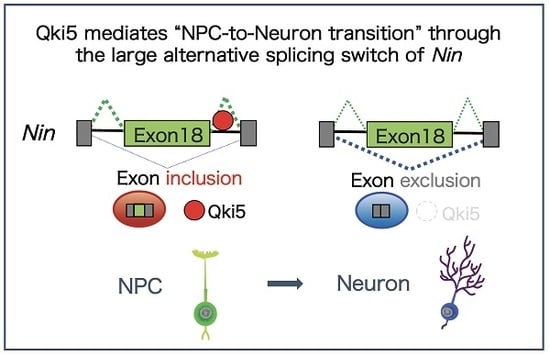
An RNA Switch of a Large Exon of Ninein Is Regulated by the Neural Stem Cell Specific-RNA Binding Protein, Qki5
Abstract
:1. Introduction
2. Results
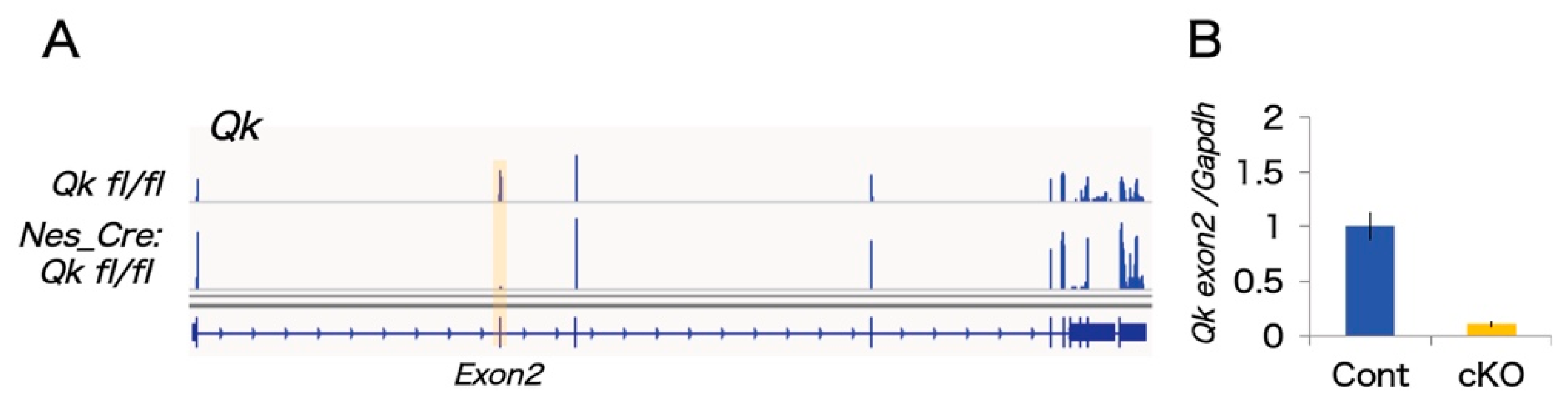
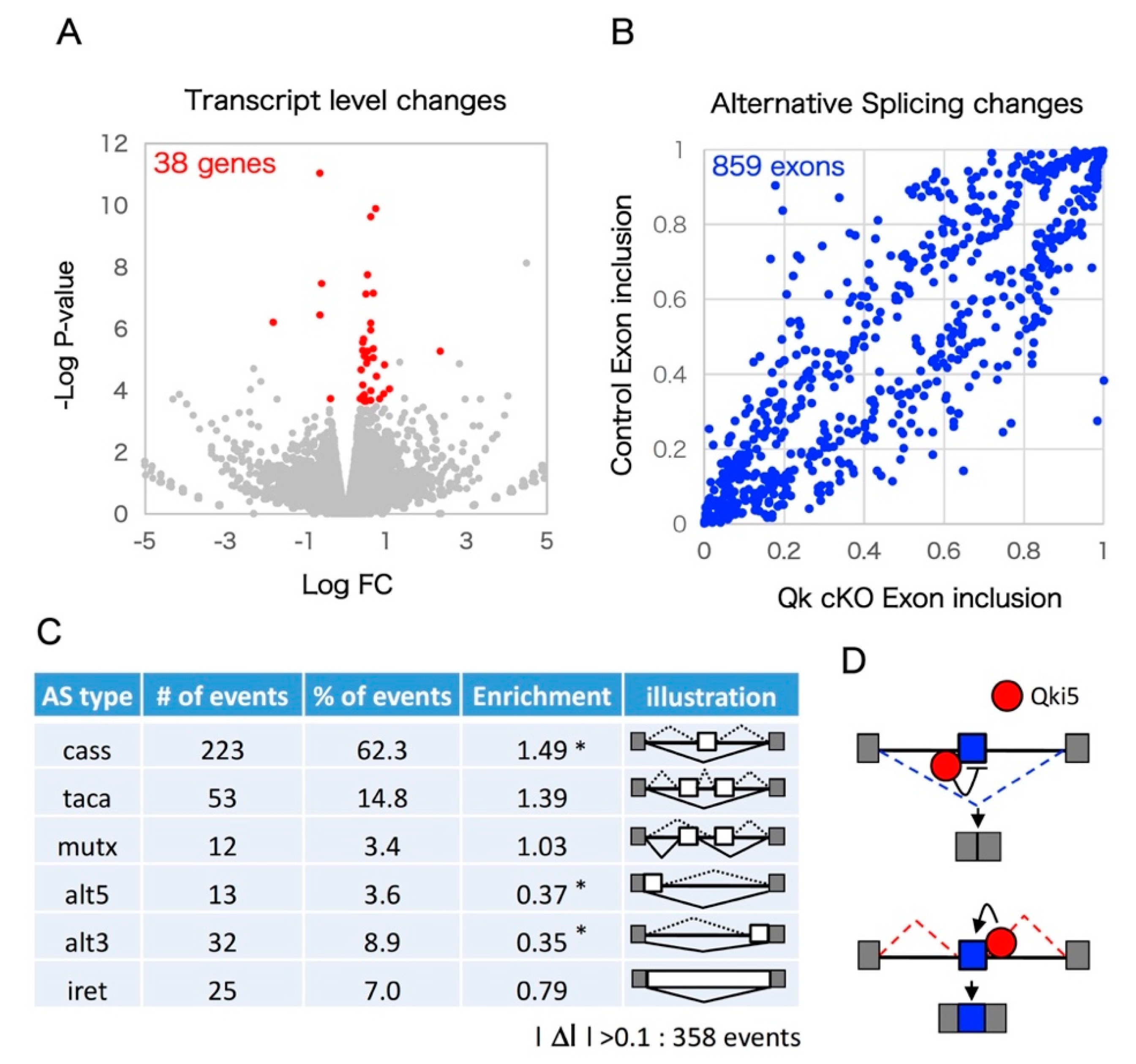
2.1. The mRNAseq Analysis of Qk Conditional Knock-Out Cortices
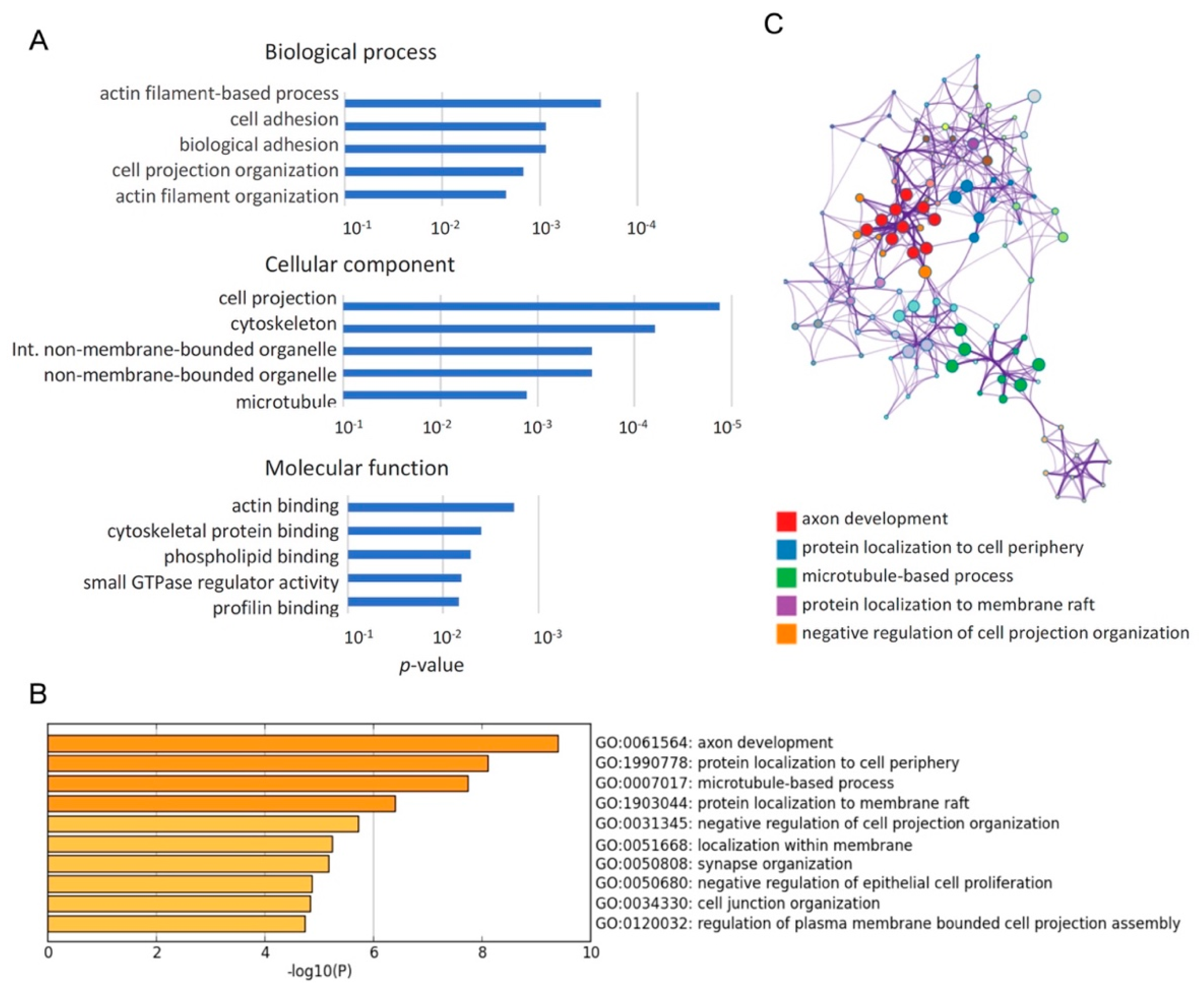
2.2. Enrichment Analyses Revealed that Qki Dependent Pre-mRNA Splicing Is Involved in Cytoskeleton-Related Pathways
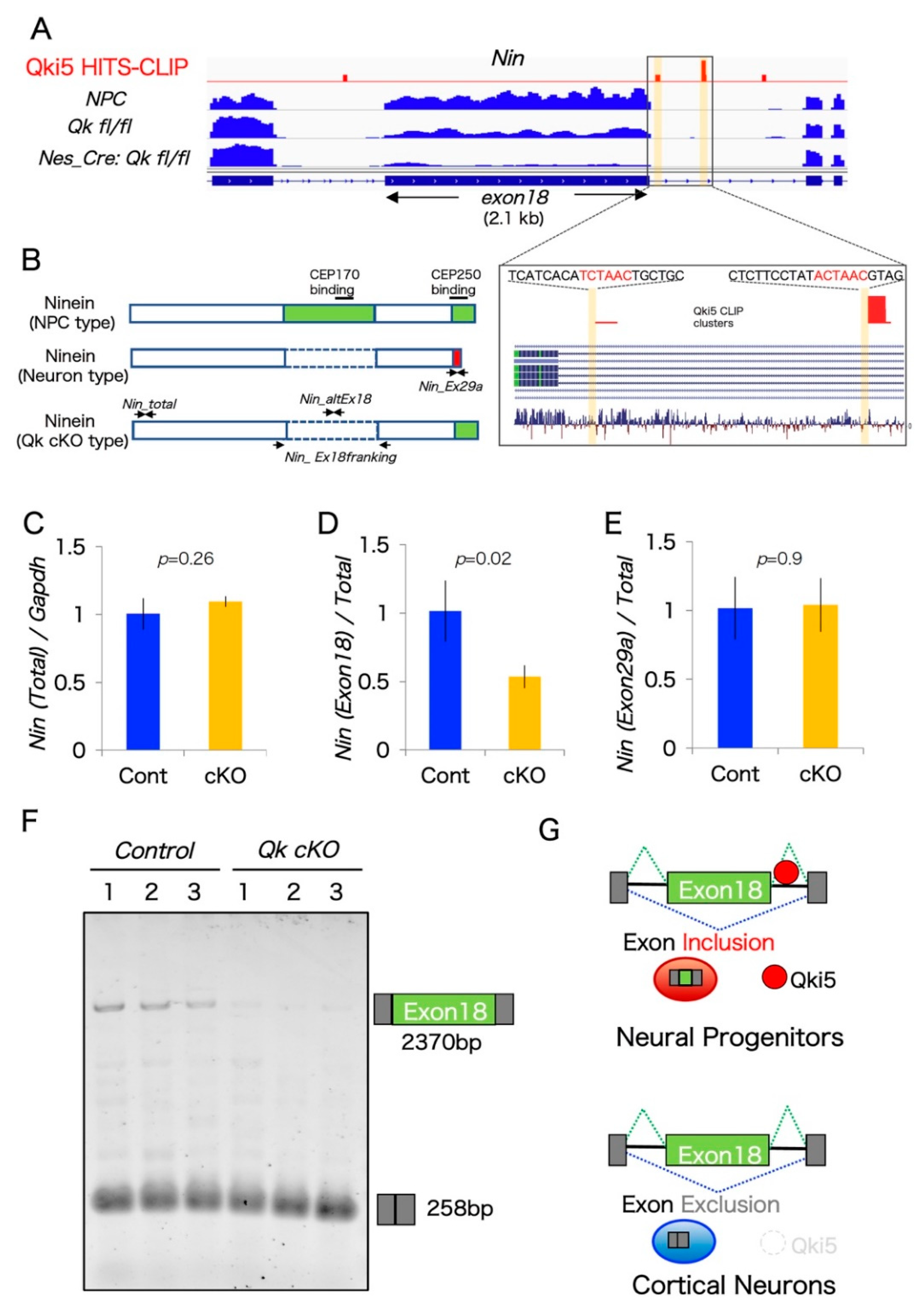
2.3. A Large Exon 18 of Nin Is Regulated by Neural Stem Cell Regulator Qki5
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. The mRNA Sequence and Data Analysis
4.3. Accession Numbers
4.4. Enrichment Analyses
4.5. RT-PCR and qRT-PCR
4.6. Primers for qRT-PCR and RT-PCR
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boutz, P.L.; Stoilov, P.; Li, Q.; Lin, C.H.; Chawla, G.; Ostrow, K.; Shiue, L.; Ares, M.J.; Black, D.L. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes Dev. 2007, 21, 1636–1652. [Google Scholar] [CrossRef] [PubMed]
- Darnell, R.B. RNA protein interaction in neurons. Annu. Rev. Neurosci. 2013, 36, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Gerstberger, S.; Hafner, M.; Tuschl, T. A census of human RNA-binding proteins. Nat. Rev. Genet. 2014, 15, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Castle, J.; Sun, S.; Johnson, J.; Krainer, A.R.; Zhang, M.Q. Defining the regulatory network of the tissue-specific splicing factors Fox-1 and Fox-2. Genes Dev. 2008, 22, 2550–2563. [Google Scholar] [CrossRef] [PubMed]
