Muscle-Saturated Bioactive Lipids Are Increased with Aging and Influenced by High-Intensity Interval Training
Abstract
1. Introduction
2. Results
2.1. Subject Characteristics
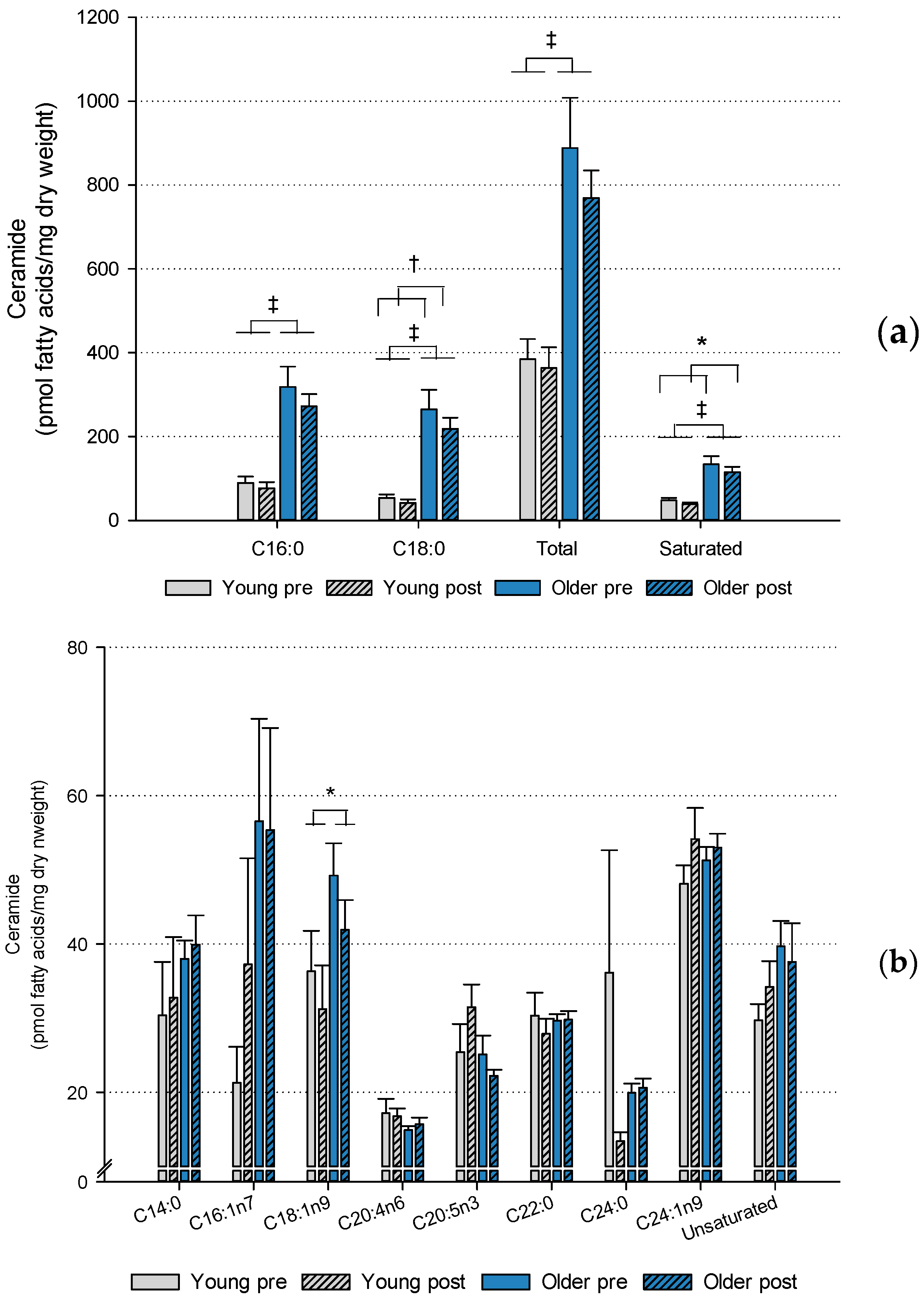
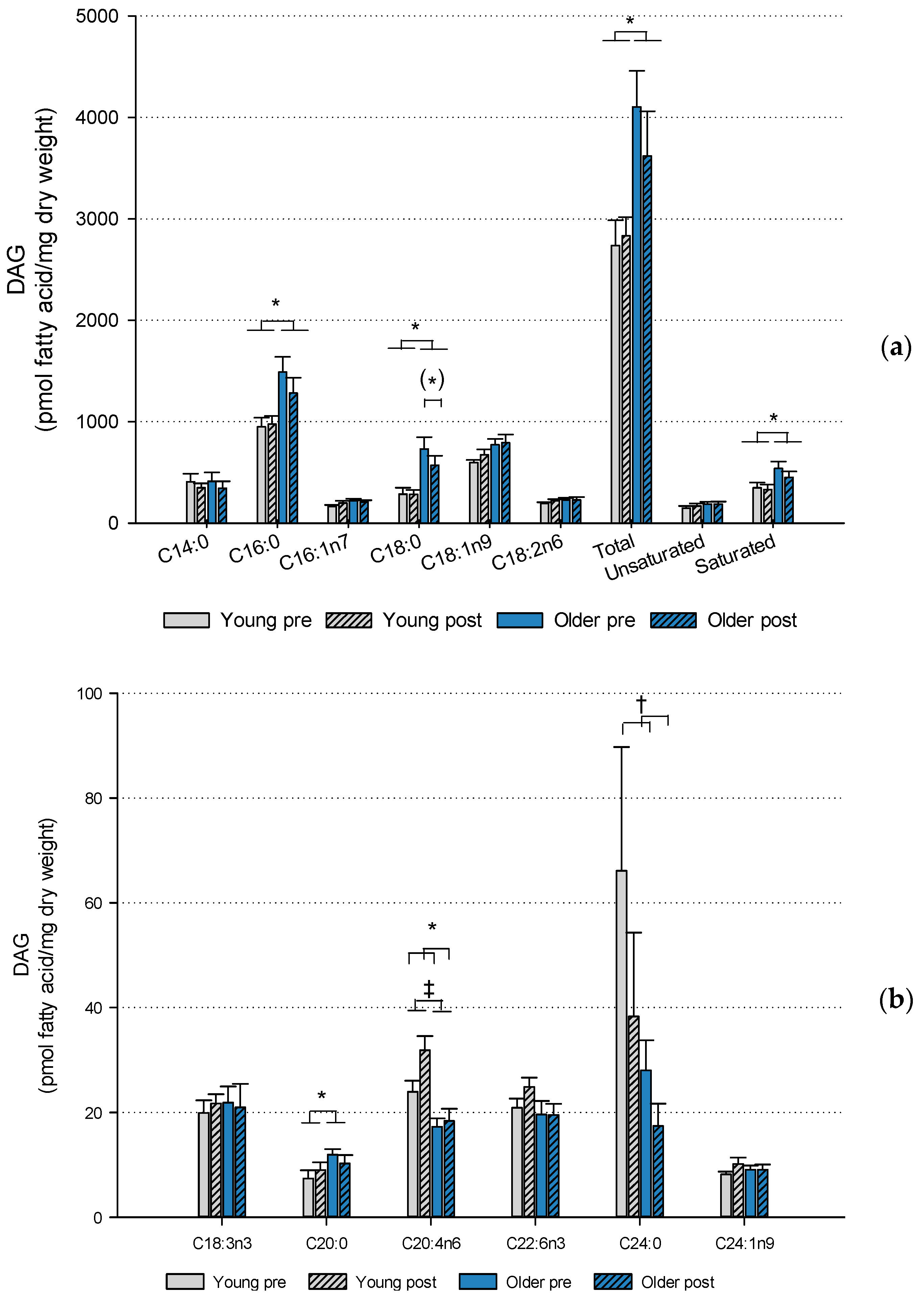
2.2. Muscle Lipids and Glycogen
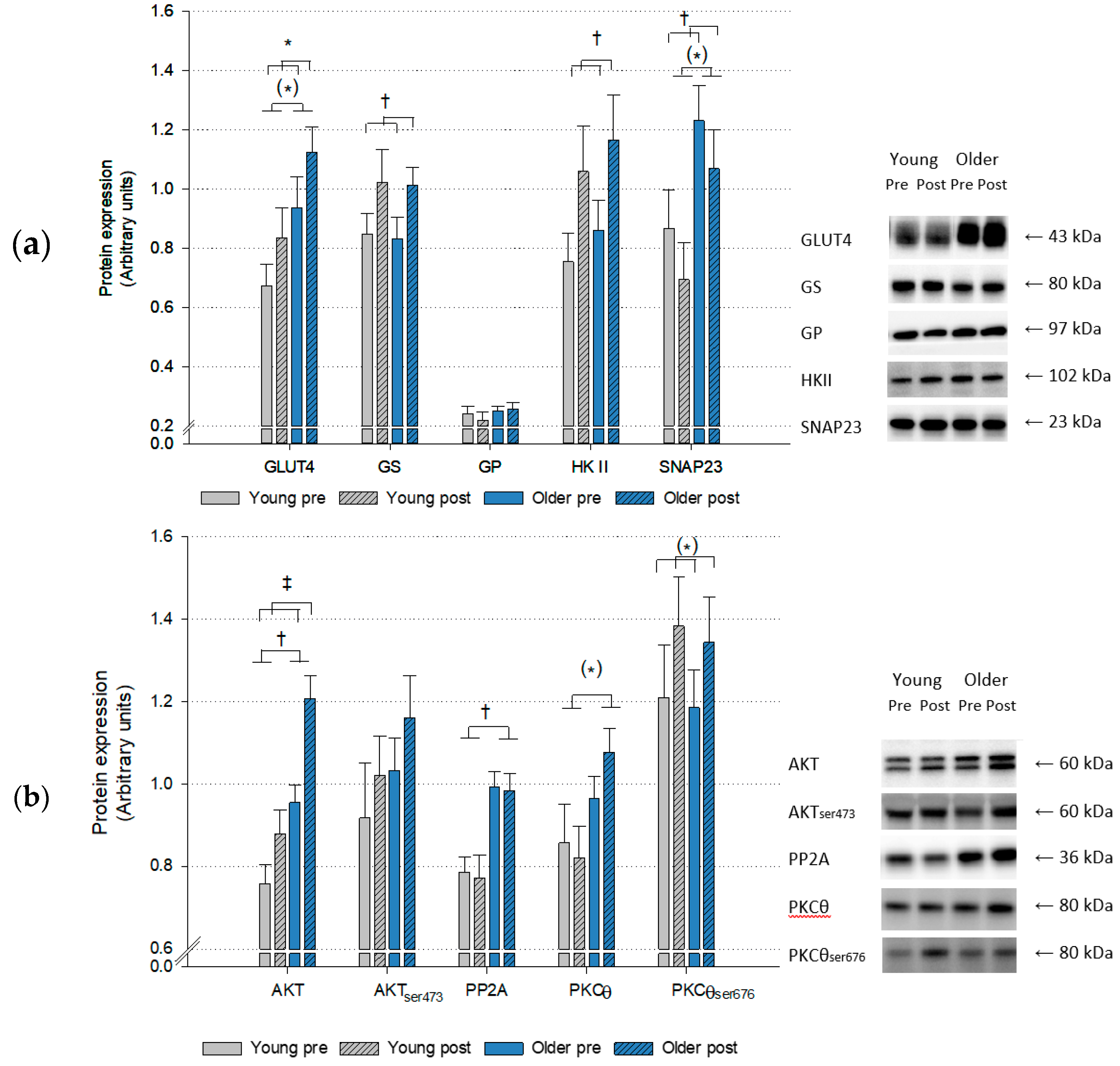
2.3. Protein Expression
3. Discussion
Limitations
4. Methods and Materials
4.1. Subjects
4.2. Study Design
4.3. High-Intensity Interval Training (HIIT) Protocol
4.4. Maximal Oxygen Uptake
4.5. Blood Analyses
4.6. Muscle Biopsies
4.7. Lipid Analyses
4.8. Intramyocellular Triglyceride and Glycogen
4.9. Citrate Synthase and β-Hydroxyacyl-CoA Dehydrogenase
4.10. Western Blot
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dela, F.; Mikines, K.J.; Larsen, J.J.; Glabo, H. Training-induced enhancement of insulin action in human skeletal muscle: The influence of aging. J. Gerontol. A Biol. Sci. Med. Sci. 1996, 51, B247–B252. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritschevsky, S.B.; Nevitt, M.; Schwarts, A.V.; Simonsick, E.M.; Tylavsky, A.V.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Bunprajun, T.; Henriksen, T.I.; Scheele, C.; Pedersen, B.K.; Green, C.J. Lifelong Physical Activity Prevents Aging-Associated Insulin Resistance in Human Skeletal Muscle Myotubes via Increased Glucose Transporter Expression. PLoS ONE 2013, 8, e66628. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Glickman, S.G.; Dengel, D.R.; Brown, M.D.; Supiano, M.A. Abdominal adiposity assessed by dual energy X-ray absorptiometry provides a sex-independent predictor of insulin sensitivity in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Roubenoff, R.; Wood, M.; Frontera, W.R.; Evans, W.J.; Fiatarone Singh, M.A. Anthropometric assessment of 10-y changes in body composition in the elderly. Am. J. Clin. Nutr. 2004, 80, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, W.M.; Kirwan, J.P.; Staten, M.A.; Bourey, R.E.; Kings, D.S.; Holloszy, J.S. Insulin resistance in aging is related to abdominal obesity. Diabetes 1993, 42, 273–281. [Google Scholar] [CrossRef]
