Effects of Caffeine Treatment on Hepatopulmonary Syndrome in Biliary Cirrhotic Rats
Abstract
1. Introduction
2. Results
2.1. Mortality Rates of Caffeine- and Vehicle-Treated CBDL Rats
2.2. Hemodynamics, Biochemistry Parameters and Blood Gas Analysis
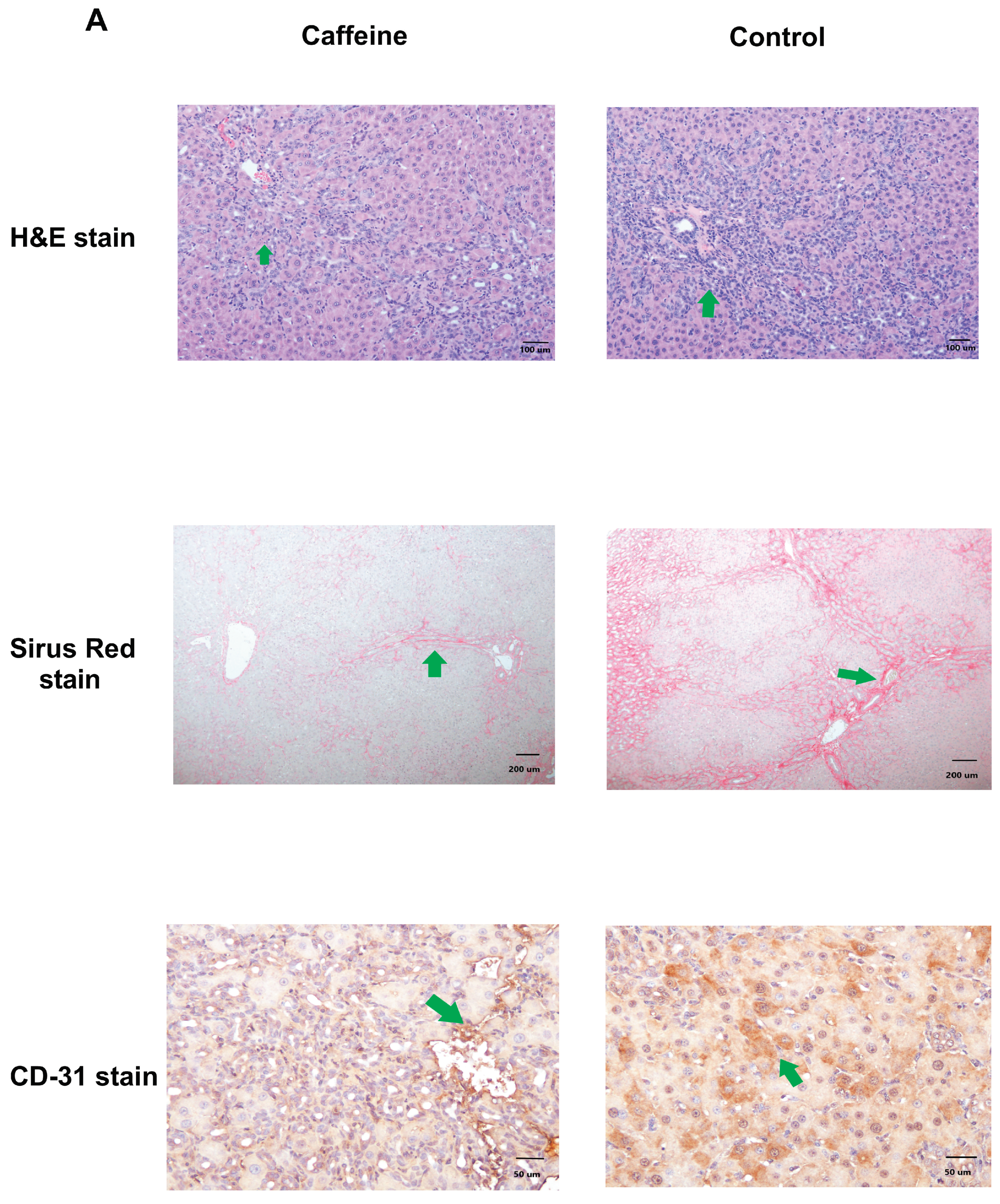
2.3. Histopathological Change and Immunochemical Staining of Liver
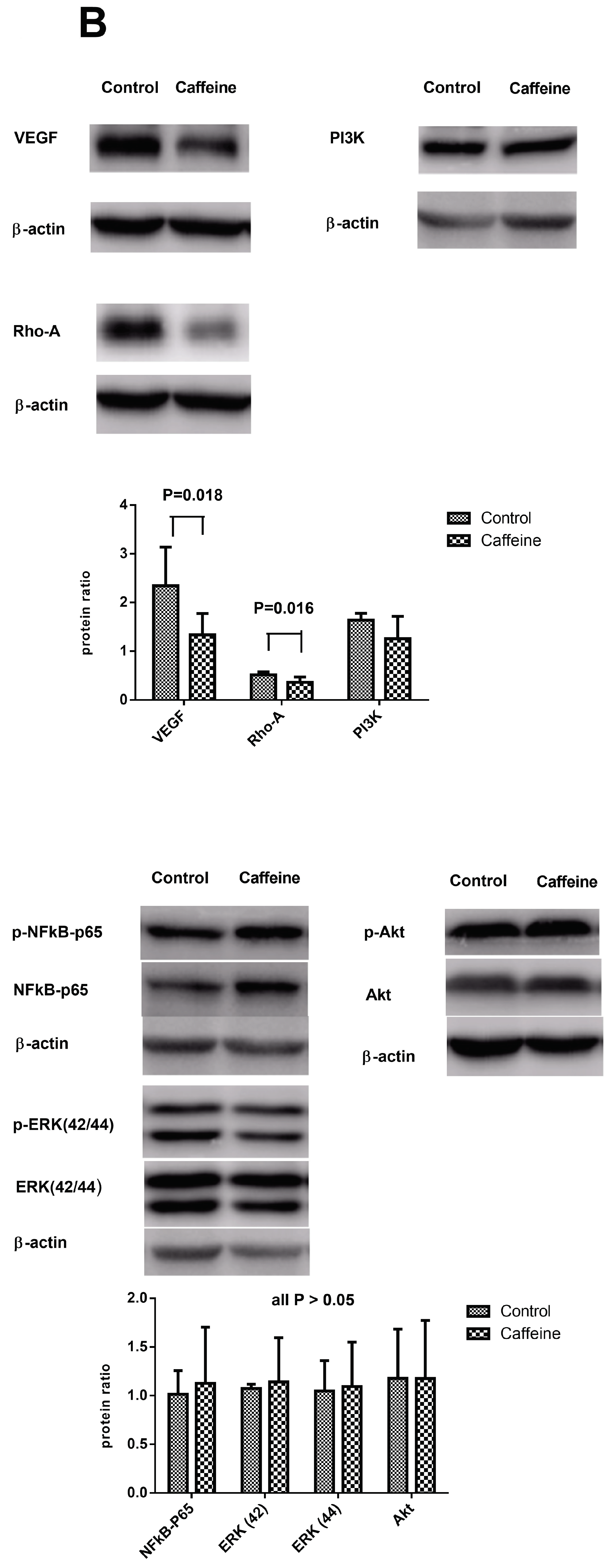
2.4. Hepatic Protein Expressions
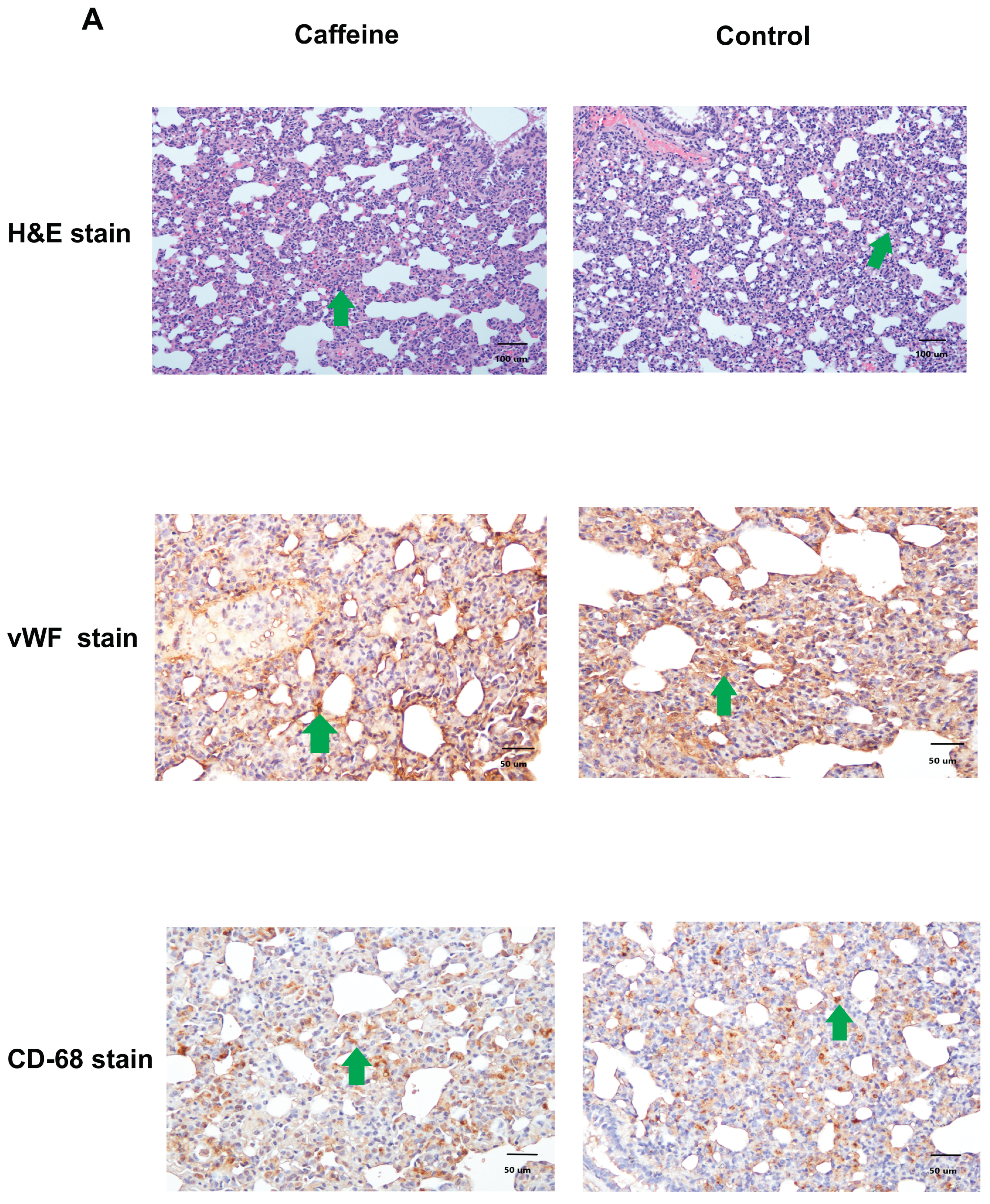
2.5. Pulmonary Inflammation and Angiogenesis
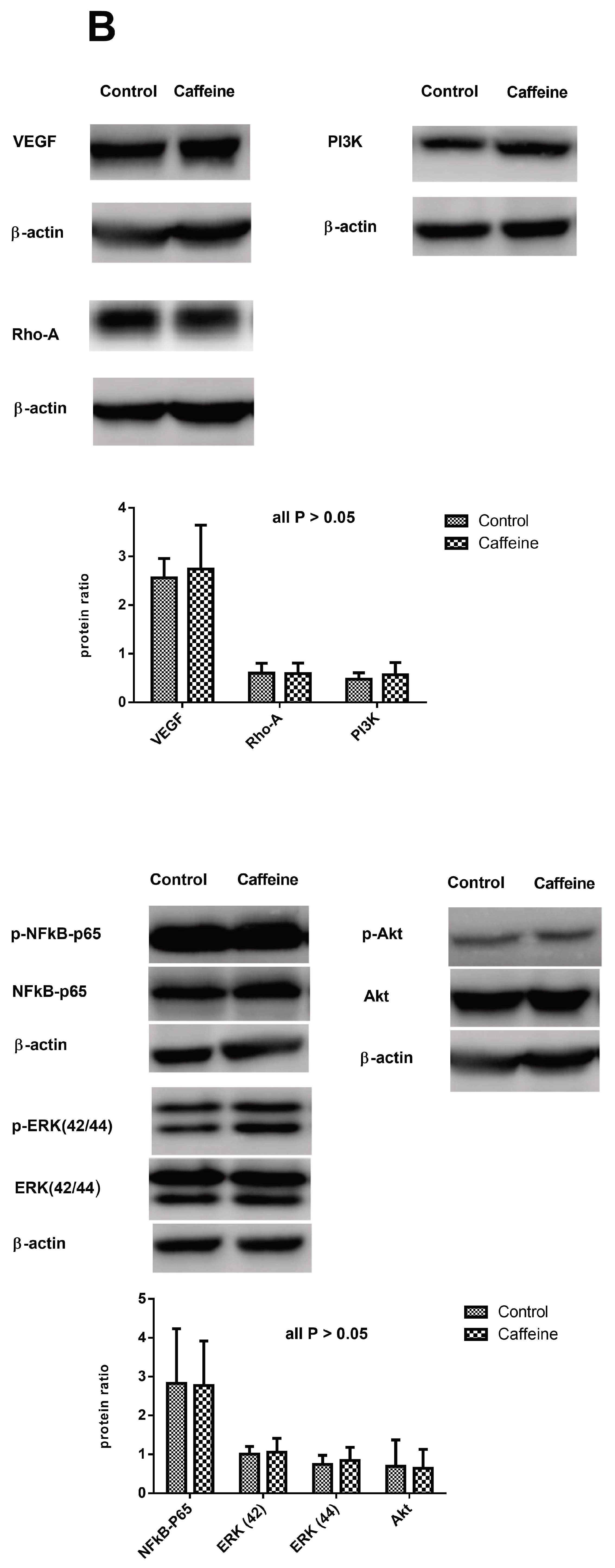
2.6. Protein Expressions in the Lungs of CBDL Rats Treated by Vehicle (n = 5) or Caffeine (n = 7)
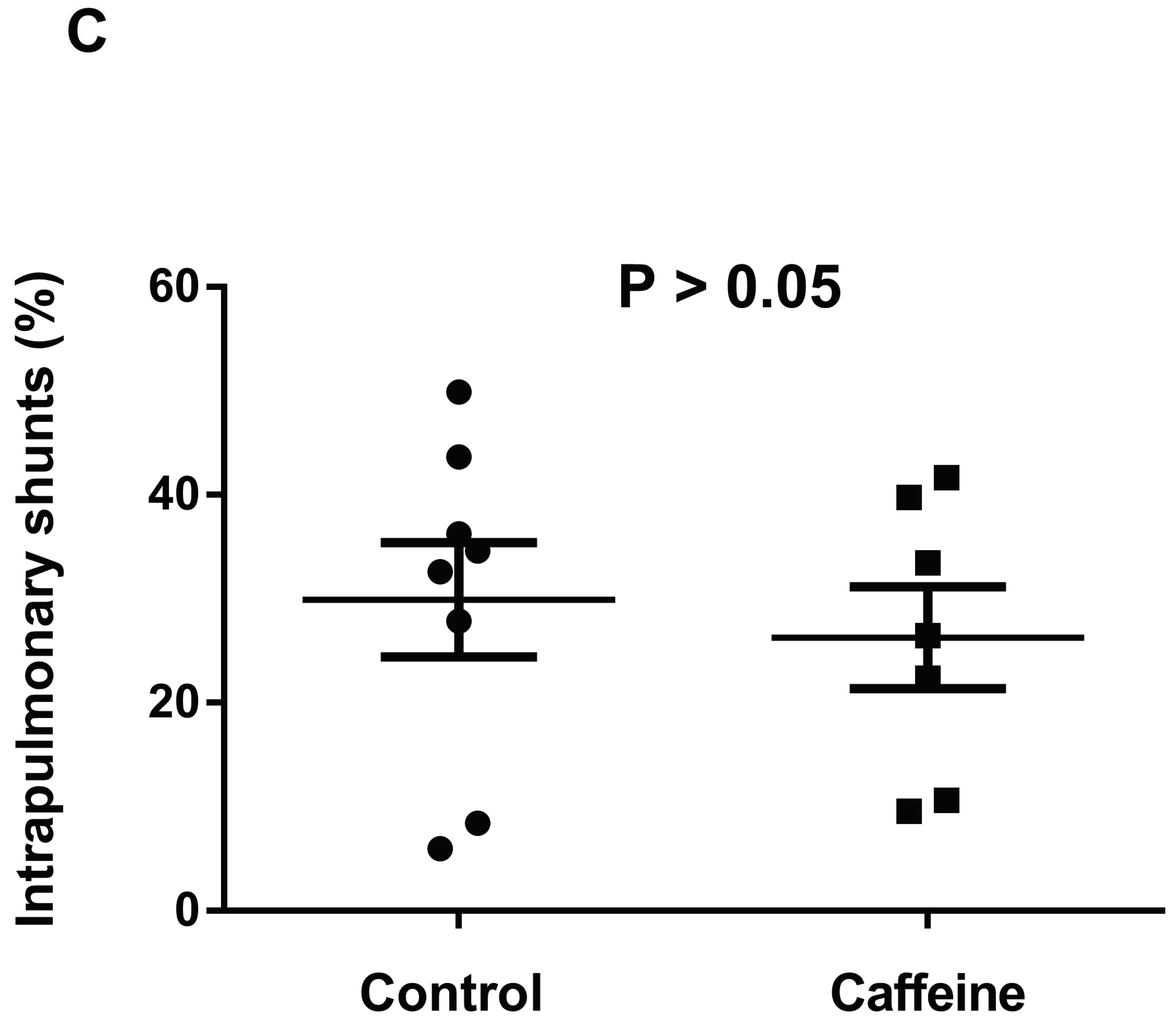
2.7. Intrapulmonary Shunting
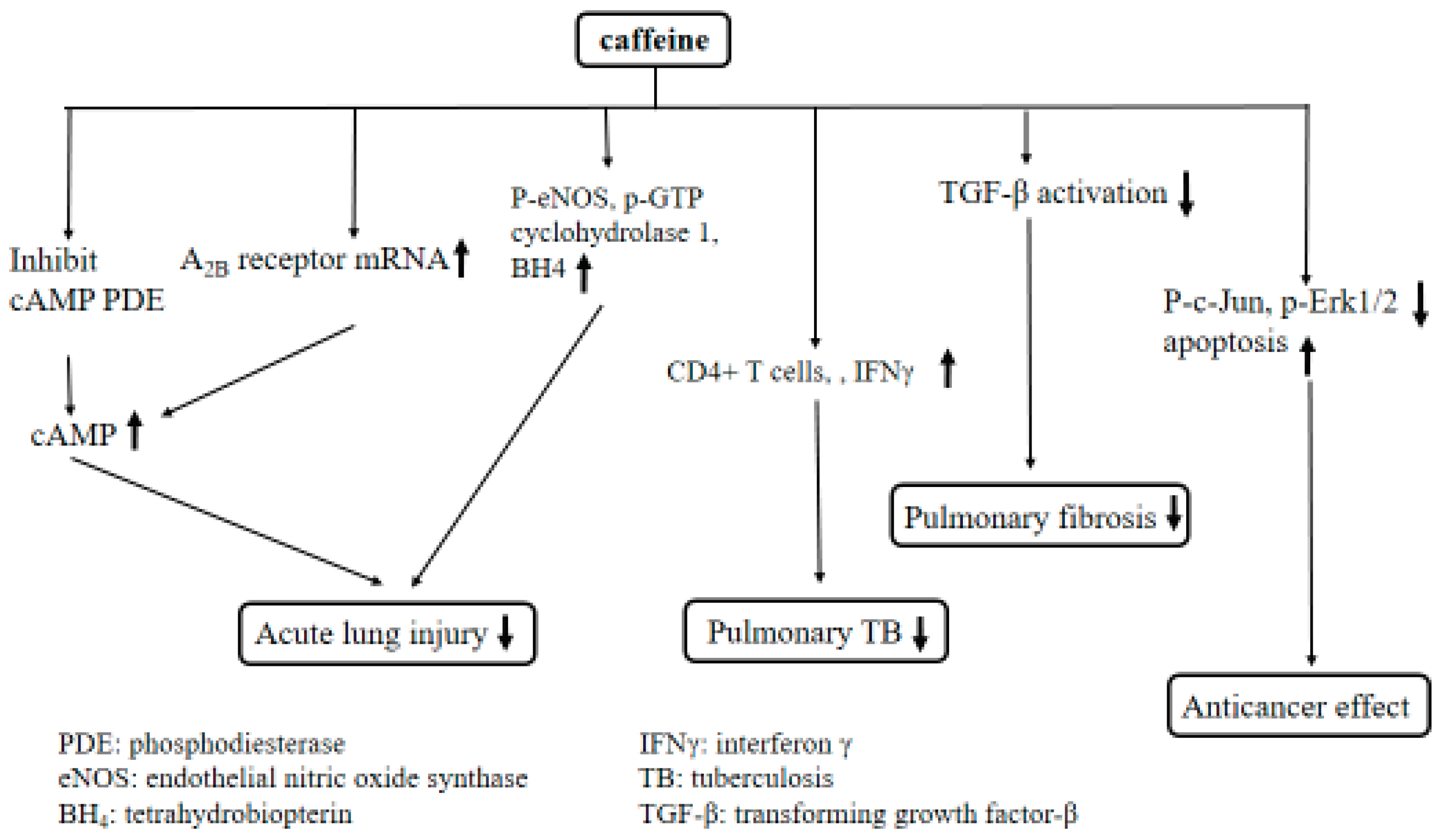
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Experimental Design
4.3. Systemic and Portal Hemodynamic Measurements
4.4. Biochemistry and Blood Gas Analysis
4.5. Histopathological and Immunochemical Staining
4.6. Western Blot Analysis for Protein Expressions
4.7. Intrapulmonary Shunting Analysis
4.8. Drugs
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruhl, C.E.; Everhart, J.E. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology 2005, 129, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.A.; Feld, J.J.; Park, Y.; Kleiner, D.E.; Everhart, J.E.; Liang, T.J.; Hoofnagle, J.H. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology 2010, 51, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Coffee consumption and risk of liver cancer: A meta-analysis. Gastroenterology 2007, 132, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, S.Y.; Son, H.J.; Seo, J.H.; Jeong, G.B. Caffeine-induced endothelial cell death and the inhibition of angiogenesis. Anat. Cell Biol. 2013, 46, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Köroğlu, O.A.; MacFarlane, P.M.; Balan, K.V.; Zenebe, W.J.; Jafri, A.; Martin, R.J.; Kc, P. Anti-inflammatory effect of caffeine is associated with improved lung function after lipopolysaccharide-induced amnionitis. Neonatology 2014, 106, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.J.; Lee, F.Y.; Wang, S.S.; Hsin, I.F.; Lin, T.Y.; Huang, H.C.; Chang, C.C.; Chuang, C.L.; Ho, H.L.; Lin, H.C.; Lee, S.D. Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology 2015, 61, 1672–1684. [Google Scholar]
- Koch, D.G.; Fallon, M.B. Hepatopulmonary syndrome. Curr. Opin. Gastroenterol. 2014, 30, 260–264. [Google Scholar] [CrossRef]