- Raj, B.; Blencowe, B.J. Alternative Splicing in the Mammalian Nervous System: Recent Insights into Mechanisms and Functional Roles. Neuron 2015, 87, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Tarn, W.Y. Alternative Splicing in Neurogenesis and Brain Development. Front. Mol. Biosci. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Conlon, E.G.; Manley, J.L. RNA-binding proteins in neurodegeneration: Mechanisms in aggregate. Genes Dev. 2017, 31, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Licatalosi, D.D.; Darnell, R.B. Splicing regulation in neurologic disease. Neuron 2006, 52, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Calarco, J.A.; Superina, S.; O’Hanlon, D.; Gabut, M.; Raj, B.; Pan, Q.; Skalska, U.; Clarke, L.; Gelinas, D.; van der Kooy, D.; et al. Regulation of vertebrate nervous system alternative splicing and development by an SR-related protein. Cell 2009, 138, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Dhananjaya, D.; Hung, K.Y.; Tarn, W.Y. RBM4 Modulates Radial Migration via Alternative Splicing of Dab1 during Cortex Development. Mol. Cell. Biol. 2018, 38, e00007-18. [Google Scholar]
- Hayakawa-Yano, Y.; Suyama, S.; Nogami, M.; Yugami, M.; Koya, I.; Furukawa, T.; Zhou, L.; Abe, M.; Sakimura, K.; Takebayashi, H.; et al. An RNA-binding protein, Qki5, regulates embryonic neural stem cells through pre-mRNA processing in cell adhesion signaling. Genes Dev. 2017, 31, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Licatalosi, D.D.; Yano, M.; Fak, J.J.; Mele, A.; Grabinski, S.E.; Zhang, C.; Darnell, R.B. Ptbp2 represses adult-specific splicing to regulate the generation of neuronal precursors in the embryonic brain. Genes Dev. 2012, 26, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, T.; Tokunaga, A.; Sakamoto, R.; Sagara, H.; Noguchi, S.; Sasaoka, T.; Yoshida, N. PTB deficiency causes the loss of adherens junctions in the dorsal telencephalon and leads to lethal hydrocephalus. Cereb. Cortex 