1. Introduction
Octopamine (OCT) is among a group of compounds that is known as biogenic amines. OCT is an invertebrate structural analog of vertebrate norepinephrine, and it is present in both vertebrate and invertebrate nervous systems [
1]. OCT occurs as a trace amine in the nervous systems of most invertebrate species, including insects, where it plays a multifunctional role. OCT controls various peripheral functions, including modulation flight activity, visceral muscle, peripheral organs, and some sense organs [
2,
3]. In the central nervous system, OCT is vital in the regulation of motivation, maintenance of various rhythmic behaviors, social behaviors, and learning and memory [
4]. OCT mediates starvation-induced hyperactivity in adult
Drosophila [
5]. OCT modulates muscle contraction and metabolism and fuel availability [
6]. As a neurohormone, OCT modulates haemocytic nodulation in immune larvae and enhances phagocytosis [
7]. OCT also increased the number of circulating hemocytes in
Spodoptera exigua [
8]. Our previous results also showed that OCT plays an important role in the larvae of
Mythimna sepatrata, from high density conditions to be resistant to pathogens [
9].
OCT is biosynthesized from tyrosine by two steps: tyrosine is decarboxylated by tyrosine decarboxylase to produce tyramine, followed by hydroxylation of tyramine by tyramine β-hydroxylase (Tβh) to form OCT [
10]. The tyramine β-hydroxylase is the last and the rate-limiting enzyme in the biosynthetic pathway of OCT [
2]. Davenport and Evans, in 1984 [
11], postulated the hypothesis that the upregulation of Tβh activity that they observed might be due to an increase in
Tβh expression. Lehman et al., 2000 [
12] have demonstrated that elevated OCT levels during
Manduca sexta development correlated with the induction of Tβh activity in the brain and ganglia during metamorphosis. Lehman et al., 2006 [
13] have demonstrated that during honey bee behavioral development, an increase OCT levels in forager honey bee brain were correlated with the elevated expression of the gene encoding
Tβh. Chatel et al., 2013 [
14] found that stressful mechanical stimulation led to a significant increase in Tβh activity after 1 and 24h., although the level of OCT did not significantly increase in the same treatments. All these data exist seem to indicate that a link between Tβh enzyme activity or transcriptional activity, and OCT levels.
The oriental armyworm,
M. separata (Lepidoptera: Noctuidae), is a major insect pest of grain crops in China and other Asian countries, and outbreaks have caused huge losses in annual crop production nationwide [
15,
16,
17]. As an insect with typical phase polymorphisms, the variation of larval color polymorphism, survival rates, development rate, feeding behavior of larvae, and flight capacity, fecundity, and energetic reserves in adults from larvae reared at different larval densities have been found [
18,
19,
20]. Meanwhile, Mitsui and Kunimi, 1988 [
21] found that gregarious (high density) phases larvae were more resistant than the solitary (low density) phase ones when orally inoculated with nuclear polyhedrosis virus (PsNPV). Furthermore, our previous results also showed that resistance to entomopathogenic fungus
Beauveria bassiana and bacteria
Bacillus thuringiensis of larvae from high densities was higher than the larvae from low densities. Therefore, larvae that experience crowded conditions are more resistant to pathogens when compared to counterparts at low-densities, and this phenomenon is now known as “density-dependent prophylaxis (DDP)” [
22]. The DDP affected the stability of the insect-parasite (or pathogen) interaction. Understanding the mechanism of DDP is of great importance in characterizing the population dynamics and controlling them by natural enemies [
23].
Wang et al., 2016 [
24] found that Tβh expression was related to aphid density, which affected wing polyphenism (winged or wingless), and aphids under high density conditions exhibited significantly higher
Tβh transcription than those that were under low-density conditions. Our previous research suggested that immune indicators, such as phenoloxidase (PO) activity and total haemocyte counts and the level of OCT, increased in the larvae of
M. separata from high-density conditions. Our previous research has demonstrated that exogenous OCT could significantly increase the PO activity and the number of total hemocytes of larvae [
9]. Nonetheless, whether larval density can influence endogenous Tβh enzyme activity and transcriptional activity remains unclear, and whether the
Tβh gene has a key role in insect density-dependent prophylaxis.
M. separata was investigated to gain further insight into OCT regulation, larval density-dependent prophylaxis of the armyworm. To accomplish this, we investigated the Tβh enzyme activity of larvae from different densities in rearing chambers. A full-length Tβh cDNA from M. separata was obtained and characterized, and mRNA expression was examined during the entire life cycle and in fifth-instar larvae that were reared under five different densities. Silencing the Tβh on the octopamine level and immune parameters were investigated. The study advances our knowledge of OCT-associated Tβh gene expression in the larval density dependent prophylaxis, and it provides information needed to explore the mechanism of octopamine on the larval immune function in M. separata.