- Schenk, P.; Fuhrmann, V.; Madl, C.; Funk, G.; Lehr, S.; Kandel, O.; Müller, C. Hepatopulmonary syndrome: Prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut 2002, 51, 853–859. [Google Scholar] [CrossRef]
- Swanson, K.L.; Wiesner, R.H.; Krowka, M.J. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology 2005, 41, 1122–1129. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, B.; Tang, L.; Wang, Y.; Stockard, C.R.; Kadish, I.; Van Groen, T.; Grizzle, W.E.; Ponnazhagan, S.; Fallon, M.B. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology 2009, 136, 1070–1080. [Google Scholar] [CrossRef]
- Zhang, J.; Fallon, M.B. Hepatopulmonary syndrome: Update on pathogenesis and clinical features. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, P.; Walker, J.G.; Sherlock, S.; Reid, L. Arterial changes in the lungs in cirrhosis of the liver-lung spider nevi. N. Engl. J. Med. 1966, 274, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Edell, E.S.; Cortese, D.A.; Krowka, M.J.; Rehder, K. Severe hypoxemia and liver disease. Am. Rev. Respir. Dis. 1989, 140, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chuang, C.L.; Lee, F.Y.; Wang, S.S.; Lin, H.C.; Huang, H.C.; Teng, T.H.; Hsu, S.J.; Hsieh, H.G.; Lee, S.D. Sorafenib treatment improves hepatopulmonary syndrome in rats with biliary cirrhosis. Clin. Sci. 2013, 124, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, W.; Luo, B.; Hu, B.; Maheshwari, A.; Fallon, M.B. The role of CX3CL1/CX3CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome. J. Hepatol. 2012, 57, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Goel, A.; Marsboom, G.; Fang, Y.H.; Toth, P.T.; Zhang, H.J.; Kajimoto, H.; Hong, Z.; Paul, J.; Wietholt, C.; et al. A central role for CD68 (+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion. Am. J. Respir. Crit. Care Med. 2011, 183, 1080–1091. [Google Scholar] [CrossRef]
- Chang, C.C.; Wang, S.S.; Hsieh, H.G.; Lee, W.S.; Chuang, C.L.; Lin, H.C.; Lee, F.Y.; Lee, S.D.; Huang, H.C. Rosuvastatin improves hepatopulmonary syndrome through inhibition of inflammatory angiogenesis of lung. Clin. Sci. 2015, 129, 449–460. [Google Scholar] [CrossRef]
- Cachón, A.U.; Quintal-Novelo, C.; Medina-Escobedo, G.; Castro-Aguilar, G.; Moo-Puc, R.E. Hepatoprotective effect of low doses of caffeine on CCl4-Induced liver damage in rats. J. Diet Suppl. 2017, 14, 158–172. [Google Scholar] [CrossRef]
- Furtado, K.S.; Prado, M.G.; Aguiar, E.; Silva, M.A.; Dias, M.C.; Rivelli, D.P.; Rodrigues, M.A.; Barbisan, L.F. Coffee and caffeine protect against liver injury induced by thioacetamide in male Wistar rats. Basic Clin. Pharmacol. Toxicol. 2012, 111, 339–347. [Google Scholar] [CrossRef]
