Prevention of Deficit in Neuropsychiatric Disorders through Monitoring of Arsenic and Its Derivatives as Well as Through Bioinformatics and Cheminformatics
Abstract
1. Arsenic Exposure Induces the Neuropsychiatric Disorders—General Aspects
2. Possible Molecular Mechanisms of Arsenic-Induced Disturbance of Cognitive Human Functions
3. Perturbance of Neuropsychiatric Treatments during Arsenic Exposure
4. Bioinformatics and Cheminformatics Tools Applied to Study Arsenic Toxicity in the Brain
- (i)
- For various chemical structures the critical molecular features for structure toxicity is different;
- (ii)
- The chemical structure constructed based on the electrophilicity index (ω) and log P (R2adj = 0.965 for acceptors, 0.888 for donors) is better than the model based on eLUMO and log P (R2 = 0.963 for acceptors, 0.842 for donors); and
- (iii)
- The QSAR model can improve molecules’ toxicity predictability, and can be developed by taking into account the electrophilic property in addition to molecule hydrophobicity.
- >3BWM:A|PDBID|CHAIN|SEQUENCE
- GDTKEQRILNHVLQHAEPGNAQSVLEAIDTYCEQKEWAMNVGDKKGKIVDAVIQEHQPSVLLELGAYCGYSAVRMARLLSPGARLITIEINPDCAAITQRMVDFAGVKDKVTLVVGASQDIIPQLKKKYDVDTLDMVFLDHWKDRYLPDTLLLEECGLLRKGTVLLADNVICPGAPDFLAHVRGSSCFECTHYQSFLEYREVVDGLEKAIYKGP
- >4FS8:A|PDBID|CHAIN|SEQUENCE
- MPCSCASGCQKSKNGGSTPSIRDHVADYYGKTLQSSADLKTSACKLAAAVPESHRKILADIADEVLEKFYGCGSTLPADGSLEGATVLDLGCGTGRDVYLASKLVGEHGKVIGVDMLDNQLEVARKYVEYHAEKFFGSPSRSNVRFLKGFIENLATAEPEGVPDSSVDIVISNCVCNLSTNKLALFKEIHRVLRDGGELYFSDVYADRRLSEAAQQDPILYGECLGGALYLEDFRRLVAEAGFRDVRLVSVGPVDVSDPQLRKLVPDVQFYSCTFRCFKVATLEATREDYGQSATYLGGIGEEFKLDRFFTFPREKPVRVDRNTAEIIRHSRLHQWFSVSAEQQHMGLFKANDSYALLHAPLSMQVEQLVSGAAALEHHHHHH
5. Arsenic Reduced Toxicity and Arsenic Nanoparticles Used as Delivery System into the Human Body
6. Conclusions and Perspective
Conflicts of Interest
References
- Andrade, V.M.; Batoréu, M.; Aschner, M.; Marreilha dos Santos, A. Lead, arsenic and manganese metal mixture exposures: Focus on biomarkers of effect. Biol. Trace Element Res. 2015, 166, 13–23. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Available online: https://www.epa.gov/ (accessed on 21 November 2018).
- Agency for Toxic Substances and Disease Registry (ATSDR). Available online: https://www.atsdr.cdc.gov/ (accessed on 18 November 2018).
- Siddiqui, S.I.; Chaudhry, S.A. Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement. Process Saf. Environ. Prot. 2017, 111, 592–626. [Google Scholar] [CrossRef]
- Kao, A.C.; Chu, Y.J.; Hsu, F.L.; Liao, V.H.C. Removal of arsenic from groundwater by using a native isolated arsenite-oxidizing bacterium. J. Contam. Hydrol. 2013, 155, 1–8. [Google Scholar] [CrossRef]
- Bowell, R.J.; Alpers, C.N.; Jamieson, H.E.; Nordstrom, D.K.; Majzlan, J. The Environmental Geochemistry of Arsenic-An Overvi. Rev. Mineral. Geochem. 2014, 79, 1–16. [Google Scholar]
- McIntyre, D.O.; Linton, T.K. Arsenic. Fish Physiol. 2011, 31, 297–349. [Google Scholar]
- Smedley, P.L. Arsenic in rural groundwater in Ghana. J. Afr. Earth Sci. 1996, 22, 459–470. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.W.J.; Hou, D.; Luan, Z.; Fan, B.; Zhao, C. Experimental study of arsenic removal by direct contact membrane distillation. J. Hazard. Mater. 2009, 163, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Gupta, A.K. As(III) removal from aqueous medium in fixed bed using iron oxide-coated cement (IOCC): Experimental and modeling studies. Chem. Eng. J. 2007, 129, 123–131. [Google Scholar] [CrossRef]
- Avram, S.; Maria, M.; Mihailescu, D.; Duda-Seiman, D.; Duda-Seiman, C. Advanced QSAR Methods Evaluated Polycyclic Aromatic Compounds Duality as Drugs and Inductors in Psychiatric Disorders. Curr. Org. Chem. 2013, 17, 2880–2890. [Google Scholar] [CrossRef]