3. Discussion
OCT is biosynthesized from tyrosine by tyrosine decarboxylase and tyramineβ-hydroxylase enzymes. The tyramineβ-hydroxylase is a rate-limiting enzyme and it catalyzes the last step in the process of OCT biosynthesis. It plays a key role in the regulation of OCT synthesis. Some researches have demonstrated that increased Tβh activity was correlated with an increasing OCT level. During the
M. sexta metamorphosis, it showed that elevated OCT level was correlated with the induction of Tβh activity in the brain and ganglia [
12]. Mechanical stimulation stress led to an increase in Tβh activity and OCT level in
Periplaneta americana [
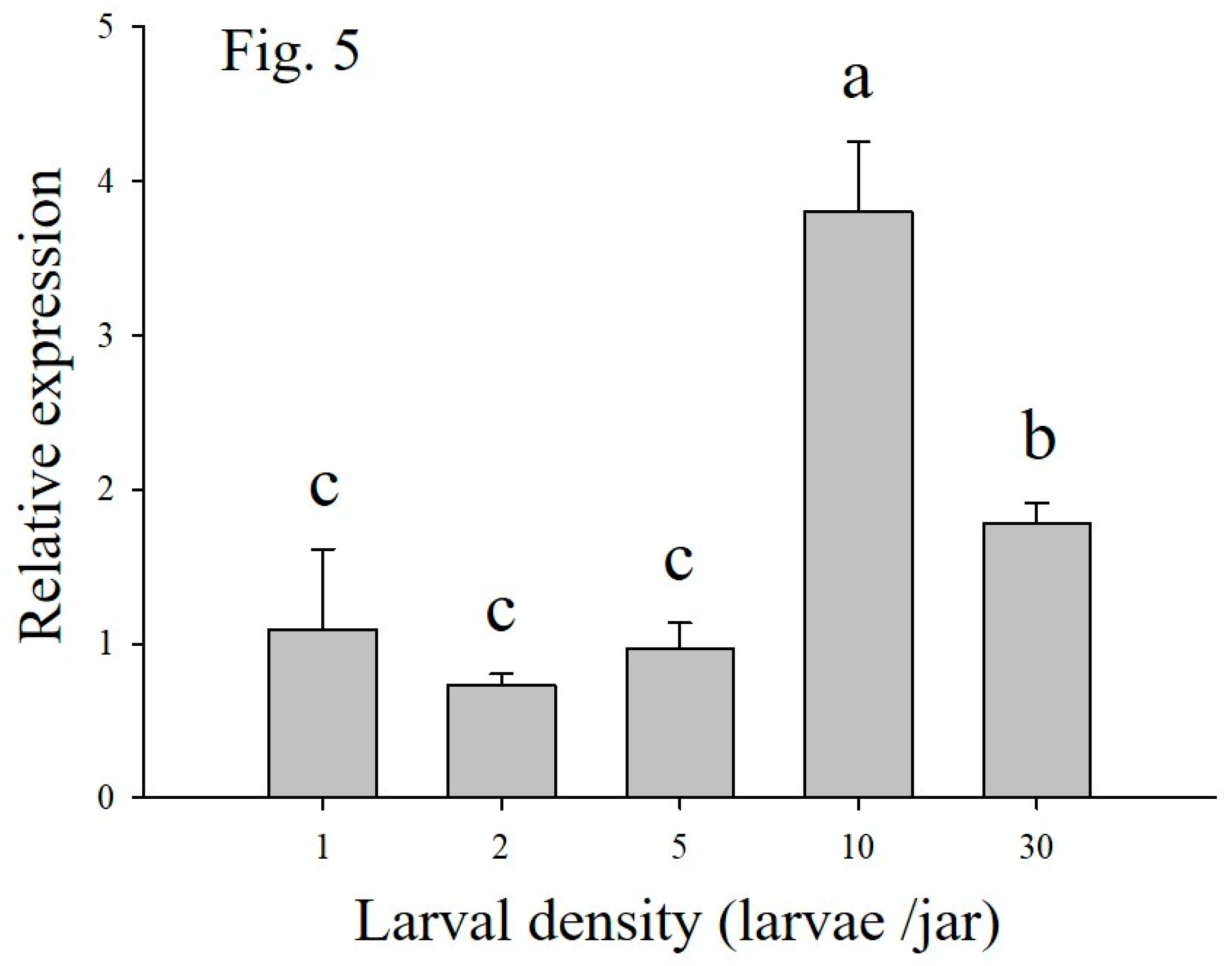
14]. Our results demonstrated that the Tβh activity of
M. separata larvae from conditions of five, 10, and 30 larvae per jar were significantly higher than those of larvae at one and two larvae per jar. Kong et al. (2018) [
9] found that, when compared with lower density larvae of
M. separata, the larvae from high density conditions had significantly higher OCT levels. We inferred that high density led to an increase in Tβh activity and OCT level in
M. separata. Our results also showed that the transcriptional levels of the
Tβh gene in
M. separata larvae from high density (10 and 30 larvae per jar) larvae were significantly higher than those of the lower density larvae (one, two, and five larvae per jar). This similar variation in the function of the density on the
Tβh expression pattern was found in the Pea aphid
Acyrthosiphon pisum [
24]. Based on these results, we concluded that a link exists between Tβh enzyme activity or transcriptional activity and OCT levels in different densities of
