Plasma Levels of Preβ1-HDL Are Significantly Elevated in Non-Dialyzed Patients with Advanced Stages of Chronic Kidney Disease
Abstract
1. Introduction
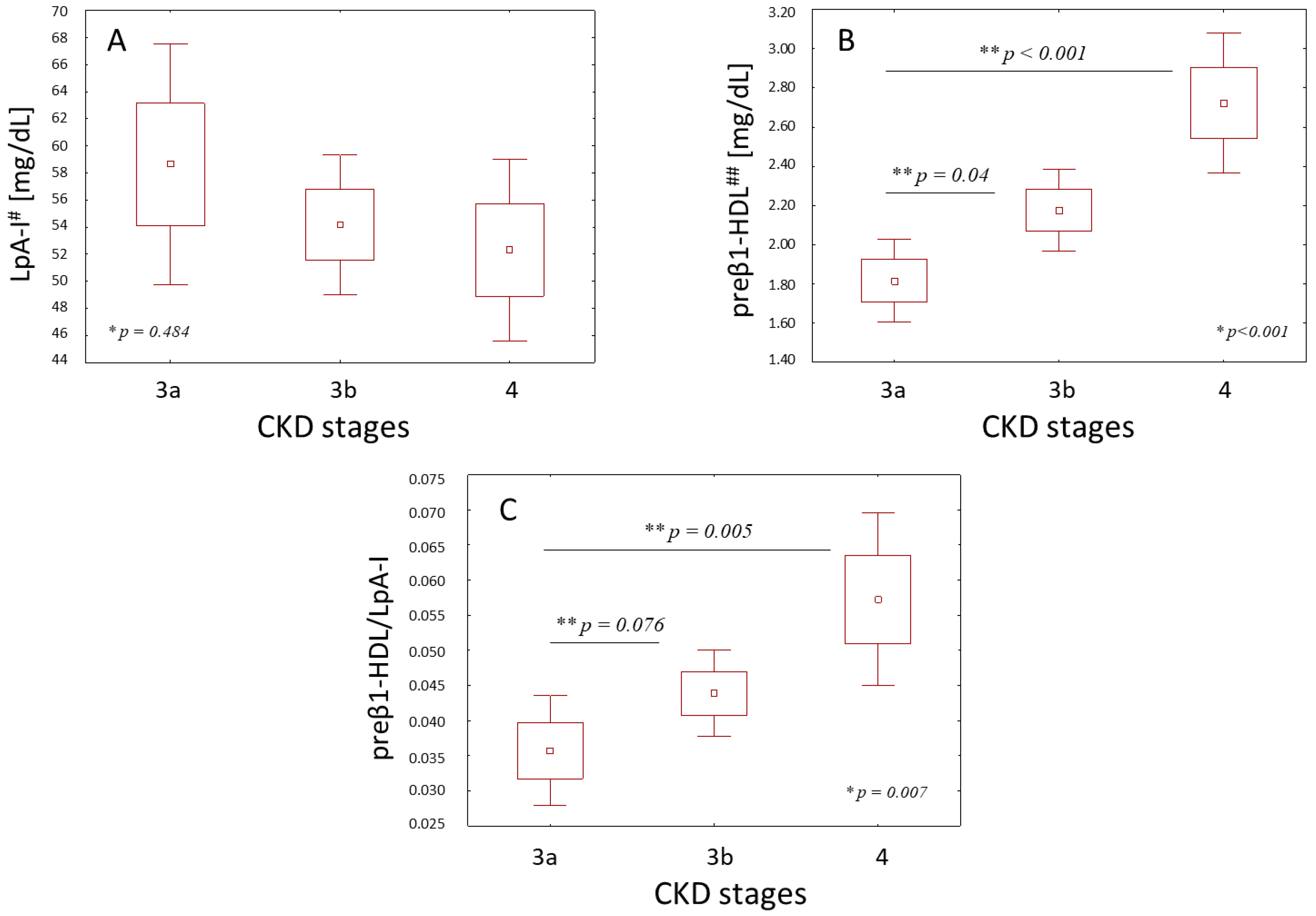
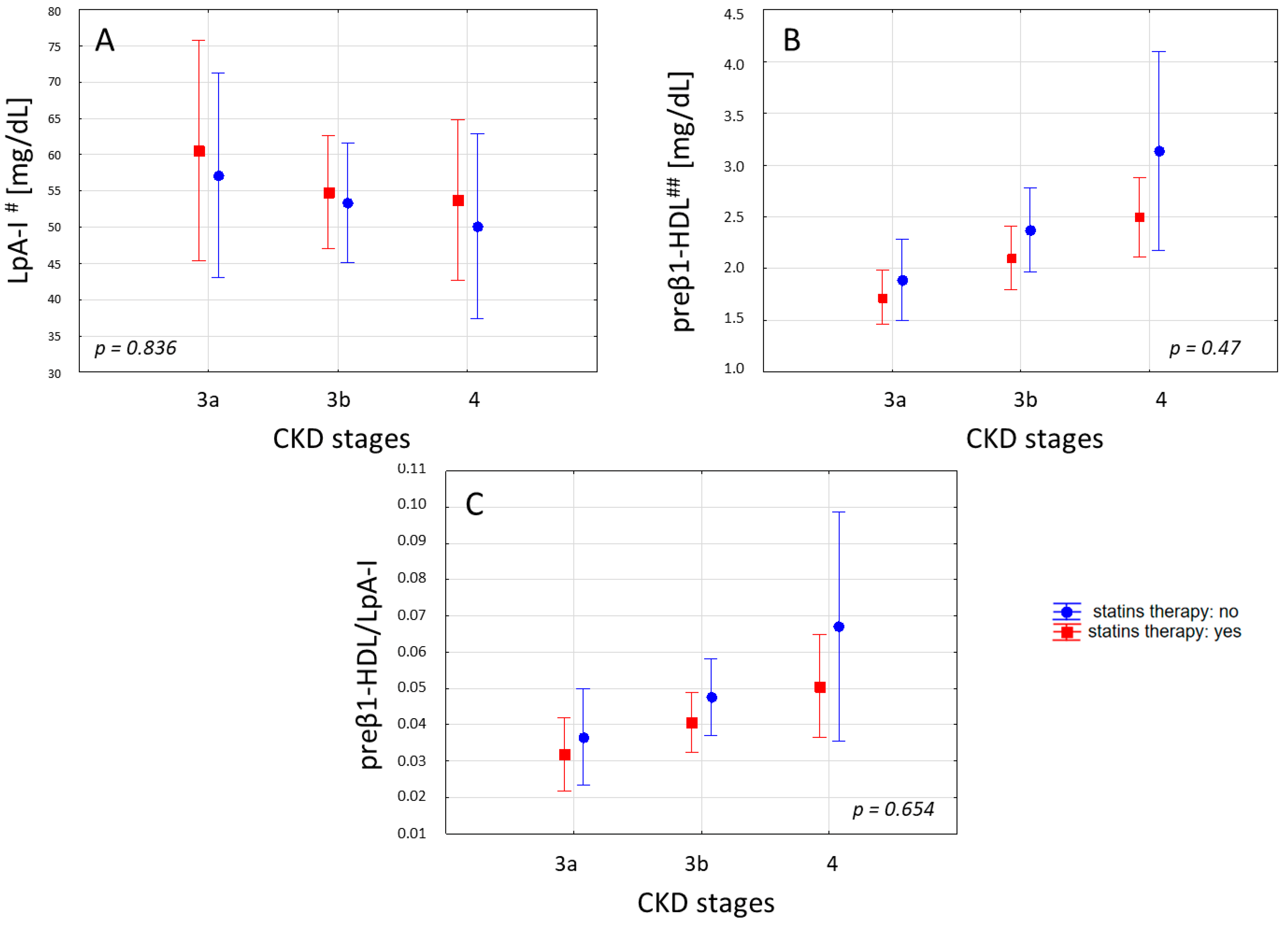
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Laboratory Measurements
4.3. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 13, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Gluba-Brzozka, A.; Michalska-Kasiczak, M.; Franczyk-Skora, B.; Nocun, M.; Banach, M.; Rysz, J. Markers of increased cardiovascular risk in patients with chronic kidney disease. Lipids Health Dis. 2014, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Gronda, E.; Genovese, S.; Padeletti, L.; Cacciatore, F.; Vitale, D.F.; Bragato, R.; Innocenti, L.; Schiano, C.; Sommese, L.; De Pascale, M.R.; et al. Renal function impairment predicts mortality in patients with chronic heart failure treated with resynchronization therapy. Cardiol. J. 2015, 4, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Navab, M.; Fogelman, A.M. HDL metabolism and activity in chronic kidney disease. Nat. Rev. Nephrol. 2010, 5, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Voloshyna, I.; DeLeon, J.; Miyawaki, N.; Mattana, J. Cholesterol Metabolism in CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2015, 6, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Vaziri, N.D.; Said, H.M.; Kalantar-Zadeh, K. Role of HDL dysfunction in end-stage renal disease: A doubl-edeged sword. J. Ren. Nutr. Off. J. Council Ren. Nutr. Natl. Kidney Found. 2013, 3, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Mangé, A.; Goux, A.; Badiou, S.; Patrier, L.; Canaud, B.; Maudelonde, T.; Cristol, J.-P.; Solassol, J. HDL Proteome in Hemodialysis Patients: A Quantitative Nanoflow Liquid Chromatography-Tandem Mass Spectrometry Approach. PLoS ONE 2012, 3, e34107. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Birner-Gruenberger, R.; Stojakovic, T.; El-Gamal, D.; Binder, V.; Wadsack, C.; Heinemann, A.; Marsche, G. Uremia Alters HDL Composition and Function. J. Am. Soc. Nephrol. JASN 2011, 9, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Sacks, F.M.; Salvini, S.; Willett, W.C.; Hennekens, C.H. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N. Engl. J. Med. 1991, 6, 373–381. [Google Scholar] [CrossRef]