- Perera, F.P.; Wheelock, K.; Wang, Y.; Tang, D.; Margolis, A.E.; Badia, G.; Cowell, W.; Miller, R.L.; Rauh, V.; Wang, S.; et al. Combined effects of prenatal exposure to polycyclic aromatic hydrocarbons and material hardship on child ADHD behavior problems. Environ. Res. 2018, 160, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Fluegge, K.; Fluegge, K. Environmental factors influencing the link between childhood ADHD and risk of adult coronary artery disease. Med. Hypotheses 2018, 110, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, A.J.; Volk, H.; Tancredi, D.J.; McConnell, R.; Lurmann, F.W.; Hansen, R.L.; Schmidt, R.J. Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res. 2018, 11, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Attademo, L.; Bernardini, F.; Garinella, R.; Compton, M.T. Environmental pollution and risk of psychotic disorders: A review of the science to date. Schizophr. Res. 2017, 181, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Hudecova, A.M.; Hansen, K.; Mandal, S.; Berntsen, H.F.; Khezri, A.; Bale, T.L.; Fraser, T.W.K.; Zimmer, K.E.; Ropstad, E. A human exposure based mixture of persistent organic pollutants affects the stress response in female mice and their offspring. Chemosphere 2018, 197, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.B.; Silva, C.; Macário, I.P.E.; Oliveira, B.; Gonçalves, F.; Pereira, J.L. Feeding inhibition in Corbicula fluminea (O.F. Muller, 1774) as an effect criterion to pollutant exposure: Perspectives for ecotoxicity screening and refinement of chemical control. Aquat. Toxicol. 2018, 196, 25–27. [Google Scholar] [CrossRef] [PubMed]
- De Vizcaya-Ruiza, A.; Barbier, O.; Ruiz-Ramos, R.; Cebrian, M.E. Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mut. Res. 2009, 674, 85–92. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Guo, H.-R.; Tsai, W.-C.; Yang, K.-L.; Lin, L.-C.; Cheng, T.-J.; Chuu, J.-J. Subchronic Arsenic Exposure Induces Anxiety-Like Behaviors in Normal Mice and Enhances Depression-Like Behaviors in the Chemically InducedChemically-induced Mouse Model of Depression. BioMed Res. Int. 2015, 2015, 159015. [Google Scholar] [CrossRef]
- Duda-Seiman, D.M.; Avram, S.; Mancas, S.; Careja, V.; Duda-Seiman, C.; Putz, M.V.; Ciubotariu, D. MTD-CoMSIA modelling of HMG-CoA reductase inhibitors. J. Serbian Chem. Soc. 2011, 76, 85–99. [Google Scholar] [CrossRef]
- Hill, D.S.; Cabrera, R.; Wallis Schultz, D.; Zhu, H.; Lu, W.; Finnell, R.H.; Wlodarczyk, B.J. Autism-Like Behavior and Epigenetic Changes Associated with Autism as Consequences of In Utero Exposure to Environmental Pollutants in a Mouse Model. Behav. Neurol. 2015, 2015, 426263. [Google Scholar] [CrossRef]
- Dickerson, A.S.; Mohammad, H.; Rahbar, I.; Han, A.V.; Bakian, D.A.; Bilder, R.A.; Harrington, S.; Pettygrove, M.; Durkin, R.S.; Kirby, M.S.; et al. Autism spectrum disorder prevalence and proximity to industrial facilities releasing arsenic, lead or mercury. Sci. Total Environ. 2015, 536, 245–251. [Google Scholar] [CrossRef]
- Rodríguez-Barranco, M.G.F.; Hernández, A.F.; Alguacil, J.; Lorca, A.; Mendoza, R.; Gómez, I.; Molina-Villalba, I.; González-Alzaga, B.; Aguilar-Garduño, C.; Rohlman, D.S.; et al. Postnatal arsenic exposure and attention impairment in school children. Cortex 2016, 74, 370–382. [Google Scholar] [CrossRef]
- Bode, M.M.D.D.; Mettelman, B.B.; Gross, S.J. Predictive validity of the Bayley, Third Edition at 2 years for intelligence quotient at 4 years in preterm infants. J. Dev. Behav. Pediatr. 2014, 35, 570–575. [Google Scholar] [CrossRef]
- Von Stackelberg, K.G.E.; Chu, T.; Henn, B.C. Exposure to Mixtures of Metals and Neurodevelopmental Outcomes: A Review. Risk Anal. 2015, 35, 971–1016. [Google Scholar] [CrossRef]
- Dassault Systemes—Biovia. Available online: http://www.3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/ (accessed on 1 November 2018).