- Costenla, A.R.; Cunha, R.A.; de Mendonça, A. Caffeine, adenosine receptors, and synaptic plasticity. J. Alzheimers. Dis. 2010, 20, S25–S34. [Google Scholar] [CrossRef]
- Chan, E.S.; Montesinos, M.C.; Fernandez, P.; Desai, A.; Delano, D.L.; Yee, H.; Reiss, A.B.; Pillinger, M.H.; Chen, J.F.; Schwarzschild, M.A.; et al. Adenosine A (2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 2006, 148, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Andersson, P.; Lacarewicz, J.; Jacobson, I.; Butcher, S.; Sandberg, M. Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J. Neurochem. 1987, 49, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Adair, T.H. Growth regulation of the vascular system: An emerging role for adenosine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R283–R296. [Google Scholar] [CrossRef] [PubMed]
- Fehrholz, M.; Glaser, K.; Speer, C.P.; Seidenspinner, S.; Ottensmeier, B.; Kunzmann, S. Caffeine modulates glucocorticoid-induced expression of CTGF in lung epithelial cells and fibroblasts. Respir. Res. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; Freedman, N.D.; Graubard, B.I.; Guertin, K.A.; Black, A.; Huang, W.Y.; Shebl, F.M.; Mayne, S.T.; Sinha, R. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am. J. Epidemiol. 2015, 182, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, G.; Hu, J.L.; Fu, X.H.; Zeng, Y.J.; Zhou, Y.G.; Xiong, G.; Yang, N.; Dai, S.S.; He, F.T. Chronic or high dose acute caffeine treatment protects mice against oleic acid-induced acute lung injury via an adenosine A2A receptor-independent mechanism. Eur. J. Pharmacol. 2011, 654, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sztrymf, B.; Rabiller, A.; Nunes, H.; Savale, L.; Lebrec, D.; Le Pape, A.; de Montpreville, V.; Mazmanian, M.; Humbert, M.; Hervé, P. Prevention of hepatopulmonary syndrome by pentoxifylline in cirrhotic rats. Eur. Respir. J. 2004, 23, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Wiltberger, G.; Wu, Y.; Lange, U.; Hau, H.M.; Tapper, E.; Krenzien, F.; Atanasov, G.; Benzing, C.; Feldbrügge, L.; Csizmadia, E.; et al. Protective effects of coffee consumption following liver transplantation for hepatocellular carcinoma in cirrhosis. Aliment. Pharmacol. Ther. 2019, 49, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, K.; Watanabe, S.; Xiao, J.; Nagatomo, R.; Ogawa, H.; Tsunematsu, T.; Umemoto, H.; Morimoto, Y.; Akatsu, H.; Inoue, K.; et al. Effect of coffee or coffee components on gut microbiome and short-chain fatty acids in a mouse model of metabolic syndrome. Sci. Rep. 2018, 8, 16173. [Google Scholar] [CrossRef]