2013, 23, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Hayakawa-Yano, Y.; Mele, A.; Darnell, R.B. Nova2 regulates neuronal migration through an RNA switch in disabled-1 signaling. Neuron 2010, 66, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Ohtsuka, T.; Okano, H. RNA-binding protein research with transcriptome-wide technologies in neural development. Cell Tissue Res. 2015, 359, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, M.H.; Wu, X.; Kodani, A.; Fan, J.; Doan, R.; Ozawa, M.; Ma, J.; Yoshida, N.; Reiter, J.F.; et al. Cell-Type-Specific Alternative Splicing Governs Cell Fate in the Developing Cerebral Cortex. Cell 2016, 166, 1147–1162.e15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, W.; Zhang, Z.; Hu, Y.; Meng, F.; Wang, F.; Lou, H.; Zhu, L.; Godbout, R.; Duan, S.; et al. Alternative Splicing of Disabled-1 Controls Multipolar-to-Bipolar Transition of Migrating Neurons in the Neocortex. Cereb. Cortex 2018, 28, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Jacko, M.; Weyn-Vanhentenryck, S.M.; Smerdon, J.W.; Yan, R.; Feng, H.; Williams, D.J.; Pai, J.; Xu, K.; Wichterle, H.; Zhang, C. Rbfox Splicing Factors Promote Neuronal Maturation and Axon Initial Segment Assembly. Neuron 2018, 97, 853–868.e6. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Yano, M.; Igarashi, M.; Okano, H.J.; Okano, H. Alternative role of HuD splicing variants in neuronal differentiation. J. Neurosci. Res. 2015, 93, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Srivatsa, S.; Parthasarathy, S.; Molnár, Z.; Tarabykin, V. Sip1 downstream Effector ninein controls neocortical axonal growth, ipsilateral branching, and microtubule growth and stability. Neuron 2015, 85, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, H.; Sakayori, N.; Takahashi, M.; Osumi, N. Ninein