- Lalia, A.Z.; Dazari, S.; Johnson, M.L.; Robinson, M.M.; Konopka, A.R.; Distelmaier, K.; Port, J.D.; Glavin, M.T.; Esponda, R.R.; Nair, K.S.; et al. Predictors of Whole-Body Insulin Sensitivity Across Ages and Adiposity in Adult Humans. J. Clin. Endocrinol. Metab. 2016, 101, 626–634. [Google Scholar] [CrossRef]
- Petersen, M.C.; Jurczak, M.J. CrossTalk opposing view: Intramyocellular ceramide accumulation does not modulate insulin resistance. J. Physiol. 2016, 594, 3171–3174. [Google Scholar] [CrossRef]
- Summers, S.A.; Goodpaster, B.H. CrossTalk proposal: Intramyocellular ceramide accumulation does modulate insulin resistance. J. Physiol. 2016, 594, 3167–3170. [Google Scholar] [CrossRef] [PubMed]
- Montell, E.; Turini, M.; Marotta, M.; Roberts, M.; Noe, V.; Ciudad, C.J.; Mace, K.; Gomez-Foix, A.M. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E229–E237. [Google Scholar] [CrossRef] [PubMed]
- Stratford, S.; Hoehn, K.L.; Lui, F.; Summers, S.A. Regulation of insulin action by ceramide: Dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 2004, 279, 36608–36615. [Google Scholar] [CrossRef]
- Griffin, M.E.; Marcucci, M.J.; Cline, G.W.; Bell, K.; Barruci, N.; Lee, D.; Goodyear, L.J.; Kraegen, E.W.; White, M.F.; Shulman, G.I. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 1999, 48, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Dzienis-Straczkowska, S.; Kinalska, I.; Baranowski, M.; Zendzian-Piotrowska, M.; Brzezinska, Z.; Gorski, J. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 2004, 53, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Sidall, P.; Wei, T.; Thomas, M.K.; et al. Muscle sphingolipids during rest and exercise: A C18:0 signature for insulin resistance in humans. Diabetologia 2016, 59, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.; Shannon, C.E.; Burns, A.; Selby, A.L.; Wilkinson, D.; Smith, K.; Greenhaff, P.L.; Stephens, F.B. Relative Contribution of Intramyocellular Lipid to Whole-Body Fat Oxidation Is Reduced With Age but Subsarcolemmal Lipid Accumulation and Insulin Resistance Are Only Associated With Overweight Individuals. Diabetes 2016, 65, 840–850. [Google Scholar] [CrossRef]