- Luc, G.; Bard, J.M.; Ferrières, J.; Evans, A.; Amouyel, P.; Arveiler, D.; Fruchart, J.C.; Ducimetière, P. Value of HDL cholesterol, apolipoprotein A-I, lipoprotein A-I, and lipoprotein A-I/A-II in prediction of coronary heart disease: The PRIME Study. Prospective Epidemiological Study of Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2002, 7, 1155–1161. [Google Scholar] [CrossRef]
- Puchois, P.; Kandoussi, A.; Fievet, P.; Fourrier, J.L.; Bertrand, M.; Koren, E.; Fruchart, J.C. Apolipoprotein A-I containing lipoproteins in coronary artery disease. Atherosclerosis 1987, 68, 35–40. [Google Scholar] [CrossRef]
- Sethi, A.A.; Sampson, M.; Warnick, R.; Muniz, N.; Vaisman, B.; Nordestgaard, B.G.; Tybjaerg-Hansen, A.; Remaley, A.T. High pre-beta1 HDL concentrations and low lecithin: Cholesterol acyltransferase activities are strong positive risk markers for ischemic heart disease and independent of HDL-cholesterol. Clin. Chem. 2010, 7, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Miyazaki, O.; Nakamura, Y.; Miyazaki, A.; Fukamachi, I.; Bujo, H.; Saito, Y. Plasma pre beta1-HDL level is elevated in unstable angina pectoris. Atherosclerosis 2009, 2, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Miida, T.; Miyazaki, O.; Hanyu, O.; Nakamura, Y.; Hirayama, S.; Narita, I.; Gejyo, F.; Ei, I.; Tasaki, K.; Kohda, Y.; et al. LCAT-dependent conversion of prebeta1-HDL into alpha-migrating HDL is severely delayed in hemodialysis patients. J. Am. Soc. Nephrol. 2003, 3, 732–738. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr. Opin. Lipidol. 2010, 4, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Nukuna, B.; Brennan, M.L.; Sun, M.; Goormastic, M.; Settle, M.; Schmitt, D.; Fu, X.; Thomson, L.; Fox, P.L.; et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Investig. 2004, 4, 529–541. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Z.; Riwanto, M.; Gao, S.; Levison, B.S.; Gu, X.; Fu, X.; Wagner, M.A.; Besler, C.; Gerstenecker, G.; et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J. Clin. Investig. 2013, 9, 3815–3828. [Google Scholar] [CrossRef]
- Matsushita, K.; Van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 9731, 2073–2081. [Google Scholar]
