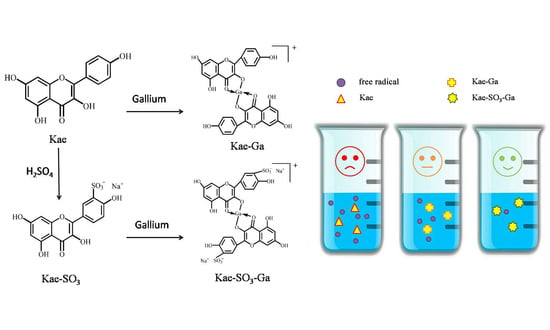
Synthesis, Spectroscopic Study and Radical Scavenging Activity of Kaempferol Derivatives: Enhanced Water Solubility and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sulfonation of Kae on Ring B
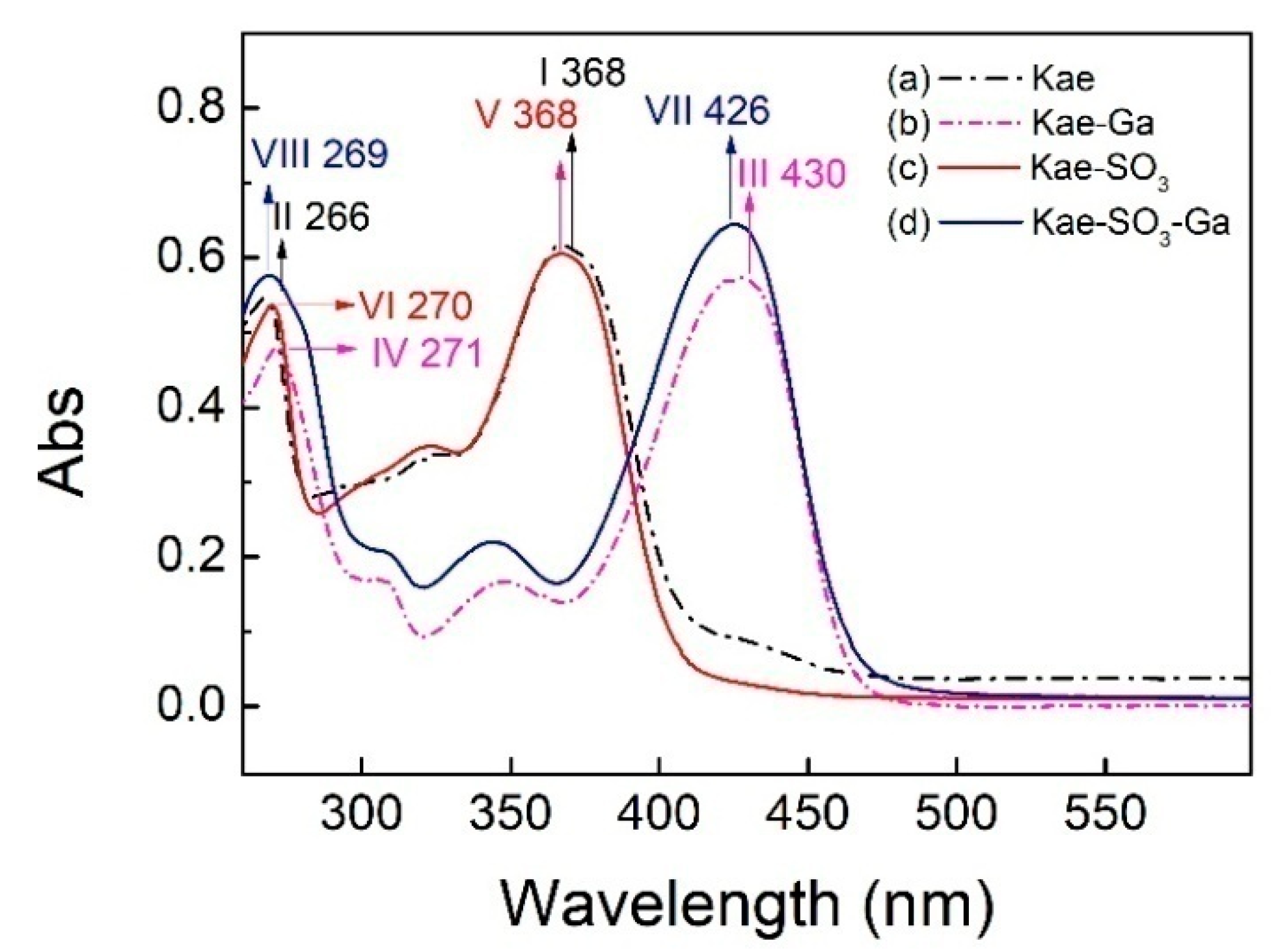
2.2. UV-Vis Spectra Analysis
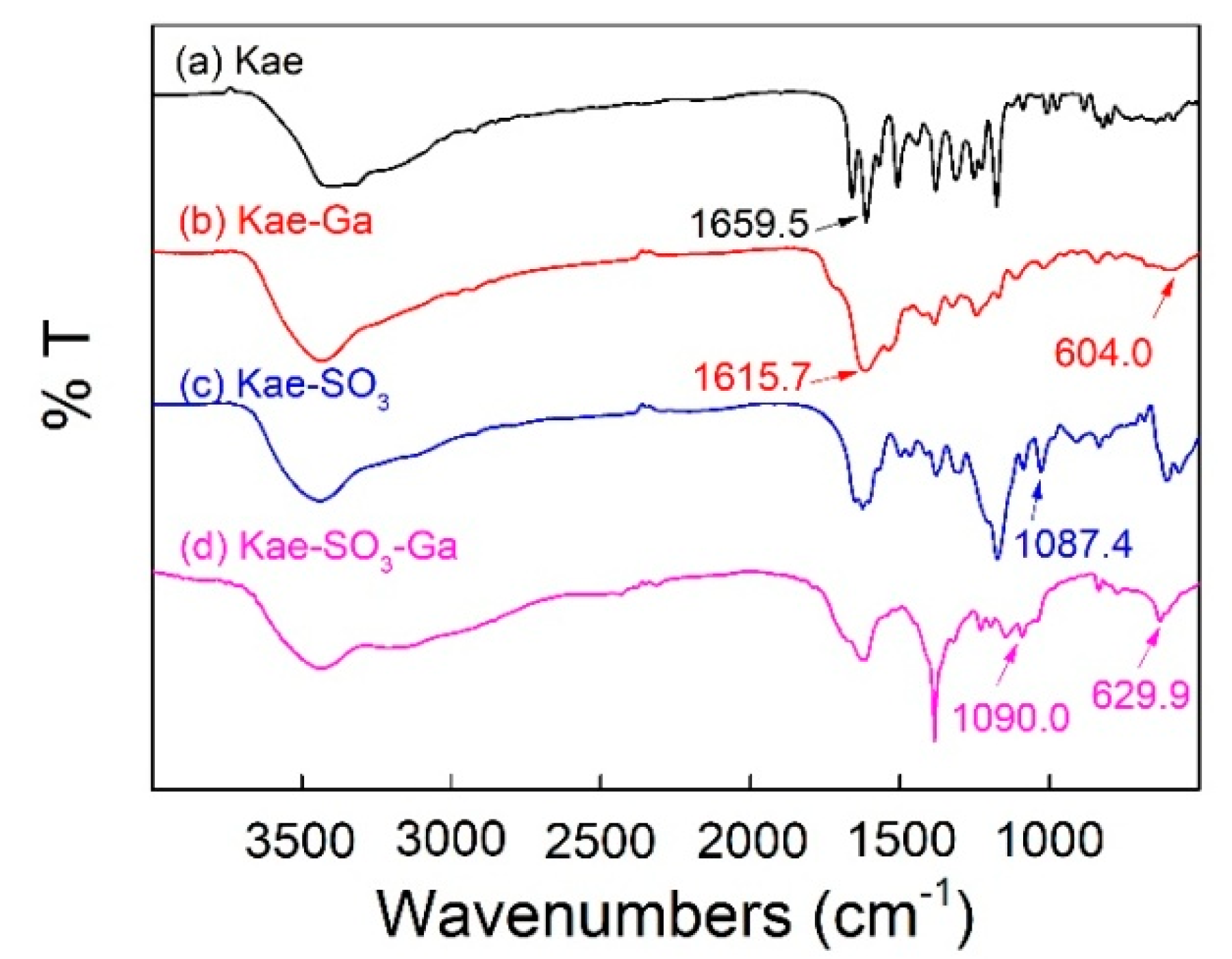
2.3. FT-IR Spectra Analysis
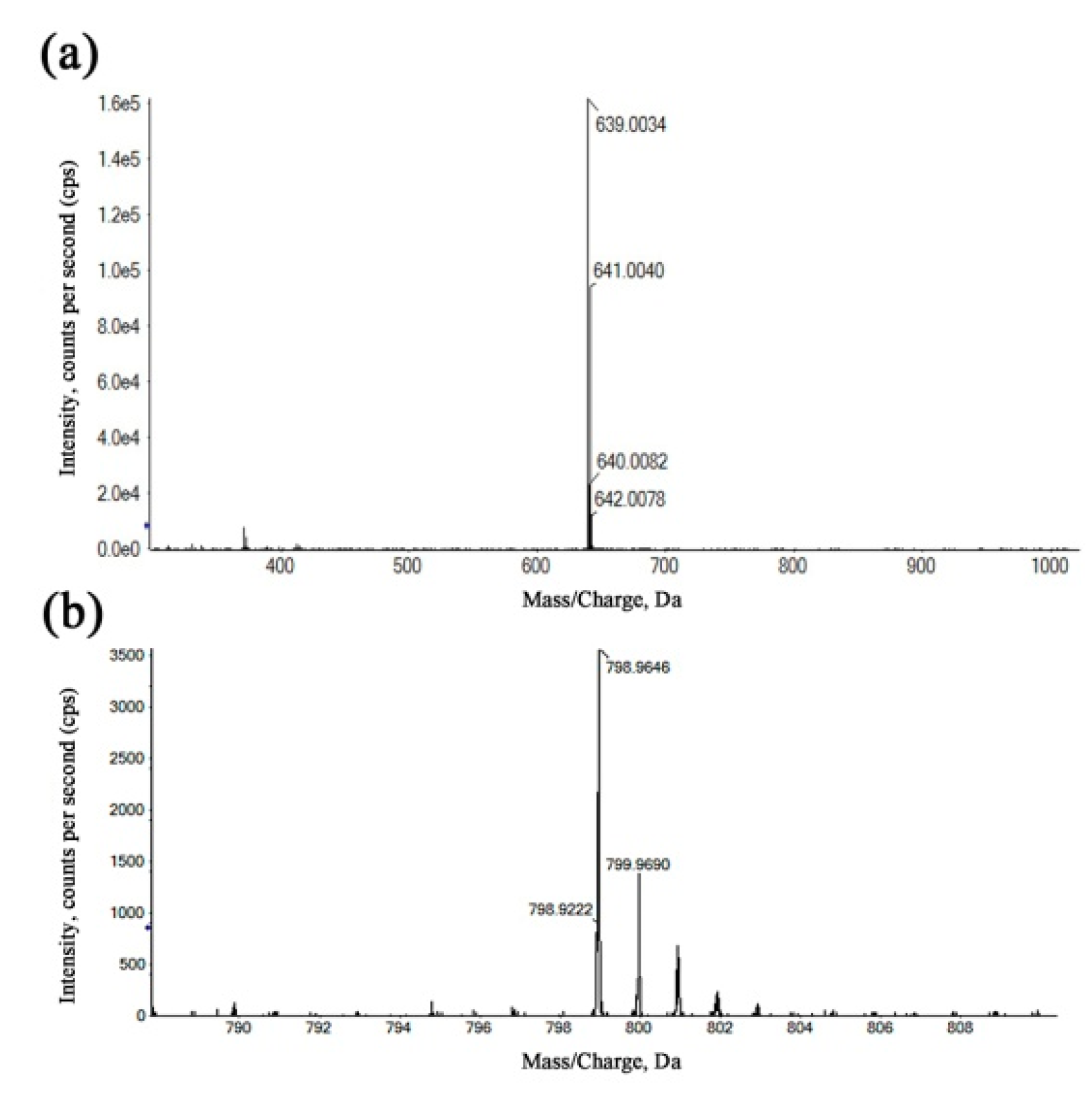
2.4. High-Resolution Mass Spectrometry (HRMS) Analysis
2.5. 1H NMR Spectrometry Analysis
2.6. Thermal Study of the Kae-Ga Complex
2.7. Enhanced Water-Solubility of Kae-SO3 and Its Complex
2.8. Antioxidant Activities of Kae, Kae-SO3 and Their Complexes
3. Conclusions
4. Materials and Methods
4.1. Materials and Instrument
4.2. Synthesis of Kae Derivatives
4.3. UV-Vis Spectra, FT-IR Spectra and TG/DSC Measurements
4.4. NMR Spectrometry
4.5. HRMS
4.6. Measurements of Antioxidant Activity by DPPH and ABTS Radicals
4.7. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–2. [Google Scholar] [CrossRef]
- Anandam, S.; Selvamuthukumar, S. Fabrication of cyclodextrin nanosponges for quercetin delivery: Physicochemical characterization, photostability, and antioxidant effects. J. Mater. Sci. 2014, 49, 8140–8153. [Google Scholar] [CrossRef]
- Balducci, V.; Incerpi, S.; Stano, P.; Tofani, D. Antioxidant activity of hydroxytyrosyl esters studied in liposome models. Biochim. Biophysi. Acta 2018, 1860, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chang, X.