M. separata larvae.
With the transcriptome data of
M. separata, a
Tβh cDNA was amplified from the larvae. The cDNA of
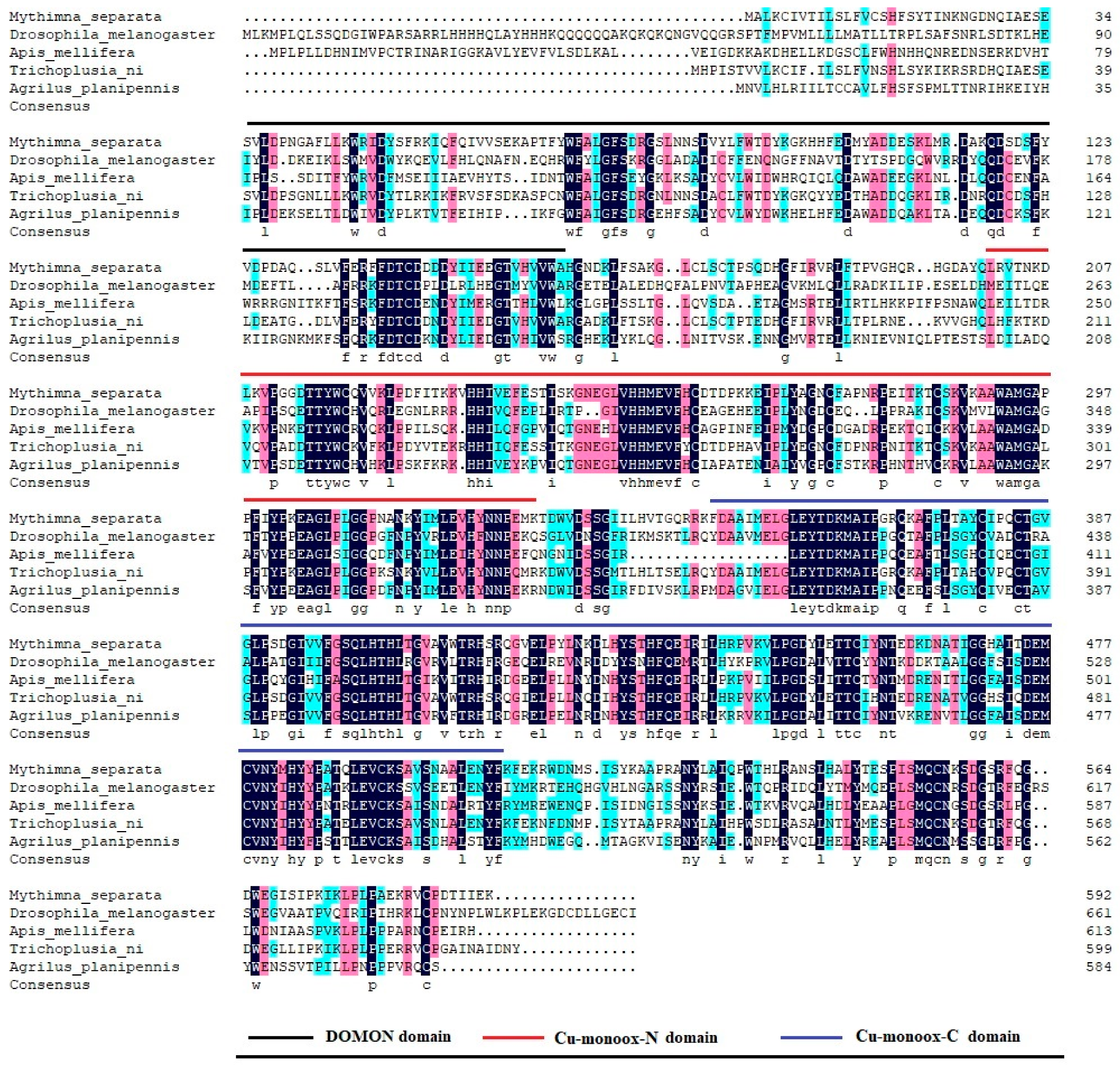
MsTβh contains an ORF of 1779 bp that encodes 593-amino acids. The alignment of multiple sequences between the
M. separata and other Diptera, Coleoptera, Hymenoptera insects, and
Tβh amino acid showed that
MsTβh is highly conserved in these insects
Tβh, including three domains: a DOMON domain, a copper type II ascorbate-dependent monooxygenase N-terminal domain (Cu-monox-N), and a copper type II ascorbate-dependent monooxygenase C-terminal domain (Cu-monox-C) (
Figure 2). This indicates that
Tβh is highly conserved across species.