- Moro, C.; Galgani, J.E.; Luu, L.; Pasarica, M.; Mairal, M.; Bajpeyi, S.; Schmitz, G.; Langin, D.; Liebisch, G.; Smith, S.R. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J. Clin. Endocrinol. Metab. 2009, 94, 3440–3447. [Google Scholar] [CrossRef]
- Skovbro, M.; Baranowski, M.; Skov-Jensen, C.; Flint, A.; Dela, F.; Gorski, J.; Helge, J.W. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 2008, 51, 1253–1260. [Google Scholar] [CrossRef]
- Coen, P.M.; Hames, K.C.; Leachman, E.M.; DeLany, J.P.; Ritov, V.B.; Menshikova, E.V.; Dube, J.J.; Stefanovic-Racic, M.; Toledo, F.G.; Goodpaster, B.H. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity 2013, 21, 2362–2371. [Google Scholar] [CrossRef]
- Straczkowski, M.; Kowalska, I.; Baranowski, M.; Nikolajuk, A.; Otziomek, E.; Zabielski, P.; Adamska, A.; Blachnio, A.; Gorski, J.; Gorska, M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 2007, 50, 2366–2373. [Google Scholar] [CrossRef]
- Sogaard, D.; Baranowski, M.; Dela, F.; Helge, J.W. The influence of age and cardiorespiratory fitness on bioactive lipids in muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2018. [Google Scholar] [CrossRef]
- Rivas, D.A.; Morris, E.P.; Harran, P.H.; Pasha, E.P.; Morais Mda, S.; Dolnikowski, G.G.; Philips, E.M.; Fielding, R.A. Increased ceramide content and NFkappaB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J. Appl. Physiol. 2012, 113, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Dela, F.; Mikines, K.J.; Sonne, B.; Galbo, H. Effect of training on interaction between insulin and exercise in human muscle. J. Appl. Physiol. 1994, 76, 2386–2393. [Google Scholar] [CrossRef]
- Gan, S.K.; Kriketos, A.D.; Ellis, B.A.; Thompson, C.H.; Kraegen, E.W.; Chisholm, D.J. Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care 2003, 26, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Thrush, A.B.; Mertz, V.A.; Bezaire, V.; Chabowski, A.; Heigenhauser, G.J.; Dyck, D.J. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E99–E107. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; Brown, A.W.; Bohan Brown, M.M.; Alcorn, A.; Noles, C.; Winwood, L.; Resuehr, H.; George, B.; Jeansonne, M.M.; Allison, D.B. High Intensity Interval- vs Moderate Intensity- Training for Improving Cardiometabolic Health in Overweight or Obese Males: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0138853. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Friesen, A.; Chahal, B.S.; Bell, G.J.; McCargar, L.J.; Boule, N.G. Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 99, 120–129. [Google Scholar] [CrossRef]
- Martins, C.; Kasakova, I.; Ludviksen, M.; Mehus, I.; Wisloff, U.; Kulseng, B.; Morgan, L.; King, B. High-Intensity Interval Training and Isocaloric Moderate-Intensity Continuous Training Result in Similar Improvements in Body Composition and Fitness in Obese Individuals. Int. J. Sport Nutr. Exerc. Metab. 2015, 26, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.C.; Johnson, T.K.; Kuzma, J.N.; Lonac, M.C.; Schweder, M.M.; Voyles, W.F.; Bell, C. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to beta-adrenergic stimulation. J. Physiol. 2010, 588, 2961–2972. [Google Scholar] [CrossRef]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Nair, K.S. Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab. 2017, 25, 581–592. [Google Scholar] [CrossRef]
- Arad, A.D.; DiMenna, F.J.; Thomas, N.; Tamis-Holland, J.; Weil, R.; Geliebter, A.; Albu, J.B. High-intensity interval training without weight loss improves exercise but not basal or insulin-induced metabolism in overweight/obese African American women. J. Appl. Physiol. 2015, 119, 352–362. [Google Scholar] [CrossRef]
- Bonen, A.; Parolin, M.L.; Steinberg, G.R.; Calles-Escandon, J.; Tandon, N.N.; Glatz, J.F.; Luiken, J.J.; Heigenhauser, G.J.; Dyck, D.J. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004, 18, 1144–1146. [Google Scholar] [CrossRef]
- Bruce, C.R.; Risis, S.; Babb, J.R.; Yang, C.; Kowalski, G.M.; Selathurai, A.; Lee-Young, R.S.; Weir, J.R.; Yoshioka, K.; Takuwa, Y.; et al. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes 2012, 61, 3148–3155. [Google Scholar] [CrossRef]