is essential for the maintenance of the cortical progenitor character by anchoring the centrosome to microtubules. Biol. Open 2013, 2, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.J.; Loushin, C.L.; Friedrich, V.L.; Chen, Q.; Ebersole, T.A.; Lazzarini, R.A.; Artzt, K. Neural cell type-specific expression of QKI proteins is altered in quakingviable mutant mice. J. Neurosci. 1996, 16, 7941–7949. [Google Scholar] [CrossRef] [PubMed]
- Licatalosi, D.D.; Mele, A.; Fak, J.J.; Ule, J.; Kayikci, M.; Chi, S.W.; Clark, T.A.; Schweitzer, A.C.; Blume, J.E.; Wang, X.; et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 2008, 456, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Zhang, C.; Gantman, E.C.; Mele, A.; Darnell, J.C.; Darnell, R.B. Mapping Argonaute and conventional RNA-binding protein interactions with RNA at single-nucleotide resolution using HITS-CLIP and CIMS analysis. Nat. Protoc. 2014, 9, 263–293. [Google Scholar] [CrossRef] [PubMed]
- Ule, J.; Hwang, H.W.; Darnell, R.B. The Future of Cross-Linking and Immunoprecipitation (CLIP). Cold Spring Harb. Perspect. Biol. 2018, 10, a032243. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Anczuków, O.; Krainer, A.R.; Zhang, M.Q.; Zhang, C. OLego: Fast and sensitive mapping of spliced mRNA-Seq reads using small seeds. Nucleic Acids Res. 2013, 41, 5149–5163. [Google Scholar] [CrossRef] [PubMed]
- Larocque, D.; Galarneau, A.; Liu, H.N.; Scott, M.; Almazan, G.; Richard, S. Protection of p27(Kip1) mRNA by quaking RNA binding proteins promotes oligodendrocyte differentiation. Nat. Neurosci. 2005, 8, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.K.; Black, D.L.; Zheng, S. The neurogenetics of alternative splicing. Nat. Rev. Neurosci. 