In
Drosophila, the knockout
Tβh strain showed a decreased level of OCT [
25]. In
Tribolium castaneum, knockdown of
Tβh led to reduced OCT level [
26]. In the current study, we found that knocked down
Tβh resulted in a decreasing OCT level in
M. separata. Our results demonstrated that
Tβh plays an essential role in the biosynthesis of OCT in
M. separata larvae.
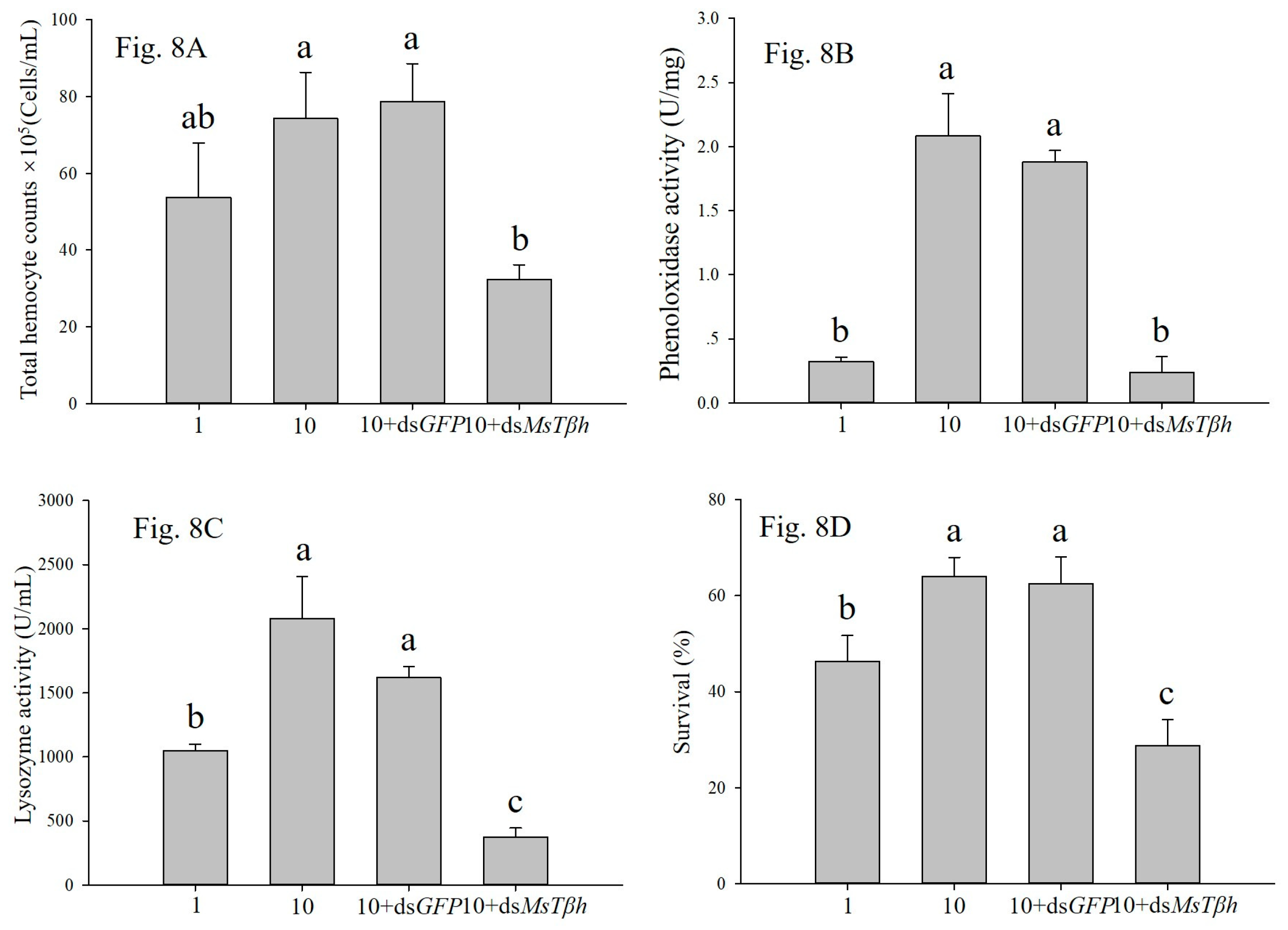
OCT is known to play critical roles in mediating insect immune response. Baines et al., 1992 [
27] found that induced haemocyte phagocytosis and nodule formation in the cockroach
Periplaneta americana. Kim and Kim, 2010 [
8] reported that an injection of octopamine induced a significant increase in the total haemocyte count in the hemolymph. In response to bacterial infection, octopamine significantly enhanced both haemocytic phagocytosis and nodule formation in the