- Veronese, N.; Notarnicola, M.; Cisternino, A.M.; Reddavide, R.; Inguaggiato, R.; Guerra, V.; Rotolo, O.; Zinzi, I.; Leandro, G.; Correale, M.; et al. Coffee intake and liver steatosis: A population study in a Mediterranean area. Nutrients 2018, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.J.; Roderick, P.; Buchanan, R.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: A systematic review and dose-response meta-analysis. BMJ Open 2017, 7, e013739. [Google Scholar] [CrossRef]
- Arauz, J.; Zarco, N.; Hernández-Aquino, E.; Galicia-Moreno, M.; Favari, L.; Segovia, J.; Muriel, P. Coffee consumption prevents fibrosis in a rat model that mimics secondary biliary cirrhosis in humans. Nutr. Res. 2017, 40, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Thongprayoon, C.; Ungprasert, P. Impact of caffeine in hepatitis C virus infection: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 17–22. [Google Scholar] [CrossRef]
- Lammert, C.; Juran, B.D.; Schlicht, E.; Xie, X.; Atkinson, E.J.; de Andrade, M.; Lazaridis, K.N. Reduced coffee consumption among individuals with primary sclerosing cholangitis but not primary biliary cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1562–1568. [Google Scholar] [CrossRef]
- Barcelos, R.P.; Souza, M.A.; Amaral, G.P.; Stefanello, S.T.; Bresciani, G.; Fighera, M.R.; Soares, F.A.; Barbosa, N.V. Caffeine supplementation modulates oxidative stress markers in the liver of trained rats. Life Sci. 2014, 96, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Farah, B.L.; Singh, B.K.; Siddique, M.M.; Li, Y.; Wu, Y.; Ilkayeva, O.R.; Gooding, J.; Ching, J.; Zhou, J.; et al. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology 2014, 59, 1366–1380. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Huang, H.; Shi, M.; Yang, W.; Kuang, J.; Yan, J. Autophagy mediated by endoplasmic reticulum stress enhances the caffeine-induced apoptosis of hepatic stellate cells. Int. J. Mol. Med. 2017, 40, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Maki, T.; Yin, G.; Kawate, H.; Adachi, M.; Ohnaka, K.; Takayanagi, R.; Kono, S. Relation of coffee consumption and serum liver enzymes in Japanese men and women with reference to effect modification of alcohol use and body mass index. Scand. J. Clin. Lab. Investig. 