- Tonks, K.T.; Coster, A.C.; Christopher, M.J.; Chaudhuri, R.; Xu, A.; Gagnon-Bartsch, J.; Chisholm, D.J.; James, D.E.; Meikle, P.J.; Greenfield, J.R.; et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity 2016, 24, 908–916. [Google Scholar] [CrossRef]
- Bergman, B.C.; Hunerdosse, D.M.; Kerege, A.; Playdon, M.C.; Perreault, L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 2012, 55, 1140–1150. [Google Scholar] [CrossRef]
- Itani, S.I.; Pories, W.J.; MacDonald, K.G.; Dohm, G.L. Increased protein kinase C theta in skeletal muscle of diabetic patients. Metabolism 2001, 50, 553–557. [Google Scholar] [CrossRef]
- Coen, P.M.; Dube, J.J.; Amati, F.; Stefanovic-Racic, M.; Ferrell, R.E.; Toledo, F.G.; Goodpaster, G.H. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 2010, 59, 80–88. [Google Scholar] [CrossRef]
- Amati, F.; Dube, J.J.; Alvarez-Carnero, E.; Edreira, M.M.; Chomentowski, P.; Coen, P.M.; Switzer, G.E.; Bickel, P.E.; Stefanovic-Racic, M.; Toledo, F.G.; et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes 2011, 60, 2588–2597. [Google Scholar] [CrossRef]
- Dube, J.J.; Amati, F.; Toledo, F.G.; Stefanovic-Racic, M.; Rossi, A.; Coen, P.; Goodpaster, B.H. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 2011, 54, 1147–1156. [Google Scholar] [CrossRef]
- Dube, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef]
- Louche, K.; Badin, P.M.; Montastier, E.; Laurens, C.; Bourlier, V.; de Glisezinski, I.; Thalamas, C.; Viguerie, N.; Langin, D.; Moro, C. Endurance exercise training up-regulates lipolytic proteins and reduces triglyceride content in skeletal muscle of obese subjects. J. Clin. Endocrinol. Metab. 2013, 98, 4863–4871. [Google Scholar] [CrossRef]
- Devries, M.C.; Samjoo, I.A.; Hamadeh, M.J.; McCready, C.; Raha, S.; Watt, M.J.; Steinberg, G.R.; Tarnapolsky, M.A. Endurance training modulates intramyocellular lipid compartmentalization and morphology in skeletal muscle of lean and obese women. J. Clin. Endocrinol. Metab. 2013, 98, 4852–4862. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Menshikova, E.V.; Distefano, G.; Zheng, D.; Tanner, C.J.; Standley, R.A.; Helbling, N.L.; Dubis, G.S.; Ritov, V.B.; Xie, H.; et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning, and Insulin Sensitivity After Gastric Bypass Surgery. Diabetes 2015, 64, 3737–3750. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.S.; Little, J.P.; Tarnapolsky, M.A.; Myslik, F.; Gibala, M.J. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med. Sci. Sports Exerc. 2011, 43, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl. Physiol. Nutr. Metab. 2008, 33, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Cermak, N.M.; Philips, S.M.; Benton, C.R.; Bonen, A.; Gibala, M.J. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am. J. Physiol. Regul. Integr. Comp Physiol. 2007, 292, R1970–R1976. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.; Kenno, K.A.; Milne, K.J. The effects of a 2 week modified high intensity interval training program on the homeostatic model of insulin resistance (HOMA-IR) in adults with type 2 diabetes. J. Sports Med. Phys. Fitness 2014, 54, 203–209. [Google Scholar] [PubMed]
- Skleryk, J.R.; Karagounis, L.G.; Hawley, J.A.; Sharman, M.J.; Laursen, P.B.; Watson, G. Two weeks of reduced-volume sprint interval or traditional exercise training does not improve metabolic functioning in sedentary obese men. Diabetes Obes. Metab. 2013, 15, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Sogaard, D.; Lund, M.T.; Scheuer, C.M.; Dehlbaek, M.S.; Dideriksen, S.G.; Abildskov, C.V.; Christensen, K.K.; Dohlmann, T.L.; Larsen, S.; Vigelso, A.H.; et al. High-intensity interval training improves insulin sensitivity in older individuals. Acta Physiol. 2018, 222, e13009. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, J. Muscle-biopsy needles. Lancet 1979, 1, 153. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Yano, M.; Kishida, E.; Muneyuki, Y.; Masusawa, Y. Quantitative analysis of ceramide molecular species by high performance liquid chromatography. J. Lipid Res. 1998, 39, 2091–2098. [Google Scholar] [PubMed]
- Nawrocki, A.; Gorski, J. Effect of plasma free fatty acid concentration on the content and composition of the free fatty acid fraction in rat skeletal muscles. Horm. Metab. Res. 2004, 36, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Maycock, P.F. Skeletal muscle triacylglycerol in the rat: Methods for sampling and measurement, and studies of biological variability. J. Lipid Res. 1980, 21, 139–144. [Google Scholar] [PubMed]