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Liu, R.H. Phenolic compounds, antioxidant activity, antiproliferative activity and bioaccessibility of Sea buckthorn (Hippophae rhamnoides L.) berries as affected by in vitro digestion. Food Funct. 2017, 8, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparå, O.K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Adhami, V.M.; Khan, N.; Khan, M.I.; Mukhtar, H. Exploring the molecular targets of dietary flavonoid fisetin in cancer. Semin. Cancer Biol. 2016, 40–41, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.V.D.; Giovani, W.F.D. Synthesis, spectral and electrochemical properties of Al(III) and Zn(II) complexes with flavonoids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Idelchik, M.D.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanput, W.; Krueyos, N.; Ritthiruangdej, P. Anti-oxidative assays as markers for anti-inflammatory activity of flavonoids. Int. Immunopharmacol. 2016, 40, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Varatharajan, K.; Pushparani, D.S. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Jabeen, E.; Janjua, N.K.; Ahmed, S.; Murtaza, I.; Ali, T.; Hameed, S. Radical scavenging propensity of Cu2+, Fe3+ complexes of flavonoids and in-vivo radical scavenging by Fe3+-primuletin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.; Jørgensen, T.Ø.; Olsen, R.L. Comparison of food antioxidants and iron chelators in two cellular free radical assays: Strong protection by luteolin. J. Agric. Food Chem. 2014, 62, 8402–8410. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.Y.; Pi, J.; Jin, H.; Cai, J.Y.; Deng, S.P. Synthesis, characterization and anticancer activity of kaempferol-zinc(II) complex. Bioorg. Med. Chem. Lett. 2016, 26, 2730–2734. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K. Synthesis and characterization of a biologically active lanthanum(III)–catechin complex and DNA binding spectroscopic studies. Spectrosc. Lett. 2009, 42, 178–185. [Google Scholar]
- Ravishankar, D.; Salamah, M.; Attina, A.; Pothi, R.; Vallance, T.M.; Javed, M.; Williams, H.F.; Alzahrani, E.M.S.; Kabova, E.; Vaiyapuri, R. Ruthenium-conjugated chrysin analogues modulate platelet activity, thrombus formation and haemostasis with enhanced efficacy. Sci. Rep. 2017, 7, 5738. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.J.M.; Naso, L.G.; Pérez, A.L.; Rizzi, A.; Okulik, N.B.; Ferrer, E.G.; Williams, P.A.M. Apigenin oxidovanadium(IV) cation interactions. Synthesis, spectral, bovine serum albumin binding, antioxidant and anticancer studies. J. Photochem. Photobiol. A Chem. 2017, 344, 84–100. [Google Scholar] [CrossRef]
- Lertanantawong, B.; Lertsathitphong, P.; O’Mullane, A.P. Chemical reactivity of Ga-based liquid metals with redox active species and its influence on electrochemical processes. Electrochem. Commun. 2018, 93, 15–19. [Google Scholar] [CrossRef]
- Kirsh, J.; Woodside, A.; Manor, B.; Carroll, P.; Rablen, P.; Graves, C. Synthesis and Characterization of (pyNO−)2GaCl: A Redox-Active Gallium Complex. Inorganics 2018, 6, 50. [Google Scholar] [CrossRef]