2010, 70, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.; Wong, V.W.; Wong, G.L.; Chan, H.L. The effect of caffeine and alcohol consumption on liver fibrosis—A study of 1045 Asian hepatitis B patients using transient elastography. Liver Int. 2011, 31, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Kurozawa, Y.; Ogimoto, I.; Shibata, A.; Nose, T.; Yoshimura, T.; Suzuki, H.; Sakata, R.; Fujita, Y.; Ichikawa, S.; Iwai, N.; et al. Coffee and risk of death from hepatocellular carcinoma in a large cohort study in Japan. Br. J. Cancer 2005, 93, 607–610. [Google Scholar] [CrossRef]
- Ohta, A.; Lukashev, D.; Jackson, E.K.; Fredholm, B.B.; Sitkovsky, M. 1,3,7-trimethylxanthine (caffeine) may exacerbate acute inflammatory liver injury by weakening the physiological immunosuppressive mechanism. J. Immunol. 2007, 179, 7431–7438. [Google Scholar] [CrossRef]
- Amaral, E.P.; Machado de Salles, É.; Barbosa Bomfim, C.C.; Salgado, R.M.; Almeida, F.M.; de Souza, P.C.; Alvarez, J.M.; Hirata, M.H.; Lasunskaia, E.B.; D’Império-Lima, M.R. Inhibiting Adenosine Receptor Signaling Promotes Accumulation of Effector CD4+ T Cells in the Lung Parenchyma During Severe Tuberculosis. J. Infect. Dis. 2019, 219, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Ranganathan, S.; Cheong, J.L.Y. Neonatal caffeine treatment and respiratory function at 11 years in children under 1.251 g at birth. Am. J. Respir. Crit. Care Med. 2017, 196, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Huang, Y.W.; Jarzembowski, J.; Shi, Y.; Konduri, G.G.; Teng, R.J. Caffeine ameliorates hyperoxia-induced lung injury by protecting GCH1 function in neonatal rat pups. Pediatr. Res. 2017, 82, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Tatler, A.L.; Barnes, J.; Habgood, A.; Goodwin, A.; McAnulty, R.J.; Jenkins, G. Caffeine inhibits TGFβ activation in epithelial cells, interrupts fibroblast responses to TGFβ, and reduces established fibrosis in ex vivo precision-cut lung slices. Thorax 2016, 71, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Kao, M.C.; Yue, C.T.; Tsai, P.S.; Huang, C.J. Caffeine mitigates lung inflammation induced by ischemia-reperfusion of lower limbs in rats. Mediators Inflamm. 2015, 2015, 361638. [Google Scholar] [CrossRef]
- Guertin, K.A.; Freedman, N.D.; Loftfield, E.; Graubard, B.I.; Caporaso, N.E.; Sinha, R. Coffee consumption and incidence of lung cancer in the NIH-AARP Diet and Health Study. Int. J. Epidemiol. 2016, 45, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Bhoopalan, V.; Wang, D.; Wang, L.; Xu, X. The effect of caffeine on cisplatin-induced apoptosis of lung cancer cells. Exp. Hematol. Oncol. 2015, 4, 5. [Google Scholar] [CrossRef]