S. exigua larvae [
7]. Similarly, octopamine accelerated the removal of bacteria from haemolymph in the greater wax moth,
Galleria mellonella [
28]. In
M. separata larvae, an injection of octopamine significantly stimulated the PO activity and total haemocyte counts [
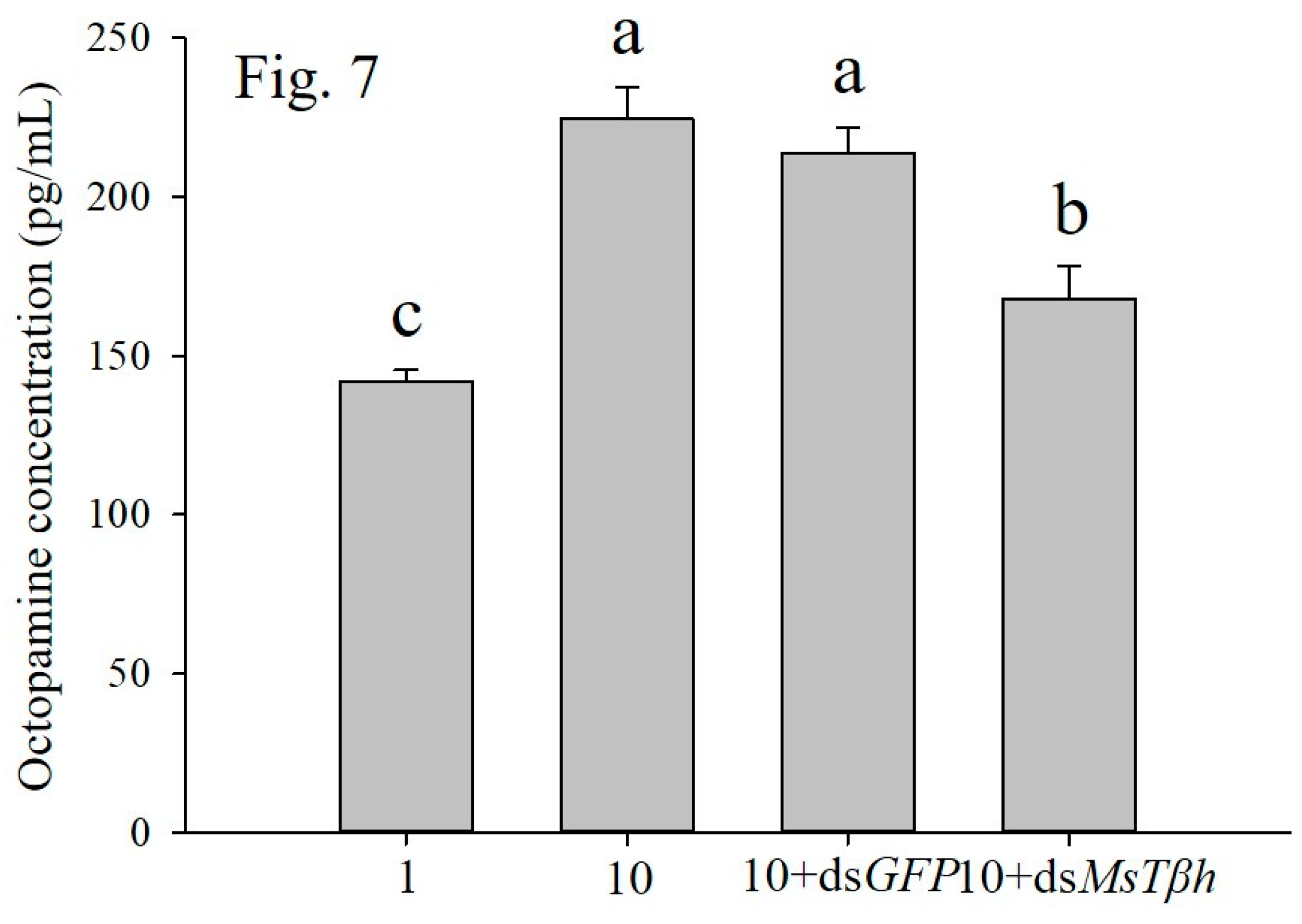
9]. We found that, in this study, that RNA interference-mediated silencing of the
MsTβh exhibited markedly decreased PO activity, total haemoyte counts, lysozyme activity, and survival rate of the larvae. All of these results indicated that increasing OCT levels of larvae resulted in enhanced immunity. Octopamine was also involved in providing locusts with mobilizing fuel or lipid mobilization in flight [
6]. In the high density conditions, improving immunity and flight capacity may cause the outbreak of