- Kuchta, A.; Strzelecki, A.; Ćwiklińska, A.; Gruchała, M.; Zdrojewski, Z.; Kortas-Stempak, B.; Wieczorek, E.; Gliwińska, A.; Dąbkowski, K.; Jankowski, M. HDL subpopulations containing apoA-I without apoA-II (LpA-I) in patients with angiographically proven coronary artery disease. J. Cardiol. 2017, 3, 523–528. [Google Scholar] [CrossRef]
- Bu, X.-M.; Niu, D.-M.; Wu, J.; Yuan, Y.-L.; Song, J.-X.; Wang, J.-J. Elevated levels of preβ1-high-density lipoprotein are associated with cholesterol ester transfer protein, the presence and severity of coronary artery disease. Lipids Health Dis. 2017, 16, 4. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, J.; Chen, X.; Jiang, H.; Bakillah, A.; Zhang, X.; Li, Z.; Yin, J.; Liang, D.; Zou, Y.; et al. Human serum preβ1-high density lipoprotein levels are independently and negatively associated with coronary artery diseases. Nutr. Metab. 2016, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Liang, K.; Parks, J.S. Down-regulation of hepatic lecithin:cholesterol acyltransferase gene expression in chronic renal failure. Kidney Int. 2001, 6, 2192–2196. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, L.; Simonelli, S.; Conca, P.; Busnach, G.; Cabibbe, M.; Gesualdo, L.; Gigante, M.; Penco, S.; Veglia, F.; Franceschini, G. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J. Intern. Med. 2015, 5, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Demissie, S.; Cupples, L.A.; Collins, D.; Cox, C.E.; Horvath, K.V.; Bloomfield, H.E.; Robins, S.J.; Schaefer, E.J. LpA-I, LpA-I:A-II HDL and CHD-risk: The Framingham Offspring Study and the Veterans Affairs HDL Intervention Trial. Atherosclerosis 2006, 1, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Hattori, S.; Nishiyama, S.; Higashi, A.; Matsuda, I. Quantitative and qualitative changes of apolipoprotein AI-containing lipoproteins in patients on continuous ambulatory peritoneal dialysis. Metabolism 1989, 9, 843–849. [Google Scholar] [CrossRef]
- Durrington, P.N.; Mackness, B.; Mackness, M.I. Paraoxonase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001, 4, 473–480. [Google Scholar] [CrossRef]