- Lu, G.; Liao, J.; Yang, G.; Reuhl, K.R.; Hao, X.; Yang, C.S. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006, 66, 11494–11501. [Google Scholar] [CrossRef]
- Yoder, B.; Thomson, M.; Coalson, J. Lung function in immature baboons with respiratory distress syndrome receiving early caffeine therapy: A pilot study. Acta. Paediatr. 2005, 94, 92–98. [Google Scholar] [CrossRef]
- Franco, F.; Gigou, M.; Szekely, A.M.; Bismuth, H. Portal hypertension after bile duct obstruction: Effect of bile diversion on portal pressure in the rat. Arch. Surg. 1979, 114, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Stavric, B.; Klassen, R.; Watkinson, B.; Karpinski, K.; Stapley, R.; Fried, P. Variability in caffeine consumption from coffee and tea: Possible significance for epidemiological studies. Food Chem. Toxicol. 1988, 26, 111118. [Google Scholar] [CrossRef]
- Whittaker, P.; Kloner, R.A.; Boughner, D.R.; Pickering, J.G. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res. Cardiol. 1994, 89, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, L.; Marcus, S.G.; Vasile, J.; Dobryansky, M.; Cohen, H.; Eng, K.; Shamamian, P.; Mignatti, P. Expression of von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: A potential method for objective assessment of tumor angiogenesis. Int. J. Cancer 2000, 85, 281–288. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lee, H.B.; Blaufox, M.D. Blood volume in the rat. J. Nucl. Med. 1985, 26, 72–76. [Google Scholar] [PubMed]

| Control (n = 9) | Caffeine (n = 8) | |
|---|---|---|
| BW (g) | 357 ± 54 | 330 ± 35 |
| MAP (mmHg) | 134 ± 17 | 133 ± 27 |
| PP (mmHg) | 17.0 ± 8.1 | 10.0 ± 3.7 * |
| AST (IU/L) | 625 ± 114 | 721 ± 78 |
| ALT (IU/L) | 216 ± 142 | 183 ± 55 |
| TB (mg/dL) | 7.9 ± 2.3 | 7.7 ± 0.5 |
| Creatinine (mg/dL) | 0.5 ± 0.2 | 0.4 ± 0.1 |
| PaO2 (mmHg) | 75.1 ± 10.2 | 78.6 ± 6.5 |
| PaCO2 (mmHg) | 37.4 ± 5.3 | 39.8 ± 5.7 |
| AaPO2 (mmHg) | 28.1 ± 7.0 | 21.7 ± 9.1 |
| References | Experimental Model | Major Findings |
|---|---|---|
| Wiltberger et al. [28] | Human | Coffee consumption was associated with a decreased risk of hepatocellular carcinoma recurrence and increased survival following orthotopic liver transplantation. |
| Nishitsuji et al. [29] | Mice | Caffeine would affect the gut dysbiosis and the disrupted plasma short-chain fatty acid profile, then subsequently prevented nonalcoholic steatohepatitis. |
| Veronese et al. [30] | Human | The consumption of coffee was not associated with liver steatosis either nonalcoholic fatty liver disease or alcoholic fatty liver disease. |