- Chou, C.T.L.W.; Kong, Z.L.; Chen, S.Y.; Hwang, D.F. Taurine prevented cell cycle arrest and restored neurotrophic gene expression in arsenite-treated SH-SY5Y cells. Amino Acids 2013, 45, 811–819. [Google Scholar] [CrossRef]
- Ademuyiwa, O.U.R.; Rotimi, S.O.; Abama, E.; Okediran, B.S.; Dosumu, O.A.; Onunkwor, B.O. Erythrocyte acetylcholinesterase activity as a surrogate indicator of lead-induced neurotoxicity in occupational lead exposure in Abeokuta, Nigeria. Environ. Toxicol. Pharmacol. 2007, 24, 183–188. [Google Scholar] [CrossRef]
- Rosemberg, D.B.; da Rocha, R.F.; Rico, E.P.; Zanotto-Filho, A.; Dias, R.D.; Bogo, M.R.; Bonan, C.D.; Moreira, J.C.F.; Klamt, F.; Souza, D.O. Taurine prevents enhancement of acetylcholinesterase activity induced by acute ethanol exposure and decreases the level of markers of oxidative stress in zebrafish brain. Neuroscience 2010, 171, 683–692. [Google Scholar] [CrossRef]
- Santos, D.M.D.; Andrade, V.; Batoréu, M.C.; Aschner, M.; Marreilha dos Santos, A.P. The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology 2012, 292, 90–98. [Google Scholar] [CrossRef]
- Kumar, M.R.R.G. Influence of age on arsenic-induced behavioral and cholinergic perturbations: Amelioration with zinc and α-tocopherol. Hum. Exp. Toxicol. 2018, 37, 295–308. [Google Scholar] [CrossRef]
- Kumar, N.K.K.; Singh, N.P. Oxidative and cellular stress as bioindicators for metal contamination in freshwater mollusk Lamellidens marginalis. Environ. Sci. Pollut. Res. Int. 2017, 24, 16137–16147. [Google Scholar] [CrossRef]
- Srivastava, P.D.Y.; Gupta, R.; Shukla, R.K.; Yadav, R.S.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K. Protective Effect of Curcumin by Modulating BDNF/DARPP32/CREB in Arsenic-Induced Alterations in Dopaminergic Signaling in Rat Corpus Striatum. Mol. Neurobiol. 2018, 55, 445–461. [Google Scholar] [CrossRef]
- Kordas, K.A.G.; Coffman, D.L.; Queirolo, E.I.; Ciccariello, D.; Mañay, N.; Ettinger, A.S. Patterns of exposure to multiple metals and associations with neurodevelopment of preschool children from Montevideo, Uruguay. J. Environ. Public Health. 2015, 2015, 493471. [Google Scholar] [CrossRef]
- Rosado, J.L.R.D.; Kordas, K.; Rojas, O.; Alatorre, J.; Lopez, P.; Garcia-Vargas, G.; Del Carmen Caamaño, M.; Cebrián, M.E.; Stoltzfus, R.J. Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ. Health Perspect. 2007, 115, 1371–1375. [Google Scholar] [CrossRef]
- Wasserman, X.L.; Faruque, P.; Habibul, A.; Pam, F.-L.; Alexander, V.G.; Vesna, S.; Nancy, J.L.; Cheng, Z.; Iftikhar, H.; Hassina, M.; et al. Water Arsenic Exposure and Children’s Intellectual Function in Araihazar, Bangladesh. Environ. Health Perspect. 2004, 112, 1329–1333. [Google Scholar] [CrossRef]
- Xi, S.S.W.; Wang, F.; Jin, Y.; Sun, G. Transplacental and early life exposure to inorganic arsenic affected development and behavior in offspring rats. Arch. Toxicol. 2009, 83, 549–556. [Google Scholar] [CrossRef]
- Jing, G.Z.; Liu, M.; Shen, X.; Zhao, F.; Wang, J.; Zhang, J.; Huang, G.; Dai, P.; Chen, Y.; Chen, J.; et al. Changes in the synaptic structure of hippocampal neurons and impairment of spatial memory in a rat model caused by chronic arsenite exposure. NeuroToxicology 2012, 33, 1230–1238. [Google Scholar] [CrossRef]
- Jiang, S.J.; Yao, S.; Zhang, Y.; Cao, F.; Wang, F.; Li, Y.; Xi, S. Fluoride and Arsenic Exposure Impairs Learning and Memory and Decreases mGluR5 Expression in the Hippocampus and Cortex in Rats. PLoS ONE 2014, 9, e96041. [Google Scholar] [CrossRef]
- Luo, J.-H.; Qiu, Z.-Q.; Shu, W.-Q.; Zhang, Y.-Y.; Zhang, L.; Chen, J.-A. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol. Lett. 2009, 184, 121–125. [Google Scholar] [CrossRef]
- Luo, J.-H.; Qiu, Z.-Q.; Shu, W.-Q. Arsenite exposure altered the expression of NMDA receptor and postsynaptic signaling proteins in rat hippocampus. Toxicol. Lett. 2012, 2111, 39–44. [Google Scholar] [CrossRef]
- Huo, T.G.L.W.; Zhang, Y.H.; Yuan, J.; Gao, L.Y.; Yuan, Y.; Yang, H.L.; Jiang, H.; Sun, G.F. Excitotoxicity Induced by Realgar in the Rat Hippocampus: The Involvement of Learning Memory Injury, Dysfunction of Glutamate Metabolism and NMDA Receptors. Mol. Neurobiol. 2015, 51, 980–994. [Google Scholar] [CrossRef]
- Sun, B.-F.; Yu, Z.-J.; Yan, Y.; Xiao, C.-L.; Kang, C.-S.; Guo, G.; Yan, L.; Zhu, J.-D.; Li, Y.-M.; Li, Q.-M.; et al. Exercise Prevents Memory Impairment Induced by Arsenic Exposure in Mice: Implication of Hippocampal BDNF and CREB. PLoS ONE 2015, 10, e0137810. [Google Scholar] [CrossRef]
- Ramos-Chávez, L.A.R.-L.C.; Zepeda, A.; Silva-Adaya, D.; Del Razo, L.M.; Gonsebatt, M.E. Neurological effects of inorganic arsenic exposure: Altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front. Cell. Neurosci. 2015, 9, 21. [Google Scholar] [CrossRef]