- Kiens, B.; Richter, E.A. Types of carbohydrate in an ordinary diet affect insulin action and muscle substrates in humans. Am. J. Clin. Nutr. 1996, 63, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Danielsen, J.H.; Sondergard, S.D.; Sogaard, D.; Vigelsoe, A.; Dybboe, R.; Skaaby, S.; Dela, F.; Helge, J.W. The effect of high-intensity training on mitochondrial fat oxidation in skeletal muscle and subcutaneous adipose tissue. Scand. J. Med. Sci. Sports 2015, 25, e59–e69. [Google Scholar] [CrossRef] [PubMed]
- Sogaard, D.; Ostergard, T.; Blachnio-Zabielska, A.U.; Baranowski, M.; Vigelso, A.H.; Andersen, J.L.; Dela, F.; Helge, J.W. Training Does Not Alter Muscle Ceramide and Diacylglycerol in Offsprings of Type 2 Diabetic Patients Despite Improved Insulin Sensitivity. J. Diabetes Res. 2016, 2016, 2372741. [Google Scholar] [CrossRef] [PubMed]

| Young (n = 14) Pre Post | Older (n = 22) Pre Post | Main Effect (p-value) Age Time | Interaction (p-value) Group x Time | ||||
|---|---|---|---|---|---|---|---|
| Gender (F/M) | 5/9 | 11/11 | |||||
| Age (yrs) | 32 ± 2 | 63 ± 1 | |||||
| Height (m) | 1.78 ± 0.02 | 1.70 ± 0.02 | 0.014 | NS | NS | ||
| Weight (kg) | 110 ± 4 | 110 ± 4 | 88.7 ± 2.6 | 88.4 ± 2.6 | <0.001 | NS | NS |
| BMI (kg·m−2) | 34.8 ± 1.0 | 34.6 ± 1.0 | 30.7 ± 0.7 | 30.6 ± 0.7 | 0.003 | NS | NS |
| LBM (kg) | 63.8 ± 2.1 | 64.7 ± 2.3 | 51.5 ± 2.1 | 51.8 ± 2.1 | <0.001 | <0.001 | 0.099 |
| Fat mass (kg) | 40.3 ± 3.1 | 39.3 ± 3.3 | 34.0 ± 1.6 | 33.3 ± 1.7 | NS | 0.016 | NS |
| Fat % | 39.2 ± 2.1 | 38.2 ± 2.3 | 39.8 ± 1.6 | 39.1 ± 1.6 | NS | <0.001 | NS |
| Visceral fat (kg) | 1.67 ± 0.25 | 1.56 ± 0.24 | 1.90 ± 0.16 | 1.81 ± 0.16 | NS | 0.024 | NS |
| HbA1c (%) | 5.3 ± 0.1 | 5.3 ± 0.1 | 5.7 ± 0.1 | 5.6 ± 0.1 | 0.002 | NS | NS |
| HOMA-IR (AU) | 2.14 ± 0.24 | 2.31 ± 0.38 | 1.88 ± 0.23 | 1.99 ± 0.30 | NS | NS | NS |
| Glucose, fasting (mmol·L−1) | 4.5 ± 0.1 | 4.5 ± 0.1 | 6.1 ± 0.2 | 6.0 ± 0.2 | <0.001 | NS | NS |