M. separata.
Taken together, we demonstrated that Tβh is a key enzyme in the biosynthetic pathway of OCT, and that the larval densities of M. separata modulated its activity. After the silencing of MsTβh in high density larvae, the larval PO activity, total haemoyte counts, lysozyme activity, and survival were significantly decreased. Therefore, we concluded that MsTβh controls the biosynthesis of OCT, which in turn modulates the immunity of the M. separata larvae from different density conditions. However, further investigation is needed to understand how the octopaminergic signaling system modulates larval immunity, and significantly more research is needed in order to decipher the density dependent prophylaxis effect in insects.
4. Materials and Methods
4.1. Experimental Insects
The M. separata that were used in this study were a gift of Professor Jiang, Institute of Plant Protection, Chinese Academy of Agricultural Sciences.
A laboratory colony of M. separata larvae was reared on corn (Zea mays L.) leaves at 23 ± 1 °C, 70% relative humidity, under a 16 h light: 8 h darkphotoperiod. Adult M. separata were put in a 2-L capacity plastic cage that was equipped with a cotton pad soaked in 10% glucose solution (w/v) and allowed to oviposit on nylon gauze lining the plastic cage.
4.2. Larval Tyramineβ-hydroxylase Activity Assay at Different Densities
Enzyme was extracted in M. separata larvae that were reared at five different densities. After hatching, the larvae were reared at five density treatments of one, two, five, 10, and 30 larvae per 650-mL-capacity jar. The number of larvae for all density treatments was maintained at a constant level throughout the feeding period. Excess food was provided by adding fresh leaves every morning. All of the insects were maintained under the same conditions described above. The larvae bodies were collected as described above, when most of the larvae at a given density had developed to the second day of the fifth instar, and larvae were cooled to torpor on ice and then sterilized by swabbing them with 75% ethanol. The head and abdomen tissues from larvae were dissected under ice-chilled saline solution, frozen in liquid nitrogen, and stored at −80 °C. The tissues were thawed immediately prior to use, placed in a ground-glass homogenizer containing saline solution, and homogenized with 20 strokes by hand. The homogenate was centrifuged (6080 g, 30min) at 4 °C, and the resulting supernatant fraction was used as the crude enzyme source. The samples that were collected from groups of three larvae per density were pooled as one replicate. Three replicates were made at each density.
Tyramine β-hydroxylase activity was determined based on the Tyramine β-hydroxylase Elisa Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). A standard curve was made that was based on different concentrations of standard solutions. To the enzyme solution from the procedure described above (10 μL), sample diluents 40 μL and 100 μL HRP-Conjugate reagent were combined and incubated for 60 min. at 37 °C in microelisa wells. Each well was aspirated and then washed with a wash solution. Subsequently, 50 μL each chromogen solution A and B were added, incubated for 15 min. at 37 °C, and then 50 μL stop solution was added. The absorbency at 450 nm was measured while using a microplate reader (Power Wave XS2, Bio Tek Instrument Company of America, Ltd., Winooski, VT, USA).
4.3. Molecular Cloning of Tyramineβ-hydroxylase (Tβh) cDNA
Total RNA was extracted with Trizol Kit (Invitrogen, Carlsbad, CA, USA) from fifth-instar larvae of M. separata and the first-strand cDNA was synthesized using an oligo(dT)15 primer (Tiangen Biotech, Beijing, China) following the manufacturer’s instructions. The integrity of RNA was checked on an 1% agarose gel and Nanophotometer NP80 (IMPLEN, München, Germany) determined the concentration of RNA.
The complete open reading frames (ORFs) of MsTβh were amplified from larval cDNA. Primer (Tβh-F: 5′ATGGCTCTAAAGTGTATAGT 3′; Tβh-R: 5′ TTTTTCAATAATCGTGTCGG 3′) were designed based on the transcriptome data of M. separata with Primer Premier 6.0. The larval transcriptome dataset of M. separata has been released in the SRA database (SRP153130).
PCR amplification was carried out under the condition of one cycle at 95 °C for 3 min, 35 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C 45 s, followed by a final extension at 72 °C for 10 min. The purified PCR products were cloned into a pMD-18T vector (Takara, Dalian, China) and subsequently sequenced by Sangon Company (Shanghai, China).