- Miljkovic, M.; Stefanovic, A.; Vekic, J.; Zeljkovic, A.; Gojkovic, T.; Simic-Ogrizovic, S.; Bogavac-Stanojevic, N.; Cerne, D.; Ilic, J.; Stefanovic, I.; et al. Activity of paraoxonase 1 (PON1) on HDL2 and HDL3 subclasses in renal disease. Clin. Biochem. 2018, 60, 52–58. [Google Scholar] [CrossRef]
- Malle, E.; Marsche, G.; Panzenboeck, U.; Sattler, W. Myeloperoxidase-mediated oxidation of high-density lipoproteins: Fingerprints of newly recognized potential proatherogenic lipoproteins. Arch. Biochem. Biophys. 2006, 2, 245–255. [Google Scholar] [CrossRef]
- Teng, N.; Maghzal, G.J.; Talib, J.; Rashid, I.; Lau, A.K.; Stocker, R. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep. 2017, 2, 51–73. [Google Scholar] [CrossRef]
- Kowalska, K.; Socha, E.; Milnerowicz, H. Review: The role of paraoxonase in cardiovascular diseases. Ann. Clin. Lab. Sci. 2015, 2, 226–233. [Google Scholar]
- Morena, M.; Cristol, J.P.; Dantoine, T.; Carbonneau, M.A.; Descomps, B.; Canaud, B. Protective effects of high-density lipoprotein against oxidative stress are impaired in haemodialysis patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2000, 3, 389–395. [Google Scholar] [CrossRef]
- Kuchta, A.; Pacanis, A.; Kortas-Stempak, B.; Cwiklińska, A.; Ziętkiewicz, M.; Renke, M.; Rutkowski, B. Estimation of Oxidative Stress Markers in Chronic Kidney Disease. Kidney Blood Press Res. 2010, 1, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.Z.; Varghese, Z.; Moorhead, J.F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 2009, 12, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Krane, V.; Marz, W.; Olschewski, M.; Mann, J.F.; Ruf, G.; Ritz, E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 2005, 3, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Krane, V.; Winkler, K.; Drechsler, C.; Lilienthal, J.; Marz, W.; Wanner, C. Effect of atorvastatin on inflammation and outcome in patients with type 2 diabetes mellitus on hemodialysis. Kidney Int. 2008, 11, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Fellstrom, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.W.; Chevaile, A.; Cobbe, S.M.; Gronhagen-Riska, C.; et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009, 14, 1395–1407. [Google Scholar] [CrossRef]