- Uehara, T.; Yokoyama, M.; Suzuki, H.; Hanaoka, H.; Arano, Y. A gallium-67/68–labeled antibody fragment for immuno-SPECT/PET shows low renal radioactivity without loss of tumor uptake. Clin. Cancer Res. 2018, 24, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, A.; Joy, A.; Pillai, M.R.; Nanabala, R.; Anees, K.M.; Jayaprakash, P.G.; Madhavan, J.; Nair, S. Diagnostic Value of 68Ga PSMA-11 PET/CT Imaging of Brain Tumors-Preliminary Analysis. Clin. Nucl. Med. 2017, 42, e41–e48. [Google Scholar] [CrossRef] [PubMed]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of atherosclerotic Inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Inden, M.; Shirai, K.; Sekine, S.; Masaki, Y.; Kurita, H.; Ichihara, K.; Inuzuka, T.; Hozumi, I. The effects of Brazilian green propolis that contains flavonols against mutant copper-zinc superoxide dismutase-mediated toxicity. Sci. Rep. 2017, 7, 2882. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid-metal ion complexes: A novel class of therapeutic agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.Y.; Bai, H.H.; Cai, J.Y.; Deng, S.P. The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Ayasa, O.; Shingo, M.; Masamori, I.; Makoto, S.; Jun, I.; Ryuichiro, S. Kaempferol stimulates gene expression of low-density lipoprotein receptor through activation of Sp1 in cultured hepatocytes. Sci. Rep. 2016, 6, 24940. [Google Scholar] [Green Version]