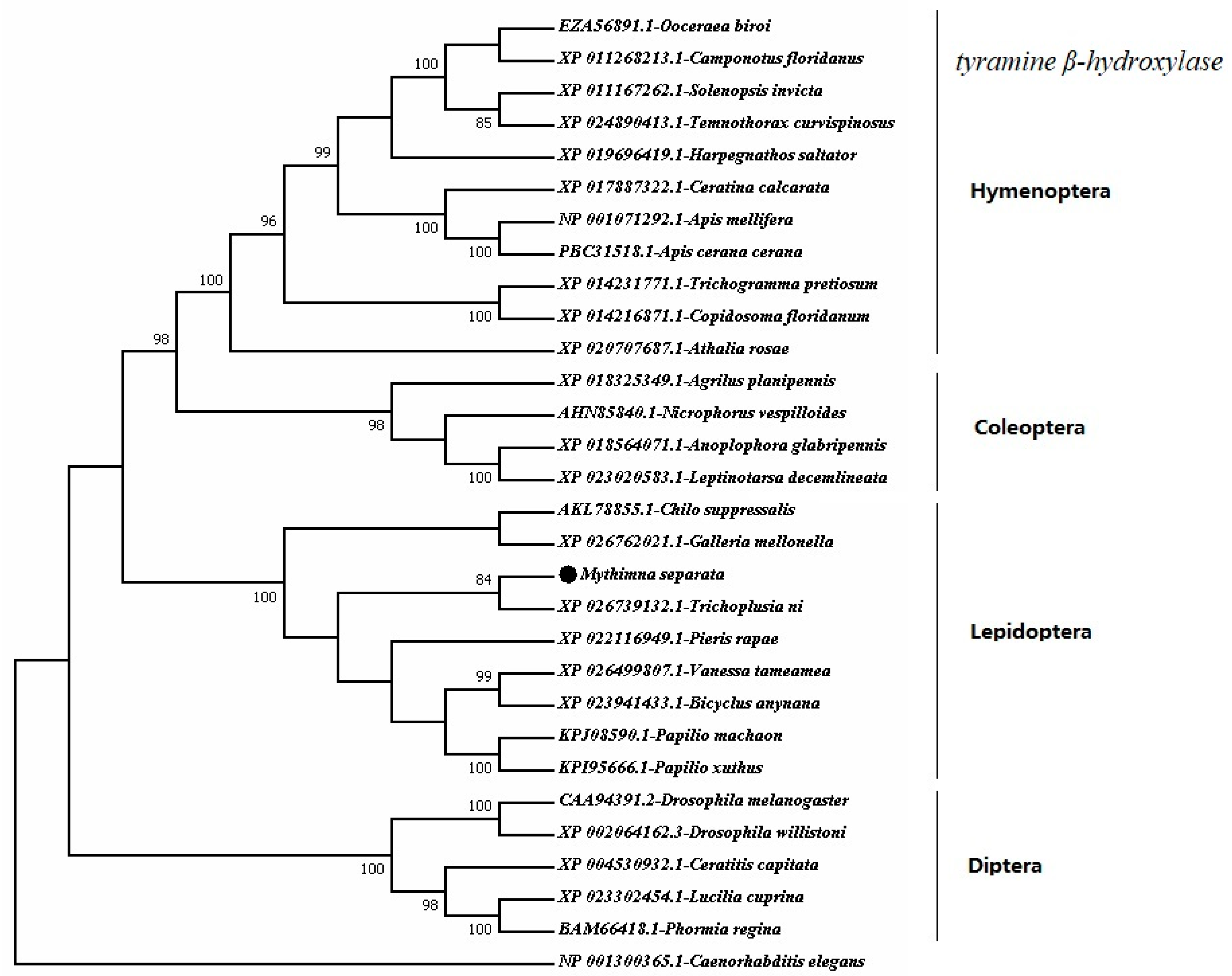
4.4. Bioinformatic Analysis
Sequence alignments were carried out while using the BLAST software (
http://www.ncbi.nlm.nih.gov). To investigate the phylogenetic relationship between
MsTβh and
Tβhs from other insect species, the amino acid sequences were aligned using the cluster algorithm ClustalW ver. 1.8, and a phylogenetic tree was constructed based on the alignment by MEGA 7.0 [
29].
4.5. Transcriptional Profiles at Different Larval Densities
The transcriptional level of the Tβh gene was evaluated in M. separata larvae that were reared at different densities using the previous methods as described above. All of the samples were snap frozen in liquid nitrogen before being stored at −80 °C for later use. The total RNA of larvae from different densities was extracted using a Trizol Kit (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was synthesized using an oligo (dT) 15 primer (Tiangen Biotech) and 1μg of total RNA as the template in a final volume of 10 μL. cDNA that was prepared from total RNA was used as the template for real-time quantitative PCR (qPCR) with an ABI PRISM 7500 Real-Time PCR System (Applied Biosystems Foster, CA, USA). MsTβh primers (F: CCAAGACCATGGCTTCATCAG; R: ACCAGTAGGTAGTGTCTCCT) were designed; β actin was selected as the reference gene (F: GCGACATCAAGGAGAAGCTC; and, R: TTCCGATGG TG AT GACTTGA).