- Aung, K.T.S.; Maekawa, F.; Nohara, K.; Nakamura, K.; Tanoue, A. Role of Environmental Chemical Insult in Neuronal Cell Death and Cytoskeleton Damage. Biol. Pharm. Bull. 2015, 38, 1109–1112. [Google Scholar] [CrossRef]
- Wang, C.B.; Huai, G.; Ruolin, C.; Xiaoxu, W.; Bingwen, W.; Hetian, J.; Fengyuan, P. Subchronic exposure to arsenic induces apoptosis in the hippocampus of the mouse brains through the Bcl-2/Bax pathway. J. Occup. Health 2015, 57, 212–221. [Google Scholar] [CrossRef]
- Caldwell, K.E.L.M.; Solomon, B.R.; Ali, A.; Allan, A.M. Prenatal arsenic exposure alters the programming of the glucocorticoid signaling system during embryonic development. Neurotoxicol. Teratol. 2015, 47, 66–79. [Google Scholar] [CrossRef]
- Ronchetti, S.N.G.; Bianchi, M.; Crocco, M.; Duvilanski, B.; Cabilla, J. In vivoxenoestrogenic actions of cadmium and arsenic in anterior pituitary and uterus. Reproduction 2016, 152, 1–10. [Google Scholar] [CrossRef]
- Wu, H.E.A.-G.N.; Gharbaoui, Y.; Teixeira, A.L.; Pigott, T.A. An Unusual Case of Acute Psychosis with Obsessive-Compulsive Features Following Arsenic Poisoning. J. Psychiatr. Pract. 2017, 23, 382–385. [Google Scholar] [CrossRef]
- Christina, R.; Tyler, B.R.S.; Adam, L.U.; Andrea, M.A. Fluoxetine treatment ameliorates depression induced by perinatal arsenic exposure via a neurogenic mechanism. Neurotoxicology 2014, 44, 98–109. [Google Scholar]
- Avram, S.; Borcan, F.; Borcan, L.C.; Milac, A.L.; Mihailescu, D. QSAR Approaches Applied to Antidepressants Induced Neurogenesis—In vivo and in silico Applications. Mini Rev. Med. Chem. 2015, 16, 230–240. [Google Scholar] [CrossRef]
- Andrade, C.H.; Pasqualoto, K.F.; Ferreira, E.I.; Hopfinger, A.J. 4D-QSAR: Perspectives in drug design. Molecules 2010, 15, 3281–3294. [Google Scholar] [CrossRef]
- Avram, S.; Maria, M.; Bagci, E.; Hritcu, L.; Borcan, L.C.; Mihailescu, D. Advanced structure-activity relationships applied to Mentha spicata L. subsp. spicata essential oil compounds as AChE and NMDA ligands, in comparison with donepezil, galantamine and memantine—New approach in brain disorders pharmacology. CNS Neurol. Disord. Drug Targets 2017, 16, 800–811. [Google Scholar] [CrossRef]
- Damale, M.G.; Harke, S.N.; Kalam Khan, F.A.; Shinde, D.B.; Sangshetti, J.N. Recent advances in multidimensional QSAR (4D–6D): A critical review. Mini Rev. Med. Chem. 2014, 14, 35–55. [Google Scholar] [CrossRef]
- Cruz-Monteagudo, M.S.S.; Tejera, E.; Pérez-Castillo, Y.; Medina-Franco, J.L.; Sánchez-Rodríguez, A.; Borges, F. Systemic QSAR and phenotypic virtual screening: Chasing butterflies in drug discovery. Drug Discov. Today 2017, 22, 994–1007. [Google Scholar] [CrossRef]
- Gramatica, P.E.; Sangion, A. QSAR modeling of cumulative environmental end-points for the prioritization of hazardous chemicals. Environ. Sci. Process. Impacts 2018, 20, 38–41. [Google Scholar] [CrossRef]
- Simões, R.S.M.V.; Oliveira, P.R.; Honorio, K.M. Transfer and Multi-task Learning in QSAR Modeling: Advances and Challenges. Front. Pharmacol. 2018, 6, 9–74. [Google Scholar] [CrossRef]
- Avram, S.; Buiu, C.; Duda-Seiman, D.; Duda-Seiman, C.; Borcan, F.; Mihailescu, D. Evaluation of the Pharmacological Descriptors Related to the Induction of Antidepressant Activity and its Prediction by QSAR/QRAR Methods. Mini Rev. Med. Chem. 2012, 12, 467–476. [Google Scholar] [CrossRef]
- Avram, S.; Buiu, C.; Duda-Seiman, D.M.; Duda-Seiman, C.; Mihailescu, D. 3D-QSAR Design of New Escitalopram Derivatives for the Treatment of Major Depressive Disorders. Sci. Pharm. 2010, 78, 233. [Google Scholar] [CrossRef]
- Avram, S.; Duda-Seiman, D.M.; Duda-Seiman, C.; Borcan, F.; Mihailescu, D. Predicted binding rate of new cephalosporin antibiotics by a 3D-QSAR method: A new approach. Monatshefte für Chemie Chem. Mon. 2010, 141, 589–597. [Google Scholar] [CrossRef]
- De Benedetti, P.G.; Fanelli, F. Multiscale quantum chemical approaches to QSAR modeling and drug design. Drug Discovery Today 2014, 19, 1921–1927. [Google Scholar] [CrossRef]
- MOE (The Molecular Operating Environment); Chemical Computing Group Inc.: Montreal, QC, Canada, 2012.
- National Library of Medicine. Available online: https://sis.nlm.nih.gov/pdf/toxnetbrochure.pdf (accessed on 10 November 2018).
- Maciejewski, A.A.C.; Guo, A.; Marcu, C.; Li, D.; Johnson, E.J.; Lo, J.R.; Grant, N.; Assempour, Y.D.; Feunang, Z.; Sayeeda, D.S.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2017, 46, D1074–D1082. [Google Scholar]
- Cheng, F.W.; Li, Y.; Zhou, S.; Jie, Z.; Wu, G.; Liu, L.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Molecular Discovery. VolSurf. Available online: http://www.moldiscovery.com/software/vsplus/ (accessed on 10 November 2018).