- Guo, M.; Perez, C.; Wei, Y.; Rapoza, E.; Su, G.; Bouabdallah, F.; Chasteen, N.D. Iron-binding properties of plant phenolics and cranberry’s bio-effects. Dalton Trans. 2007, 4951–4961. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yang, X.; He, J.; Cai, S.; Xiao, K.; Zhu, L. Enhanced antioxidant activity, antibacterial activity and hypoglycemic effect of luteolin by complexation with manganese(II) and its inhibition kinetics on xanthine oxidase. RSC Adv. 2017, 7, 53385–53395. [Google Scholar] [CrossRef]
- Gao, L.G.; Wang, H.; Song, X.L.; Cao, W. Research on the chelation between luteolin and Cr(III) ion through infrared spectroscopy, UV-vis spectrum and theoretical calculations. J. Mol. Struct. 2013, 1034, 386–391. [Google Scholar] [CrossRef]
- Apicella, B.; Bruno, A.; Wang, X.; Spinelli, N. Fast Fourier Transform and autocorrelation function for the analysis of complex mass spectra. Int. J. Mass Spectrom. 2013, 338, 30–38. [Google Scholar] [CrossRef]
- Roy, S.; Mallick, S.; Chakraborty, T.; Ghosh, N.; Singh, A.K.; Manna, S.; Majumdar, S. Synthesis, characterisation and antioxidant activity of luteolin-vanadium(II) complex. Food Chem. 2015, 173, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhu, J.; Pan, Y.; Chen, Z.; Liang, H.; Liu, H.; Wang, H. Synthesis, cytotoxic activity, and DNA binding properties of copper (II) complexes with hesperetin, naringenin, and apigenin. Bioinorg. Chem. Appl. 2009, 2009, 347872. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.W.; Yen, F.L.; Wu, T.H.; Ko, H.H.; Lee, C.W.; Tzeng, W.S.; Lin, C.C. Enhancement of dissolution and antioxidant activity of kaempferol using a nanoparticle engineering process. J. Agric. Food Chem. 2011, 59, 5073–5080. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, H.; Park, S.; Jung, S. Enhancement of solubility and antioxidant activity of some flavonoids based on the inclusion complexation with sulfobutylether β-cyclodextrin. Bull. Korean Chem. Soc. 2010, 31, 3035–3037. [Google Scholar] [CrossRef]
- Teles, Y.C.F.; Souza, M.S.R.; Souza, M.D.F.V.D. Sulphated flavonoids: biosynthesis, structures, and biological activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Cheng, G.; Liu, Y.; Yang, H.; Hu, Y.T.; Huang, W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food Chem. Toxicol. 2012, 50, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Avallone, L.; Menichini, F. Radical scavenging, antioxidant and metal chelating activities of Annona cherimola Mill. (cherimoya) peel and pulp in relation to their total phenolic and total flavonoid contents. J. Food Compos. Anal. 2012, 25, 179–184. [Google Scholar] [CrossRef]
- Pu, W.; Wang, D.; Zhou, D. Structural characterization and evaluation of the antioxidant activity of phenolic compounds from astragalus taipaishanensis and their structure-activity relationship. Sci. Rep. 2015, 5, 13914. [Google Scholar] [CrossRef] [PubMed]
- Dejian, H.; Boxin, O.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar]
- Wangchuk, P.; Apte, S.H.; Smout, M.J.; Groves, P.L.; Loukas, A.; Doolan, D.L. Defined small molecules produced by Himalayan medicinal plants display immunomodulatory properties. Int. J. Mol. Sci. 2018, 19, 3490. [Google Scholar] [CrossRef] [PubMed]
