H. Molecular-level understanding of the carbonisation of polysaccharides. Chem.—A Eur. J. 2013, 19, 9351–9357. [Google Scholar] [CrossRef] [PubMed]
- Falco, C.; Perez Caballero, F.; Babonneau, F.; Gervais, C.; Laurent, G.; Titirici, M.-M.; Baccile, N. Hydrothermal carbon from biomass: Structural differences between hydrothermal and pyrolyzed carbons via 13c solid state nmr. Langmuir 2011, 27, 14460–14471. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, G.; Zhao, D.; He, G. Xafs study of starch-stabilized magnetite nanoparticles and surface speciation of arsenate. Environ. Pollut. 2011, 159, 3509–3514. [Google Scholar] [CrossRef]
- Liang, Q.; Zhao, D. Immobilization of arsenate in a sandy loam soil using starch-stabilized magnetite nanoparticles. J. Hazard. Mater. 2014, 271, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Janković, B. Thermal characterization and detailed kinetic analysis of cassava starch thermo-oxidative degradation. Carbohydr. Polym. 2013, 95, 621–629. [Google Scholar] [CrossRef]
- Šárka, E.; Dvořáček, V. New processing and applications of waxy starch (a review). J. Food Eng. 2017, 206, 77–87. [Google Scholar] [CrossRef]
- Hernández-Jaimes, C.; Utrilla-Coello, R.G.; Carrillo-Navas, H.; García-Márquez, E.; Meraz, M.; Bello-Pérez, L.A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Corn starch acid hydrolysis at the onset gelatinization temperature: Morphology, crystallinity, viscoelasticity, and thermal properties. Starch—Stärke 2014, 66, 636–644. [Google Scholar]
- Han, J.-A.; BeMiller, J.N. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydr. Polym. 2007, 67, 366–374. [Google Scholar] [CrossRef]
- Durães, L.; Moutinho, A.; Seabra, I.J.; Costa, B.F.O.; de Sousa, H.C.; Portugal, A. Characterization of iron(iii) oxide/hydroxide nanostructured materials produced by sol–gel technology based on the fe(no3)3·9h2o–c2h5oh–ch3chch2o system. Mater. Chem. Phys. 2011, 130, 548–560. [Google Scholar] [CrossRef]
- Yazdani, F.; Seddigh, M. Magnetite nanoparticles synthesized by co-precipitation method: The effects of various iron anions on specifications. Mater. Chem. Phys. 2016, 184, 318–323. [Google Scholar] [CrossRef]
- Bluthardt, C.; Fink, C.; Flick, K.; Hagemeyer, A.; Schlichter, M.; Volpe, A. Aqueous synthesis of high surface area metal oxides. Catal. Today 2008, 137, 132–143. [Google Scholar] [CrossRef]
- Gharibshahian, M.; Mirzaee, O.; Nourbakhsh, M.S. Evaluation of superparamagnetic and biocompatible properties of mesoporous silica coated cobalt ferrite nanoparticles synthesized via microwave modified pechini method. J. Magn. Magn. Mater. 2017, 425, 48–56. [Google Scholar] [CrossRef]
- Yang, X.; Jin, D.; Zhang, M.; Wu, P.; Jin, H.; Li, J.; Wang, X.; Ge, H.; Wang, Z.; Lou, H. Fabrication and application of magnetic starch-based activated hierarchical porous carbon spheres for the efficient removal of dyes from water. Mater. Chem. Phys. 2016, 174, 179–186. [Google Scholar] [CrossRef]
- Chang, P.R.; Yu, J.; Ma, X.; Anderson, D.P. Polysaccharides as stabilizers for the synthesis of magnetic nanoparticles. Carbohydr. Polym. 2011, 83, 640–644. [Google Scholar] [CrossRef]
- Knight, R.J.; Sylva, R.N. Precipitation in hydrolysed iron(iii) solutions. J. Inorg. Nucl. Chem. 1974, 36, 591–597. [Google Scholar] [CrossRef]
- Minzatu, V.; Negrea, A.; Davidescu, C.M.; Seiman Duda, C.; Ciopec, M.; Duţeanu, N.; Negrea, P.; Seiman, D.D.; Pascu, B.I. Arsenic adsorption into the fixed bed column from drinking groundwater. WIT Trans. Ecol. Environ. 2018. [Google Scholar] [CrossRef]
- Institute of Chemistry University of Tartu, Estonia. Atr-ft-ir Spectrum of Starch. Available online: http://lisa.chem.ut.ee/IR_spectra/paint/binders/starch/ (accessed on 25 September 2018).