- Law, V.C.; Knox, Y.; Djoumbou, T.; Jewison, A.C.; Guo, Y.; Liu, A.; Maciejewski, D.; Arndt, M.; Wilson, V.; Neveu, A.; et al. DrugBank 4.0: Shedding new light on drug metabolism. Nucleic Acids Res. 2014, 42, D1091–D1097. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Berman, H.M.W.J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Parker, L.J.; Parker, M.W.; Morton, C.J.; Bocedi, A.; Ascher, D.B.; Aitken, J.B.; Harris, H.H.; Lo Bello, M.; Ricci, G. Visualisation of Organoarsenic Human Glutathione Transferase P1-1 Complexes: Metabolism of Arsenic-based Therapeutics. Available online: https://www.rcsb.org/structure/5dak (accessed on 25 September 2018).
- Canaval, L.R.; Lutz, O.M.; Weiss, A.K.; Huck, C.W.; Hofer, T.S. A Dissociative Quantum Mechanical/Molecular Mechanical Molecular Dynamics Simulation and Infrared Experiments Reveal Characteristics of the Strongly Hydrolytic Arsenic(III). Inorg. Chem. 2014, 53, 11861–11870. [Google Scholar] [CrossRef]
- Roy, D.R.G.S.; Chattaraj, P.K. Arsenic toxicity: An atom counting and electrophilicity-based protocol. Mol. Divers. 2009, 13, 551–556. [Google Scholar] [CrossRef]
- Zhang, Z.L.H.; Zhou, H.; Zhu, X.; Zhao, Z.; Chi, X.; Shan, H.; Gao, J. A facile route to core-shell nanoparticulate formation of arsenic trioxide for effective solid tumor treatment. Nanoscale 2016, 8, 4373–4380. [Google Scholar] [CrossRef]
- Teoh, W.K.S.F.; Shahir, S. Characterization of Thiomonas delicata arsenite oxidase expressed in Escherichia coli. 3 Biotech 2017, 7, 97. [Google Scholar] [CrossRef][Green Version]
- Tsai, H.H.L.J.; Huang, J.M.; Juwita, R. A molecular dynamics study of the structural and dynamical properties of putative arsenic substituted lipid bilayers. Int. J. Mol. Sci. 2013, 9, 7702–7715. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Kale, L.; Skeel, R.; Bhandarkar, M.; Brunner, R.; Gursoy, A.; Krawetz, N.; Phillips, J.; Shinozaki, A.; Varadarajan, K.; Schulten, K. NAMD2: Greater scalability for parallel molecular dynamics. J. Comp. Phys. 1999, 151, 283–312. [Google Scholar] [CrossRef]
- Altaf Hussain Pandith, S.G.; Chattaraj, K. A Comparative Study of Two Quantum Chemical Descriptors in Predicting Toxicity of Aliphatic Compounds towards Tetrahymena pyriformis. Organ. Chem. Int. 2010, 2010, 545087. [Google Scholar] [CrossRef]
- Dong, H.M.M.; Nefzi, A.; Houghten, R.A.; Giulianotti, M.A.; Rosen, B.P. Identification of Small Molecule Inhibitors of Human As(III) S-Adenosylmethionine Methyltransferase (AS3MT). Chem. Res. Toxicol. 2015, 28, 2419–2425. [Google Scholar] [CrossRef]
- Chen, S.C.S.G.; Rosen, B.P.; Zhang, S.Y.; Deng, Y.; Zhu, B.K.; Rensing, C.; Zhu, Y.G. Recurrent horizontal transfer of arsenite methyltransferase genes facilitated adaptation of life to arsenic. Sci. Rep. 2017, 7, 7741. [Google Scholar] [CrossRef]