2016, 17, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Gehman, L.T.; Meera, P.; Stoilov, P.; Shiue, L.; O’Brien, J.E.; Meisler, M.H.; Ares, M.J.; Otis, T.S.; Black, D.L. The splicing regulator Rbfox2 is required for both cerebellar development and mature motor function. Genes Dev. 2012, 26, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, S.; Han, A.; Lin, C.H.; Stoilov, P.; Fu, X.D.; Black, D.L. The splicing regulator PTBP2 controls a program of embryonic splicing required for neuronal maturation. Elife 2014, 3, e01201. [Google Scholar] [CrossRef] [PubMed]
- Quesnel-Vallieres, M.; Irimia, M.; Cordes, S.P.; Blencowe, B.J. Essential roles for the splicing regulator nSR100/SRRM4 during nervous system development. Genes Dev. 2015, 29, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Ruggiu, M.; Herbst, R.; Kim, N.; Jevsek, M.; Fak, J.J.; Mann, M.A.; Fischbach, G.; Burden, S.J.; Darnell, R.B. Rescuing Z+ agrin splicing in Nova null mice restores synapse formation and unmasks a physiologic defect in motor neuron firing. Proc. Natl. Acad. Sci. USA 2009, 106, 3513–3518. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Lamperti, E.D.; Ekşioğlu, Y.Z.; Hong, S.E.; Feng, Y.; Graham, D.A.; Scheffer, I.E.; Dobyns, W.B.; Hirsch, B.A.; Radtke, R.A.; et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 1998, 21, 1315–1325. [Google Scholar] [CrossRef]
- Bolisetty, M.T.; Beemon, K.L. Splicing of internal large exons is defined by novel cis-acting sequence elements. Nucleic Acids Res. 2012, 40, 9244–9254. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.T.; Chasin, L.A. Large exon size does not limit splicing in vivo. Mol. Cell. Biol. 1994, 14, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Pohl, M.O.; Zhou, Y.; Rodriguez-Frandsen, A.; Wang, G.; Stein, D.A.; Moulton, H.M.; DeJesus, P.; Che, J.; Mulder, L.C.; et al. Meta- and Orthogonal Integration of Influenza “OMICs” Data Defines a Role for UBR4 in Virus Budding. Cell Host Microbe 2015, 18, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Yano, M.; Okano, H. Acute reduction of neuronal RNA binding Elavl2 protein and Gap43 mRNA in mouse hippocampus after kainic acid treatment. Biochem. Biophys. Res. Commun. 2015, 466, 46–51. [Google Scholar] [CrossRef] [PubMed]
| Sample | Total Reads | Pair.gapless.bed | Mappable (%) | Junction Reads |
|---|---|---|---|---|
| E14.5_ Qk fl/fl_#1 | 83,931,860 | 28,953,888 | 68.9 | 13,050,933 |
| E14.5_Qk fl/fl_#2 | 80,674,030 | 26,878,815 | 66.6 | 11,895,843 |
| E14.5_Nes Qk fl/fl_#1 | 79,970,980 | 29,534,795 | 73.8 | 13,383,801 |
| E14.5_Nes Qk fl/fl_#2 | 80,218,216 | 28,907,399 | 72.1 | 12,642,383 |
| Term | Description | Log P | Log (q-Value) | InTerm_InList | Symbols |
|---|---|---|---|---|---|
| GO:0061564 | axon development | −9.397 | −5.617 | 26/468 | Aatk,Apc,Ddr1,Dab1,Dst,Dvl1,Evl,Fn1,Nfib,Nin,Numb,Ptprf, Ptprm,Ptprs,Ptprz1,Cyfip1,Tnc,Tsc2,Golga4,Brsk2,Foxp1,Boc, Picalm,Nrcam,Auts2,Kalrn,Cask,Cln3,Dab2,Lrp8,Nrxn1,Dennd5a, Map4k4,Nckap1,Grip1,Ccp110,Ccdc88a,Flna,Bcas3,Ripor2,Ttc3, Prpf40a,Actn4,Qrich1,Fmnl2,Triobp,Zmym5,Fat1,Mff,Bnip2, Tenm4,Clstn1,Adgrl2,Adgrb1,Sorbs2,Celf1,Pcnt,Pkm,Zfx,Brd4, Kmt2c,Ankrd26,Sbf1,Atad3a,Hp1bp3,Slc12a7,Arfip2,Cacna1g,Pla2g6 |
| GO:1990778 | protein localization to cell periphery | −8.110 | −4.934 | 19/291 | Cask,Cln3,Dab2,Flot2,Kif13a,Myo5a,Nrxn1,Numb,Sorbs1,Snap23, Tsc2,Golga4,Clip1,Rer1,Slmap,Flna,Picalm,Dennd4c,Kalrn,Dvl1, Reep2,Apc,Gcc2,Ccdc88a,Bcas3,Ripor2,Sec16a |