- Pérez-Rodríguez, S.; Torres, D.; Lázaro, M.J. Effect of oxygen and structural properties on the electrical conductivity of powders of nanostructured carbon materials. Powder Technol. 2018, 340, 380–388. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Noble metal-free catalysts supported on carbon for CO2 electrochemical reduction. J. CO2 Util. 2017, 18, 41–52. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Gavis, J. A review of the arsenic cycle in natural waters. Water Res. 1972, 6, 1259–1274. [Google Scholar] [CrossRef]
- Roberts, L.C.; Hug, S.J.; Ruettimann, T.; Billah, M.M.; Khan, A.W.; Rahman, M.T. Arsenic removal with iron(ii) and iron(iii) in waters with high silicate and phosphate concentrations. Environ. Sci. Technol. 2004, 38, 307–315. [Google Scholar] [CrossRef]
- Saha, B.; Bains, R.; Greenwood, F. Physicochemical characterization of granular ferric hydroxide (gfh) for arsenic(v) sorption from water. Sep. Sci. Technol. 2005, 40, 2909–2932. [Google Scholar] [CrossRef]
- Wilkie, J.A.; Hering, J.G. Adsorption of arsenic onto hydrous ferric oxide: Effects of adsorbate/adsorbent ratios and co-occurring solutes. Colloids Surf. A-Physicochem. Eng. Asp. 1996, 107, 97–110. [Google Scholar] [CrossRef]
- Altundogan, H.S.; Altundogan, S.; Tumen, F.; Bildik, M. Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag. 2000, 20, 761–767. [Google Scholar] [CrossRef]
- Negrea, A.; Muntean, C.; Botnarescu, I.; Ciopec, M.; Motoc, M. Effect of matrix solution on as(v) adsorption onto iron-containing materials ph and kinetic studies. Rev. De Chim. 2013, 64, 397–406. [Google Scholar]
- Negrea, A.; Lupa, L.; Ciopec, M.; Lazau, R.; Muntean, C.; Negrea, P. Adsorption of as(iii) ions onto iron-containing waste sludge. Adsorpt. Sci. Technol. 2010, 28, 467–484. [Google Scholar] [CrossRef]