- Bürli, R.W.H.; Wei, G.; Ernst, A.; Mariga, I.M.; Hardern, K.; Herlihy, A.J.; Cross, S.S.; Wesolowski, H.; Chen, R.D.G.; McKay, D.R.; et al. Novel inhibitors of As(III) S-adenosylmethionine methyltransferase (AS3MT) identified by virtual screening. Bioorg. Med. Chem. Lett. 2018, 28, 3231–3235. [Google Scholar] [CrossRef]
- Dheeman, D.S.; Packianathan, C.; Pillai, J.K.; Rosen, B.P. Pathway of human AS3MT arsenic methylation. Chem. Res. Toxicol. 2014, 27, 1979–1989. [Google Scholar] [CrossRef]
- Ellison, P.A.B.T.; Chen, F.; Hong, H.; Zhang, Y.; Theuer, C.P.; Cai, W.; Nickles, R.J.; DeJesus, O.T. High Yield Production and Radiochemical Isolation of Isotopically Pure Arsenic-72 and Novel Radioarsenic Labeling Strategies for the Development of Theranostic Radiopharmaceuticals. Bioconjug. Chem. 2016, 27, 179–188. [Google Scholar] [CrossRef]
- Benjamin, M.M.S.R.S.; Bailey, R.P.; Bennett, T. Sorption and filtration of metals using iron-oxide-coated sand. Water Resour. 1996, 30, 2609–2620. [Google Scholar] [CrossRef]
- Dambies, L.V.T.; Guibal, E. Treatment of arsenic-containing solutions using chitosan derivatives: Uptake mechanism and sorption performance. Water Resour. 2002, 36, 3699–3710. [Google Scholar] [CrossRef]
- Perrich, J.R. Activated Carbon Adsorption for Wastewater Treatment; CRC Press, Inc.: Boca Raton, FL, USA, 1981. [Google Scholar]
- Radovic, L.R. Chemistry and Physics of Carbon; Marcel Dekker, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Mohan, D.; Singh, K.P. Granular Activated Carbon. In Water Encyclopedia: Domestc, Municipal, and Industrial Water Supply andWaste Disposal; Lehr, J., Keeley, J., Lehr, J., Eds.; Wiley–Interscience: New York, NY, USA, 2005. [Google Scholar]
- Zhimang, G.F.J.; Deng, B. Preparation and evaluation of GAC-based iron containing adsorbent for arsenic removal. Environ. Sci. Technol. 2005, 39, 3833–3843. [Google Scholar]
- Cortina, J.L.; Warshawsky, A. Solvent Extraction Ion Exchange; Marinsky, J.A., Marcus, Y., Eds.; Marcel Dekker: New York, NY, USA, 1997; Volume 13. [Google Scholar]
- Saha, B.G.R.J.; Bailey, D.G.; Kabay, N.; Arda, M. Sorption of Cr(VI) from aqueous solution by Amberlite XAD-7 resin impregnated with Aliquat 336. React. Funct. Polym. 2004, 60, 222–244. [Google Scholar] [CrossRef]
- Mendoza, R.N.M.I.S.; Vera, A.; Rodriguez, M.A. Study of the sorption of Cr(III) with XAD-2 resin impregnated with di-(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272). Solvent Extr. Ion Exch. 2000, 18, 319–343. [Google Scholar] [CrossRef]
- Muraviev, D.G.L.; Valiente, M. Stabilization of solvent impregnated resin capacities by different techniques. React. Funct. Polym. 1998, 38, 259–268. [Google Scholar] [CrossRef]
- Cotna, J.; Miralles, N.; Aguilar, M.; Sastre, A.M. Solvent impregnated resins containing di(2-ethylhexyl) phosphoric acid. I. Preparation and stdy of the extractant on the resin. Solv. Extr. Exch. 1994, 12, 349–369. [Google Scholar]