- Asztalos, B.F.; Horvath, K.V.; McNamara, J.R.; Roheim, P.S.; Rubinstein, J.J.; Schaefer, E.J. Comparing the effects of five different statins on the HDL subpopulation profiles of coronary heart disease patients. Atherosclerosis 2002, 2, 361–369. [Google Scholar] [CrossRef]
- Franceschini, G.; Calabresi, L.; Colombo, C.; Favari, E.; Bernini, F.; Sirtori, C.R. Effects of fenofibrate and simvastatin on HDL-related biomarkers in low-HDL patients. Atherosclerosis 2007, 2, 385–391. [Google Scholar] [CrossRef]
- Tian, L.; Chen, Y.; Li, C.; Zeng, Z.; Xu, Y.; Long, S.; Fu, M. Statin treatment improves plasma lipid levels but not HDL subclass distribution in patients undergoing percutaneous coronary intervention. Lipids. 2013, 2, 127–137. [Google Scholar] [CrossRef]
- Miyazaki, O.; Kobayashi, J.; Fukamachi, I.; Miida, T.; Bujo, H.; Saito, Y. A new sandwich enzyme immunoassay for measurement of plasma pre-beta1-HDL levels. J. Lipid Res. 2000, 12, 2083–2088. [Google Scholar]
- Nakanishi, M.; Takanami, Y.; Maruyama, T.; Murata, M.; Motohashi, Y.; Nakano, S.; Uchida, K.; Maruyama, C.; Kyotani, S.; Tsushima, M. The ratio of serum paraoxonase/arylesterase activity using an improved assay for arylesterase activity to discriminate PON1(R192) from PON1(Q192). J. Atheroscler. Thromb. 2003, 6, 337–342. [Google Scholar] [CrossRef]
| Parameter | Stages of CKD | |||
|---|---|---|---|---|
| 3a | 3b | 4 | p-Value | |
| Gender (M/F) | 12/5 | 19/15 | 12/5 | 0.531 ** |
| Age (years) | 69 ± 5 | 70 ± 9 | 63 ± 5 | 0.06 * |
| BMI (kg/m2) | 28 ± 3 | 29 ± 5 | 26 ± 4 | 0.306 * |
| eGFR | 50 ± 3 | 37 ± 4 | 22 ± 4 | <0.001 * |
| Albumin (g/L) | 43.6 ± 3.2 | 42.8 ± 2.9 | 42.9 ± 2.9 | 0.674 * |
| Statin therapy (%) | 41 | 53 | 64 | 0.333 ** |
| TAG (mg/dL) | 102 ± 30 | 117 ± 45 | 135 ± 63 | 0.215 * |
| TC (mg/dL) | 199 ± 42 | 200 ± 53 | 215 ± 36 | 0.345 * |
| HDL-C (mg/dL) | 51 ± 11 | 50 ± 12 | 48 ± 11 | 0.761 * |
| LDL-C (mg/dL) | 127 ± 39 | 127 ± 49 | 140 ± 33 | 0.335 * |
| ApoA-I (mg/dL) | 172 ± 27 | 164 ± 27 | 160 ± 23 | 0.297 * |
| ApoA-II (mg/dL) | 33 ± 7 | 31 ± 6 | 31 ± 5 | 0.499 * |
| LCAT (390/470 nm) | 1.34 ± 0.03 | 1.32 ± 0.05 | 1.32 ± 0.04 | 0.458 * |
| FC/TC | 0.283 ± 0.05 | 0.283 ± 0.03 | 0.290 ± 0.03 | 0.234 * |
| Parameter | R | p |
|---|---|---|
| HDL-C (mg/dL) | 0.104 | 0.382 |
| ApoA-I (mg/dL) | 0.153 | 0.202 |
| ApoA-II (mg/dL) | 0.109 | 0.358 |