- Negrea, A.; Lupa, L.; Ciopec, M.; Muntean, C.; Lazau, R.; Motoc, M. Arsenic removal from aqueous solutions using a binary mixed oxide. Rev. De Chim. 2010, 61, 691–695. [Google Scholar]
- Amin, M.N.; Kaneco, S.; Kitagawa, T.; Begum, A.; Katsumata, H.; Suzuki, T.; Ohta, K. Removal of arsenic in aqueous solutions by adsorption onto waste rice husk. Ind. Eng. Chem. Res. 2006, 45, 8105–8110. [Google Scholar] [CrossRef]
- Ranjan, D.; Talat, M.; Hasan, S.H. Rice polish: An alternative to conventional adsorbents for treating arsenic bearing water by up-flow column method. Ind. Eng. Chem. Res. 2009, 48, 10180–10185. [Google Scholar] [CrossRef]
- Wasiuddin, N.M.; Tango, M.; Islam, M.R. A novel method for arsenic removal at low concentrations. Energy Sources 2002, 24, 1031–1041. [Google Scholar] [CrossRef]
- Maiti, A.; DasGupta, S.; Basu, J.K.; De, S. Batch and column study: Adsorption of arsenate using untreated laterite as adsorbent. Ind. Eng. Chem. Res. 2008, 47, 1620–1629. [Google Scholar] [CrossRef]
- Elizalde-Gonzalez, M.P.; Mattusch, J.; Wennrich, R.; Morgenstern, P. Uptake of arsenite and arsenate by clinoptilolite-rich tuffs. Microporous Mesoporous Mater. 2001, 46, 277–286. [Google Scholar] [CrossRef]
- Guo, H.M.; Stuben, D.; Berner, Z. Removal of arsenic from aqueous solution by natural siderite and hematite. Appl. Geochem. 2007, 22, 1039–1051. [Google Scholar] [CrossRef]
- Mohapatra, D.; Mishra, D.; Chaudhury, G.R.; Das, R.P. Arsenic(v) adsorption mechanism using kaolinite, montmorillonite and illite from aqueous medium. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 463–469. [Google Scholar] [CrossRef]