- Benamor, M.B.Z.; Belaid, T.; Draa, M.T. Kinetic studies on cadmium ions by Amberlite XAD7 impregnated resin containing di(20ethylhexyl) phosphoric acid as extractant. Sep. Purif. Technol. 2008, 59, 74–84. [Google Scholar] [CrossRef]
- Juang, R.-S. Preparation, Properties and Sorption Behavior of Impregnated Resins Containing Acidic Organophosphorus Extractants. Proc. Natl. Sci. Counc. ROC(A.) 1999, 23, 353–364. [Google Scholar]
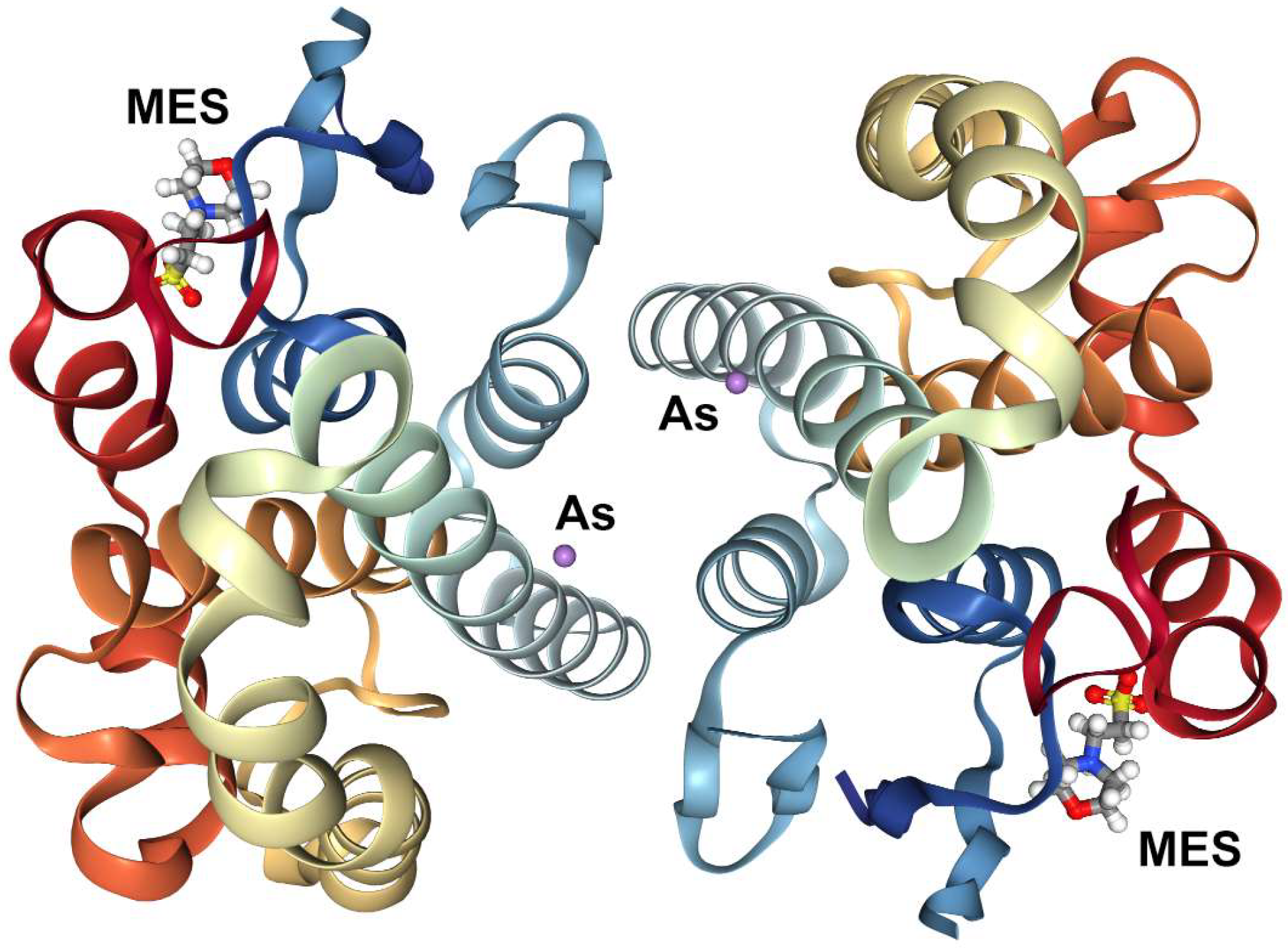
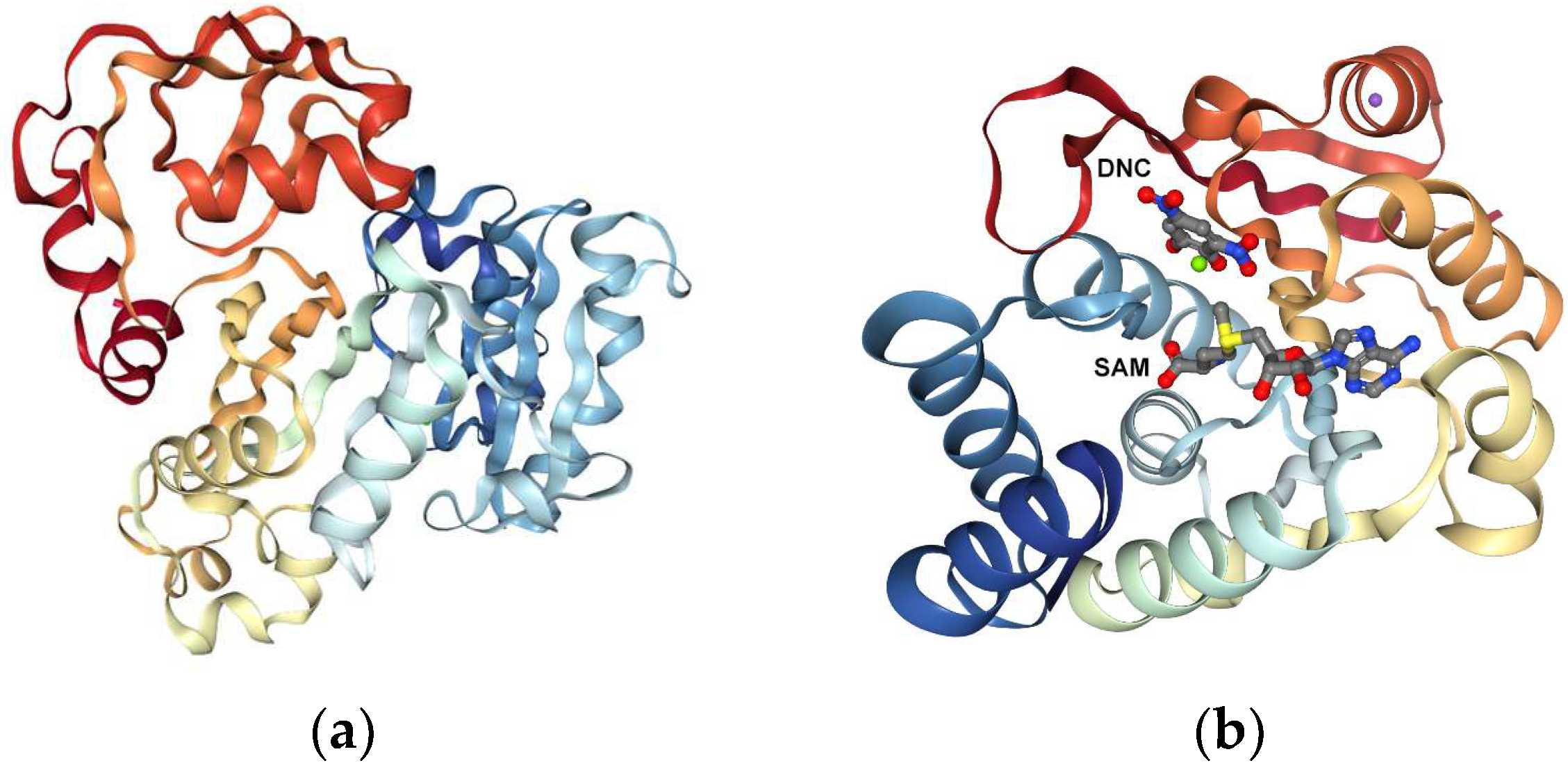
| Exposures | Cognitive Score | Fine Motor Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Sirajdikhan, n = 239 Children | Pabna, n = 286 Children | Sirajdikhan, n = 239 Children | Pabna, n = 285 Children | |||||
| Statistical Values | ||||||||
| β (SE) | p-Value | β (SE) | p-Value | β (SE) | p-Value | β (SE) | p-Value | |
| Water and Arsenic | −0.002 (0.02) | 0.93 | −0.06 (0.03) | 0.05 | −0.05 (0.03) | 0.09 | 0.02 (0.03) | 0.48 |
| Water and Manganese (Mn) | 0.02 (0.02) | 0.35 | −0.06 (0.07) | 0.33 | −0.04 (0.03) | 0.21 | 0.85 (0.39) | 0.03 |
| Water and Manganese (Mn2) | - | - | - | - | - | - | −0.08 (0.03) | 0.02 |
| Blood and Lead metal (Pb) | −0.17 (0.09) | 0.05 | 0.02 (0.12) | 0.87 | 0.07 (0.11) | 0.50 | −0.07 (0.11) | 0.50 |
| ChemIDplus | Chemical names, formulas, structures |
| CCRIS | Carcinogenicity, mutagenicity |
| CPDB | Cancer testing |
| CTD | Toxicogenomics information |
| GENE-TOX | Mutagenicity test data |
| IRIS | Human health risk assessment |
| ITER | Risk information |
| TOXLINE | Toxicology journal literature |
| DART | Reproductive toxicology journal literature |
| Haz-Map | Occupational health |
| HSDB | Health effects, toxicity, regulations |
| TOXMAP | Interactive U.S. maps of chemical releases |
| ID arsenic Trioxide | DB01169 |
| Chemical Formula | As2O3 |
| SMILES | O=[As]O[As]=O |
| Therapeutic Indication | “For induction of remission and consolidation in patients with acute promyelocytic leukemia (APL), and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression” |
| Pharmacodynamics | “Arsenic Trioxide is indicated for induction of remission and consolidation in patients with acute promyelocytic leukemia (APL) who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy.” |
| Absorption | Not available |
| Volume of Distribution | Not available |
| Protein Binding | 75% bound |
| Route of Elimination | Trivalent arsenic is mostly methylated in humans and excreted in urine |
| Half Life | Not Available |
| Clearance | Not Available |
| Toxicity | “Symptoms of overdose include convulsions, muscle weakness and confusion” |
| Arsenic Trioxide | ||
|---|---|---|
| ADME-Tox | Features | Unit |
| Absorption | Water solubility (log S) | 0.84 (log mol/L) |
| Intestinal absorption | 100% | |
| Distribution | Volume of distribution (human) | −0.48 log (L/Kg) |
| Blood-brain Barrier Permeability (BBB) | −0.361(log BB) | |
| Central Nervous System permeability (CNS) | −2.70 (log PS) | |