| GO:0007017 | microtubule-based process | −7.738 | −4.630 | 31/770 | Apc,Bnip2,Cln3,Dctn1,Dst,Dvl1,Dyrk1a,Kif13a,Kif16b,Kif21a,Mark3, Mdm1,Myo1b,Myo5a,Nin,Pcnt,Fbxw5,Clip1,Haus2,Camsap3,Gcc2, Eml2,Brsk2,Eml4,Ccp110,Phldb1,Ccdc88a,Flna,Bcas3,Ripor2,Prc1, Evl,Cyfip1,Nckap1,Afap1,Arfip2,Triobp,Mtss1,Fat1,Sorbs1,Actn4, Foxp1,Sorbs2,Nrxn1,Clstn1,Adgrl2,Adgrb1,Auts2,Pla2g6,Hnrnpa2b1, Mff,Pot1a,Ddhd1,Eif4g3 |
| GO:1903044 | protein localization to membrane raft | −6.406 | −3.430 | 4/6 | Flot2,Tsc2,Clip1,Reep2,Snap23,Cln3,Dctn1,Dvl1,Nrxn1,Rer1, Tmem175,Adgrb1,Ccdc88a,Mtss1,Sec24c,Sec16a,Picalm,Nrcam |
| GO:0031345 | negative regulation of cell projection organization | −5.720 | −2.864 | 13/199 | Aatk,Dab1,Dab2,Evl,Nrxn1,Ptprf,Ptprs,Ptprz1,Dennd5a,Tsc2, Map4k4,Ccp110,Flna,Apc,Dyrk1a,Mdm1,Ttc3,Map3k7,Actn4, Camsap3,Eml2,Eml4,Pot1a,Bcas3,Picalm,Foxp1,Npr2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayakawa-Yano, Y.; Yano, M. An RNA Switch of a Large Exon of Ninein Is Regulated by the Neural Stem Cell Specific-RNA Binding Protein, Qki5. Int. J. Mol. Sci. 2019, 20, 1010. https://doi.org/10.3390/ijms20051010
Hayakawa-Yano Y, Yano M. An RNA Switch of a Large Exon of Ninein Is Regulated by the Neural Stem Cell Specific-RNA Binding Protein, Qki5. International Journal of Molecular Sciences. 2019; 20(5):1010. https://doi.org/10.3390/ijms20051010
Chicago/Turabian StyleHayakawa-Yano, Yoshika, and Masato Yano. 2019. "An RNA Switch of a Large Exon of Ninein Is Regulated by the Neural Stem Cell Specific-RNA Binding Protein, Qki5" International Journal of Molecular Sciences 20, no. 5: 1010. https://doi.org/10.3390/ijms20051010
APA StyleHayakawa-Yano, Y., & Yano, M. (2019). An RNA Switch of a Large Exon of Ninein Is Regulated by the Neural Stem Cell Specific-RNA Binding Protein, Qki5. International Journal of Molecular Sciences, 20(5), 1010. https://doi.org/10.3390/ijms20051010