- Vitela-Rodriguez, A.V.; Rangel-Mendez, J.R. Arsenic removal by modified activated carbons with iron hydro(oxide) nanoparticles. J. Environ. Manag. 2013, 114, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Malana, M.A.; Qureshi, R.B.; Ashiq, M.N. Adsorption studies of arsenic on nano aluminium doped manganese copper ferrite polymer (ma, va, aa) composite: Kinetics and mechanism. Chem. Eng. J. 2011, 172, 721–727. [Google Scholar] [CrossRef]
- Thirunavukkarasu, O.S.; Viraraghavan, T.; Subramanian, K.S. Arsenic removal from drinking water using iron oxide-coated sand. Water Air Soil Pollut. 2003, 142, 95–111. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Westerhoff, P.; Knappe, D.R.U. Intraparticle diffusion and adsorption of arsenate onto granular ferric hydroxide (gfh). Water Res. 2004, 38, 4002–4012. [Google Scholar] [CrossRef]
- Hódi, M.; Polyák, K.; Hlavay, J. Removal of pollutants from drinking water by combined ion exchange and adsorption methods. Environ. Int. 1995, 21, 325–331. [Google Scholar] [CrossRef]
- Gu, Z.; Fang, J.; Deng, B. Preparation and evaluation of gac-based iron-containing adsorbents for arsenic removal. Environ. Sci. Technol. 2005, 39, 3833–3843. [Google Scholar] [CrossRef] [PubMed]
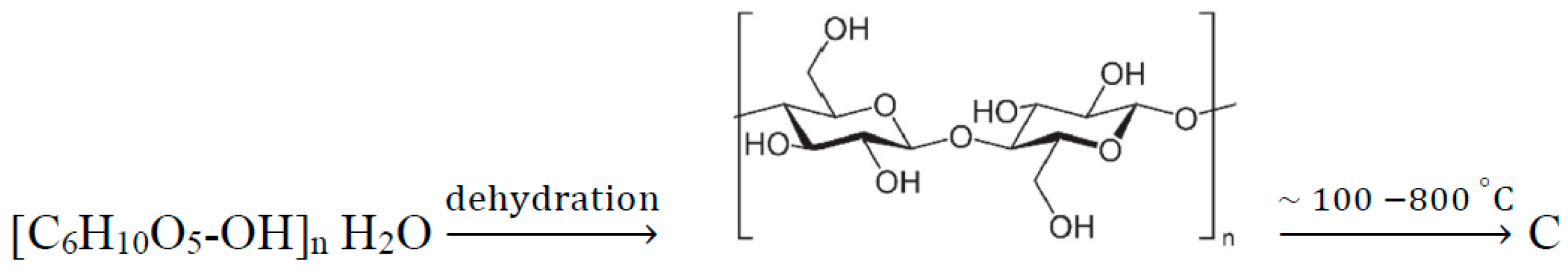
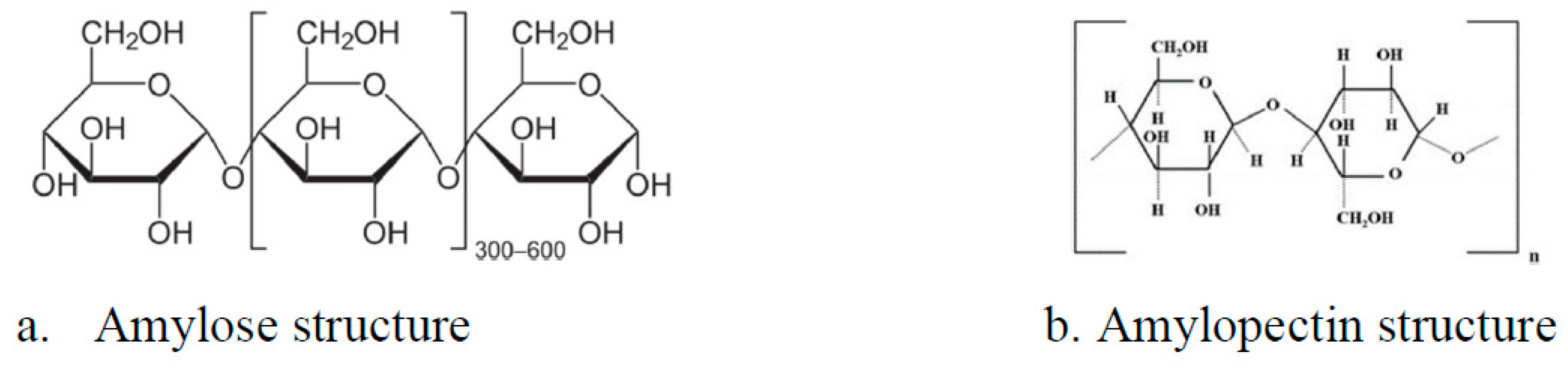
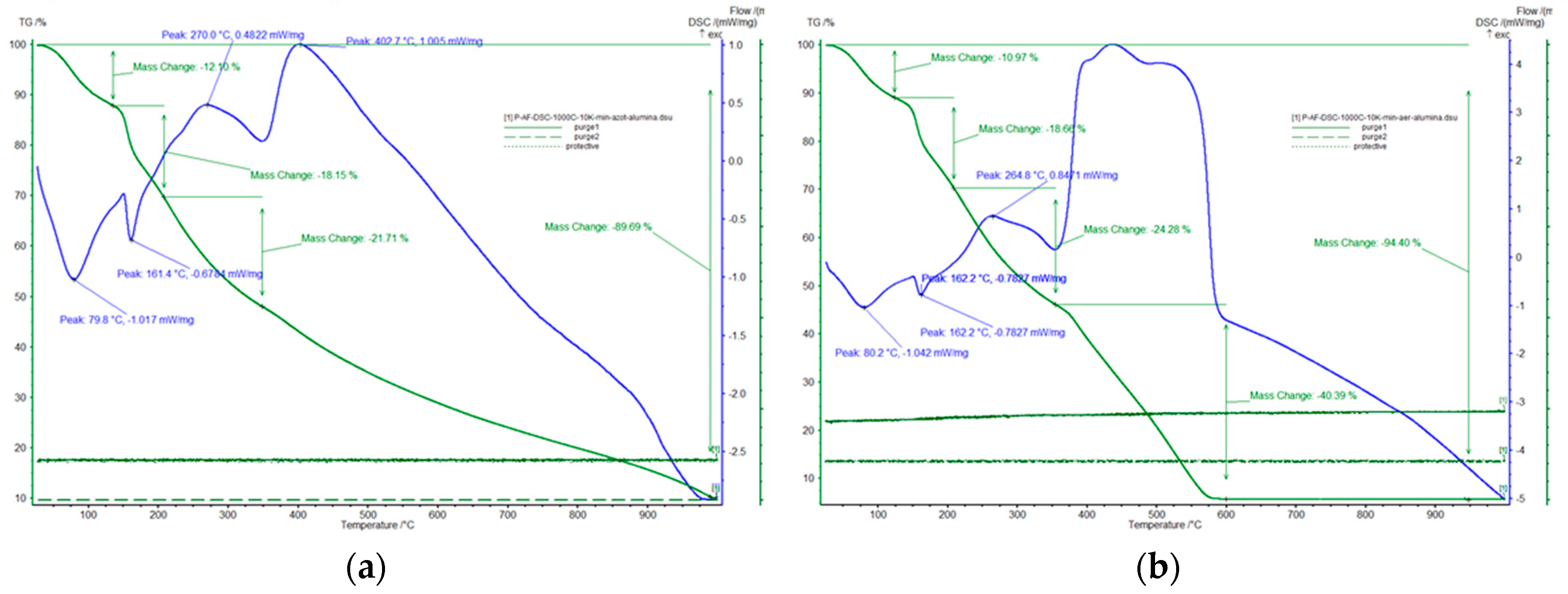
| Sample Name | Stirring Time [min] | Synthesis Temperature [°C] | Thermal Decomposition Temperature [°C] |
|---|---|---|---|
| S 1 | 45 | 55 | 600 |
| S 2 | 240 | 45 | 600 |
| S 3 | 20 | 58 | 600 |
| Materials | Adsorbtion Capacity, q (µgAs(V)/g) | References |
|---|---|---|
| S1 | 261 | Present paper |
| S2 | 160 | Present paper |
| S3 | 26 | Present paper |
| Waste rice husk | 7 | [46] |
| Rice polish | 79 | [47] |
| Human hair | 12 | [48] |
| Natural laterite | 147 | [49] |
| Clinoptilolite–rich tuff | 50 | [50] |
| Hematite | 202 | [51] |
| Montmorillonite | 64 | [52] |