| Fraction unbounded of plasma proteins | 75% | |
| Metabolism | CYP2D6 substrate/inhibitor | NO |
| Excretion | Total clearance | 1.064 (log mL/min/kg) |
| Toxicity | AMES (mutagenic feature) | NO |
| Hepatotoxicity | NO | |
| Skin permeability | NO | |
| Max. tolerated dose (human) | 1.125 (log mg/kg/day) | |
| Oral rat acute toxicity (LD50) | 2.331 (mol/kg) | |
| Arsenic | ||
| Distribution | Blood-brain Barrier Permeability (BBB) | 0.003 (log BB) |
| Central Nervous System permeability (CNS) | −2.30 (log PS) | |
| Toxicity | AMES (mutagenic mutagenic) | NO |
| Hepatotoxicity | NO | |
| Skin permeability | NO | |
| Max. tolerated dose (human) | 1.18 (log mg/kg/day) | |
| Oral rat acute toxicity (LD50) | 2.21(mol/kg) | |
| Compound | hAS3MT Binding Affinity (kcal/mol) | hAS3MT Kd (μM) | Catachol O-Methyltransferase (COMT) Binding Affinity (kcal/mol) | Catachol O-Methyltransferase COMT Kd (μM) |
|---|---|---|---|---|
| TPI-1 | −7.3 | 4.5 | −4.3 | 704 |
| TPI-2 | −8.4 | 0.7 | −5.6 | 79 |
| TPI-3 | −9.3 | 0.2 | −4.6 | 424 |
| TPI-4 | −8.0 | 1.3 | −3.6 | 229 |
| TPI-5 | −9.4 | 0.1 | −1.4 | 9410 |
| TPI-6 | −7.2 | 5.3 | −4.7 | 358 |
| TPI-7 | −8.5 | 0.6 | −5.0 | 216 |
| TPI-8 | −9.2 | 0.2 | −2.2 | 2440 |
| TPI-9 | −8.8 | 0.5 | −0.7 | 306,800 |
| TPI-10 | −9.0 | 0.3 | −5.5 | 93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avram, S.; Udrea, A.M.; Negrea, A.; Ciopec, M.; Duteanu, N.; Postolache, C.; Duda-Seiman, C.; Duda-Seiman, D.; Shaposhnikov, S. Prevention of Deficit in Neuropsychiatric Disorders through Monitoring of Arsenic and Its Derivatives as Well as Through Bioinformatics and Cheminformatics. Int. J. Mol. Sci. 2019, 20, 1804. https://doi.org/10.3390/ijms20081804
Avram S, Udrea AM, Negrea A, Ciopec M, Duteanu N, Postolache C, Duda-Seiman C, Duda-Seiman D, Shaposhnikov S. Prevention of Deficit in Neuropsychiatric Disorders through Monitoring of Arsenic and Its Derivatives as Well as Through Bioinformatics and Cheminformatics. International Journal of Molecular Sciences. 2019; 20(8):1804. https://doi.org/10.3390/ijms20081804
Chicago/Turabian StyleAvram, Speranta, Ana Maria Udrea, Adina Negrea, Mihaela Ciopec, Narcis Duteanu, Carmen Postolache, Corina Duda-Seiman, Daniel Duda-Seiman, and Sergey Shaposhnikov. 2019. "Prevention of Deficit in Neuropsychiatric Disorders through Monitoring of Arsenic and Its Derivatives as Well as Through Bioinformatics and Cheminformatics" International Journal of Molecular Sciences 20, no. 8: 1804. https://doi.org/10.3390/ijms20081804
APA StyleAvram, S., Udrea, A. M., Negrea, A., Ciopec, M., Duteanu, N., Postolache, C., Duda-Seiman, C., Duda-Seiman, D., & Shaposhnikov, S. (2019). Prevention of Deficit in Neuropsychiatric Disorders through Monitoring of Arsenic and Its Derivatives as Well as Through Bioinformatics and Cheminformatics. International Journal of Molecular Sciences, 20(8), 1804. https://doi.org/10.3390/ijms20081804