| Insulin, fasting (pmol L−1) | 69.7 ± 9,5 | 67.2 ± 8.9 | 40.9 ± 4.8 | 42.6 ± 5.8 | 0.008 | NS | NS |
| IMTG (mmol·kg−1 dw) | 126 ± 27 | 118 ± 21 | 156 ± 23 | 119 ± 12 | NS | NS | NS |
| Glycogen (nmol·kg−1 dw) | 236 ± 30 | 474 ± 46 | 323 ± 24 | 483 ± 23 | NS | <0.001 | NS |
| HAD (µmol·g−1·min−1) | 116 ± 7 | 130 ± 7 | 112 ± 10 | 141 ± 5 | NS | <0.001 | NS |
| CS (µmol·g−1·min−1) | 132 ± 7 | 165 ± 8 | 122 ± 10 | 169 ± 10 | NS | <0.001 | NS |
| VO2max (mL·min−1) | 3068 ± 131 | 3186 ± 118 | 2234 ± 106 | 2361 ± 134 | <0.001 | 0.021 | NS |
| VO2max (mL·min−1·kg−1) | 28.3 ± 1.2 | 29.7 ± 1.5 | 25.2 ± 1.0 | 26.7 ± 1.1 | NS | 0.007 | NS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Søgaard, D.; Baranowski, M.; Larsen, S.; Taulo Lund, M.; Munk Scheuer, C.; Vestergaard Abildskov, C.; Greve Dideriksen, S.; Dela, F.; Wulff Helge, J. Muscle-Saturated Bioactive Lipids Are Increased with Aging and Influenced by High-Intensity Interval Training. Int. J. Mol. Sci. 2019, 20, 1240. https://doi.org/10.3390/ijms20051240
Søgaard D, Baranowski M, Larsen S, Taulo Lund M, Munk Scheuer C, Vestergaard Abildskov C, Greve Dideriksen S, Dela F, Wulff Helge J. Muscle-Saturated Bioactive Lipids Are Increased with Aging and Influenced by High-Intensity Interval Training. International Journal of Molecular Sciences. 2019; 20(5):1240. https://doi.org/10.3390/ijms20051240
Chicago/Turabian StyleSøgaard, Ditte, Marcin Baranowski, Steen Larsen, Michael Taulo Lund, Cathrine Munk Scheuer, Carina Vestergaard Abildskov, Sofie Greve Dideriksen, Flemming Dela, and Jørn Wulff Helge. 2019. "Muscle-Saturated Bioactive Lipids Are Increased with Aging and Influenced by High-Intensity Interval Training" International Journal of Molecular Sciences 20, no. 5: 1240. https://doi.org/10.3390/ijms20051240
APA StyleSøgaard, D., Baranowski, M., Larsen, S., Taulo Lund, M., Munk Scheuer, C., Vestergaard Abildskov, C., Greve Dideriksen, S., Dela, F., & Wulff Helge, J. (2019). Muscle-Saturated Bioactive Lipids Are Increased with Aging and Influenced by High-Intensity Interval Training. International Journal of Molecular Sciences, 20(5), 1240. https://doi.org/10.3390/ijms20051240