| Illite | 52 | [52] |
| Activated carbons modified with iron hydro (oxide) nanoparticles | 25 | [53] |
| Nano-aluminum doped manganese copper ferrite polymer composite | 50 | [54] |
| Iron oxide coated sand | 43 | [55] |
| Granular ferric hydroxide (GFH) | 4 | [56] |
| Al2O3/Fe(OH)3 | 90 | [57] |
| Untreated GAC (granular activated carbon) | 38 | [58] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mînzatu, V.; Davidescu, C.-M.; Negrea, P.; Ciopec, M.; Muntean, C.; Hulka, I.; Paul, C.; Negrea, A.; Duțeanu, N. Synthesis, Characterization and Adsorptive Performances of a Composite Material Based on Carbon and Iron Oxide Particles. Int. J. Mol. Sci. 2019, 20, 1609. https://doi.org/10.3390/ijms20071609
Mînzatu V, Davidescu C-M, Negrea P, Ciopec M, Muntean C, Hulka I, Paul C, Negrea A, Duțeanu N. Synthesis, Characterization and Adsorptive Performances of a Composite Material Based on Carbon and Iron Oxide Particles. International Journal of Molecular Sciences. 2019; 20(7):1609. https://doi.org/10.3390/ijms20071609
Chicago/Turabian StyleMînzatu, Vasile, Corneliu-Mircea Davidescu, Petru Negrea, Mihaela Ciopec, Cornelia Muntean, Iosif Hulka, Cristina Paul, Adina Negrea, and Narcis Duțeanu. 2019. "Synthesis, Characterization and Adsorptive Performances of a Composite Material Based on Carbon and Iron Oxide Particles" International Journal of Molecular Sciences 20, no. 7: 1609. https://doi.org/10.3390/ijms20071609
APA StyleMînzatu, V., Davidescu, C.-M., Negrea, P., Ciopec, M., Muntean, C., Hulka, I., Paul, C., Negrea, A., & Duțeanu, N. (2019). Synthesis, Characterization and Adsorptive Performances of a Composite Material Based on Carbon and Iron Oxide Particles. International Journal of Molecular Sciences, 20(7), 1609. https://doi.org/10.3390/ijms20071609