| Kennedy et al. [31] | Human | Increased consumption of caffeinated and decaffeinated coffee is associated with reduced risk of hepatocellular carcinoma, including in pre-existing liver disease. |
| Arauz et al. [31] | Rats | Coffee prevented liver cirrhosis by attenuating the oxidant process, blocking hepatic stellate cell activation, and down-regulated the profibrotic molecules. |
| Wijarnpreecha et al. [33] | Human | Coffee consumption showed a decreased risk of advanced liver fibrosis and inflammation among hepatitis C-infected patients. |
| Hsu et al. [6] | Rats | Caffeine decreased portal pressures, ameliorated hyperdynamic circulation, protosystemic collateral shunting, mesenteric angiogenesis and liver fibrosis in cirrhotic rats. |
| Lammert et al. [34] | Human | Coffee consumption was lower among patients with primary sclerosing cholangitis, but no primary biliary cholangitis, compared with controls. |
| Barcelos et al. [35] | Rats | Caffeine modified the hepatic responses associated to exercise-induced oxidative stress of trained rats. |
| Sinha et al. [36] | Mice | The caffeine’s lipolytic action was through autophage in mammalian liver and it was a potent stimulator of hepatic autophagic flux. |
| Li et al. [37] | Cells | The caffeine-enhanced autophagic flux in hepatic stellate cell (HSC) was stimulated by endoplasmic reticulum stress, which further weakened HSC viability via the induction of apoptosis. |
| Ikeda et al. [38] | Human | There was an inverse association between coffee consumption and elevated aminotransferase in men, and it was more evident in those with high alcohol consumption and in those with low body mass index. |
| Ong et al. [39] | Human | Caffeine intake does not affect liver stiffness in chronic hepatitis B-infected patients. |
| Kurozawa et al. [40] | Human | An inverse association between coffee consumption and HCC mortality was found in a large cohort study in Japan. |
| Ohta et al. [41] | Rats | A high dose of caffeine (100 mg/kg) completely blocked both liver damage and proinflammatory cytokine responses through an A2AR-independent mechanism. |
| References | Experimental Model | Major Findings |
|---|---|---|
| Amaral et al. [42] | Mice | Caffeine enhanced the frequency and number of parenchymal CD4+ T-cell and stimulated immune function in severe tuberculosis. |
| Doyle et al. [43] | Human | Caffeine reduced the incidence of brochopulmonary dysplasia in the new-born period and improved pulmonary function at follow-up. |