Three biological replications were used for qPCR analysis while using a 20-µL total reaction system containing 0.1 µg total RNA, 0.8 µl primer mix containing 10 µM of each forward and reverse gene-specific primers, 0.4 µL ROX Reference Dye II (50×), 2 µL cDNA, 10 µl SYBR Premix EX TaqTM II, and 6 µL H
2O, following the One Step SYBR Premix Ex TaqTM II Kit instructions (Takara Biotechnology Dalian Co., Ltd., Dalian, China). The thermal cycling conditions were 15 min. at 95 °C, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and elongation at 72 °C for 35 s. Relative quantifications were calculated using the 2
−ΔΔCt method [
30].
4.6. RNA Interference of Tβh
Gene-specific primers tailed with T7 promotors were used to amplify the target region for the synthesis of double strand RNA (dsRNA) of Tβh. The amplified products were used as the templates in the synthesis of ds MsTβh using the Transcript Aid T7 High Yield Transcrition Kit (Thermo Fisher Scientific, Waltham, MA, USA) based on the manufacturer’s instructions. The 1% agarose gel electrophoresis tested the integrity of dsRNA, and a Nanophotometer NP80 measured the concentration (IMPLEN). The dsRNA was diluted with DEPC-treated water to four concentrations, 4000, 2000, 1000, and 500 ng/µL, from which 2000 ng/µL was selected as the final concentration, according to the mortality and efficiency of gene silencing data.
On the second day of the fourth larval instar, larvae from the density of 1 and 10 larvae per jar were used for the RNAi experiment. Three microgramsof
dsMsTβh or
dsGFP were injected into the proleg of larval third abdominal segment while using a microinjector. Four treatments were set as following: (1) one larva per jar (control); (2) ten larvae per jar; and, (3) ten larvae per jar + ds
GFP (4) ten larvae per jar + ds
Tβh. To confirm the dsRNA expressed, the differently treated larvae were used for the RNAi assessment by qRT-PCR after 72 h. The method of qRT-PCR was performed, as described above. For the RNAi, ds
GFP-injected larvae served as control.
Table 1 lists all of the primers used.
On the 72 h after dsRNA injection, the three aspects of immune function were assayed for treatment larvae. To perform the assay, the larvae were cooled to torpor on ice and then sterilized by swabbing with 75% ethanol. An abdominal proleg was cut with a fine scalpel, and haemolymph was collected with microcapillary pipette and then transferred into a 1.5-mL centrifuge tube on ice to prevent melanization. Haemolymph samples were collected from the injected larvae, and haemolymph from the same treated larvae were pooled as one biological replicate for the assays to determine PO and lysozyme activities and for haemocyte counts. Three biological replicates were established for every treatment. All of the samples were then frozen at −80 °C until evaluation for the immune function.
PO activity was assayed as described by Kong et al., (2018) [
9]. 50 µL haemolymph was added to 150 µL phosphate buffer (pH = 7) in a plastic tube. PO activity was assayed spectrophotometrically by adding 1500 µL 0.1M L-dopa and 1000 µL phosphate buffer (pH = 7) to 200 µL of haemolymph phosphate solution. Changes in the absorbance of the mixture within 3 min. at 492nm were measured while using a time-dependent program and a lambda 25UV/VIS spectrometer (Perkin Elmer Company, USA). The protein content was measured in the samples according to the method that was described by Bradford et al. (1976) [
31]. PO activity is expressed as PO units per milligram of protein, where 1U was the amount of enzyme that was required to increase the absorbance by 0.001min
−1. The total haemocyte count was determined by adding 5 µL haemolymph that had been stained with Giemsa for 5 min. to the counting chambers of a haemocytometer. The haemocytes in the central square and four corners were counted under phase-contrast illumination and then averaged to give an estimate of the number of haemocytes. Lysozyme activity was determined with a lysozyme kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the user’s instructions. First, 0.2mL haemolymph was mixed in a test tube with 2mL bacterial culture medium, and the two absorbance values at 530nm were determined in a UV-2000 spectrophotometer (Unico Instrument Company of Shanghai, Shanghai, China) after 20 and 140 s, respectively. The lysozyme activity was determined by the two values, according to the user’s instructions.
For resistance bioassays after RNAi, four groups of 180 different treatment larvae were exposed to the LC50 dose of B. bassiana (3.6 × 107 spore per mL) by collectively dipping them in the spore solution for 5s, followed by air-drying at room temperature. The survival rate was recorded daily until all larvae either died or pupated.
4.7. Data Analysis
Data for different density treatments to test larval enzyme activity, gene expression, and RNA interference were analyzed using one-way analysis of variance (ANOVA), with the Duncan’s multiple range test for multiple testing. All of the statistical analyses were performed with SPSS 10.0.