| LpA-I (mg/dL) | 0.025 | 0.837 |
| preβ1-HDL (mg/dL) | −0.456 | <0.001 |
| preβ1-HDL/LpA-I | −0.322 | 0.008 |
| PON-1 (U/L) | 0.068 | 0.566 |
| MPO (ng/mL) | 0.079 | 0.531 |
| LCAT (390/470 nm) | 0.080 | 0.484 |
| Parameter | β | SE | p |
|---|---|---|---|
| preβ1-HDL | −0.41 | 0.105 | <0.001 |
| preβ1-HDL/LpA-I | −0.33 | 0.09 | 0.001 |
| Stages of CKD | ||||
|---|---|---|---|---|
| 3a | 3b | 4 | p-Value * | |
| PON-1 (U/L) | 102 (53–150) | 83 (52–152) | 113 (80–130) | 0.890 |
| MPO (ng/mL) | 235 (136–392) | 199 (139–347) | 273 (160–327) | 0.377 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchta, A.; Ćwiklińska, A.; Czaplińska, M.; Wieczorek, E.; Kortas-Stempak, B.; Gliwińska, A.; Dąbkowski, K.; Sałaga-Zaleska, K.; Mickiewicz, A.; Dębska-Ślizień, A.; et al. Plasma Levels of Preβ1-HDL Are Significantly Elevated in Non-Dialyzed Patients with Advanced Stages of Chronic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1202. https://doi.org/10.3390/ijms20051202
Kuchta A, Ćwiklińska A, Czaplińska M, Wieczorek E, Kortas-Stempak B, Gliwińska A, Dąbkowski K, Sałaga-Zaleska K, Mickiewicz A, Dębska-Ślizień A, et al. Plasma Levels of Preβ1-HDL Are Significantly Elevated in Non-Dialyzed Patients with Advanced Stages of Chronic Kidney Disease. International Journal of Molecular Sciences. 2019; 20(5):1202. https://doi.org/10.3390/ijms20051202
Chicago/Turabian StyleKuchta, Agnieszka, Agnieszka Ćwiklińska, Monika Czaplińska, Ewa Wieczorek, Barbara Kortas-Stempak, Anna Gliwińska, Kamil Dąbkowski, Kornelia Sałaga-Zaleska, Agnieszka Mickiewicz, Alicja Dębska-Ślizień, and et al. 2019. "Plasma Levels of Preβ1-HDL Are Significantly Elevated in Non-Dialyzed Patients with Advanced Stages of Chronic Kidney Disease" International Journal of Molecular Sciences 20, no. 5: 1202. https://doi.org/10.3390/ijms20051202
APA StyleKuchta, A., Ćwiklińska, A., Czaplińska, M., Wieczorek, E., Kortas-Stempak, B., Gliwińska, A., Dąbkowski, K., Sałaga-Zaleska, K., Mickiewicz, A., Dębska-Ślizień, A., Król, E., & Jankowski, M. (2019). Plasma Levels of Preβ1-HDL Are Significantly Elevated in Non-Dialyzed Patients with Advanced Stages of Chronic Kidney Disease. International Journal of Molecular Sciences, 20(5), 1202. https://doi.org/10.3390/ijms20051202