| Jing et al. [44] | Rats | Early caffeine treatment could protect immature lungs from hyperoxia-induced lung injury. |
| Fehrholz et al. [24] | Cells | Glucocorticoid had adverse effects on long-term remodeling by induction of connective tissue growth factor in lung cells, however, co-treatment with caffeine attenuated connective tissue growth factor expression and promoting restoration of lung homeostatsis. |
| Tatler et al. [45] | Cells | Caffeine owned anti-fibrotic capacities for the epithelial cells and fibroblasts in the lung. |
| Chou et al. [46] | Rats | Caffeine could mitigate lung inflammation induced by ischemia-reperfusion of the lower limbs. |
| Guertin et al. [47] | Human | Coffee drinking was positively associated with lung cancer. |
| Wang et al. [48] | Cells | Caffeine administration increased the cisplatin-induced lung cancer killings and cellular apoptosis. |
| Li et al. [26] | Mice | Caffeine either enhanced lung damage by antagonizing A2A receptor or exerted protection against lung damage via A2A receptor-independent mechanisms, depending on the timing of exposure and dose of administration. |
| Lu et al. [49] | Cells | Caffeine and tea polyphenos inhibited the progression of lung adenoma to adenocarcinoma. |
| Yoder et al. [50] | Baboons | Early caffeine treatments were associated with better lung function in immature baboons. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Chuang, C.-L.; Tsai, M.-H.; Hsin, I.-F.; Hsu, S.-J.; Huang, H.-C.; Lee, F.-Y.; Lee, S.-D. Effects of Caffeine Treatment on Hepatopulmonary Syndrome in Biliary Cirrhotic Rats. Int. J. Mol. Sci. 2019, 20, 1566. https://doi.org/10.3390/ijms20071566
Chang C-C, Chuang C-L, Tsai M-H, Hsin I-F, Hsu S-J, Huang H-C, Lee F-Y, Lee S-D. Effects of Caffeine Treatment on Hepatopulmonary Syndrome in Biliary Cirrhotic Rats. International Journal of Molecular Sciences. 2019; 20(7):1566. https://doi.org/10.3390/ijms20071566
Chicago/Turabian StyleChang, Ching-Chih, Chiao-Lin Chuang, Ming-Hung Tsai, I.-Fang Hsin, Shao-Jung Hsu, Hui-Chun Huang, Fa-Yauh Lee, and Shou-Dong Lee. 2019. "Effects of Caffeine Treatment on Hepatopulmonary Syndrome in Biliary Cirrhotic Rats" International Journal of Molecular Sciences 20, no. 7: 1566. https://doi.org/10.3390/ijms20071566
APA StyleChang, C.-C., Chuang, C.-L., Tsai, M.-H., Hsin, I.-F., Hsu, S.-J., Huang, H.-C., Lee, F.-Y., & Lee, S.-D. (2019). Effects of Caffeine Treatment on Hepatopulmonary Syndrome in Biliary Cirrhotic Rats. International Journal of Molecular Sciences, 20(7), 1566. https://doi.org/10.3390/ijms20071566