- Vellosa, J.C.R.; Regasini, L.O.; Khalil, N.M.; Bolzani, V.D.S.; Khalil, O.A.K.; Manente, F.A.; Netto, H.P.; Oliveira, O.M. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclética Quim. 2011, 36, 7–20. [Google Scholar] [CrossRef]
- Vimalraj, S.; Rajalakshmi, S.; Raj, D.P.; Vinoth, S.K.; Deepak, T.; Gopinath, V.; Murugan, K.; Chatterjee, S. Mixed-ligand copper(II) complex of quercetin regulate osteogenesis and angiogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M.I. Synthesis, characterization and antioxidant activity copper-quercetin complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 71, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Y.; Feng, X.; Khaskheli, A.A.; Xiang, Y.; Huang, W. The effects of alcohol fermentation on the extraction of antioxidant compounds and flavonoids of pomelo peel. LWT-Food Sci. Technol. 2018, 89, 763–769. [Google Scholar] [CrossRef]
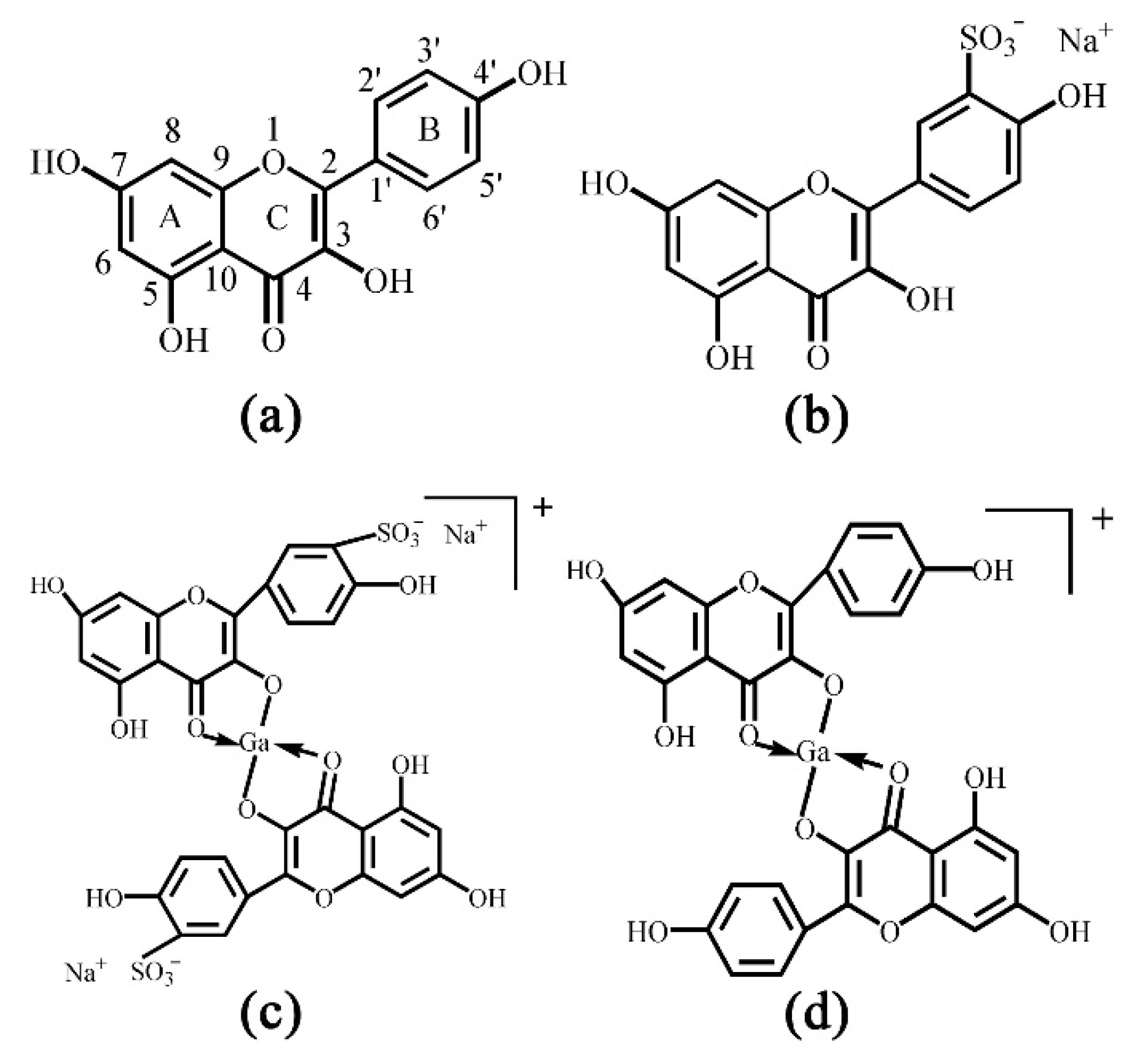


| Kae | Kae-SO3 | Δ | |
|---|---|---|---|
| C2 | 156.65 | 155.35 | −1.3 |
| C3 | 136.13 | 136.56 | 0.43 |
| C4 | 176.38 | 176.42 | 0.04 |
| C5 | 164.36 | 164.52 | 0.16 |
| C6 | 98.67 | 98.76 | 0.09 |
| C7 | 161.18 | 161.2 | 0.02 |
| C8 | 93.95 | 93.9 | −0.05 |
| C9 | 147.29 | 146.36 | −0.93 |
| C10 | 103.52 | 103.55 | 0.03 |
| C1’ | 122.14 | 122.01 | −0.13 |
| C2’ | 129.98 | 131.08 | 1.1 |
| C3’ | 115.91 | 127.63 | 11.72 |
| C4’ | 159.66 | 156.65 | −3.01 |
| C5’ | 115.91 | 117.32 | 1.41 |
| C6’ | 129.98 | 129.18 | −0.8 |
| Compound | 3-OH | 7-OH | 5-OH | 4’-OH | H6’ | H2’ | H5’ | H3’ | H8 | H6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Kae | 12.49 | 10.80 | 10.12 | 9.42 | 8.06 | 8.03 | 6.95 | 6.92 | 6.44 | 6.19 |
| Kae-Ga | — | 10.32 | 10.23 | 7.92 | 7.80 | 7.77 | 6.84 | 6.81 | 6.55 | 6.36 |
| Kae-SO3 | 12.50 | 10.99 | 10.89 | 9.61 | 8.06 | 8.42 | 6.96 | — | 6.46 | 6.20 |
| Kae-SO3-Ga | — | 11.04 | 8.55 | 8.08 | 7.79 | 7.40 | 7.24 | — | 7.07 | 6.87 |
| Compound | Peak I (nm) | Peak II (nm) |
|---|---|---|
| Kae | 368 | 266 |
| Kae-Ga | 430 | 271 |
| Kae-SO3 | 368 | 270 |
| Kae-SO3-Ga | 426 | 269 |
| Compound | –OH | C=O | C=C | C–OH (Phenolic Hydroxyl) | C–O–C | O–M | –SO3Na |
|---|---|---|---|---|---|---|---|
| Kae | 3386.1 | 1659.5 | 1507.3 | 1380.1 | 1176.4 | — | — |
| Kae-Ga | 3434.9 | 1615.7 | 1533.3 | 1384.8 | 1170.0 | 604.0 | — |
| Kae-SO3 | 3438.9 | 1654.7 | 1502.2 | 1378.9 | 1174.2 | — | 1087.4 |
| Kae-SO3-Ga | 3438.9 | 1617.6 | 1513.9 | 1384.4 | 1197.2 | 629.9 | 1090.0 |
| Compound | Solubility (g/100 g soln.) | Concentration (mol·L−1) |
|---|---|---|
| Kae | 0.0113 ± 0.0026 | 3.95 × 10−4 |
| Kae-SO3 | 2.49 ± 0.20 | 6.42 × 10−2 |
| Kae-Ga | 0.0398 ± 0.0040 | 6.22 × 10−4 |
| Kae-SO3-Ga | 14.34 ± 0.34 | 1.70 × 10−1 |
| (a) | |||||
| Concentration (×10−6 mol·L−1) | Kae (%) | Kae-Ga (%) | Kae-SO3 (%) | Kae-SO3-Ga (%) | Vc (%) |
| 5 | 5.07 | 6.24 | 8.60 | 11.53 | 15.29 |
| 15 | 12.85 | 16.26 | 17.51 | 20.85 | 36.79 |
| 30 | 27.34 | 30.63 | 33.89 | 36.75 | 45.44 |
| 45 | 41.84 | 43.90 | 48.48 | 53.92 | 63.93 |
| 60 | 54.13 | 56.36 | 63.90 | 65.68 | 78.65 |
| 75 | 65.75 | 72.77 | 80.85 | 82.47 | 88.37 |
| (b) | |||||
| Concentration (×10−6 mol·L−1) | Kae (%) | Kae-Ga (%) | Kae-SO3 (%) | Kae-SO3-Ga (%) | Vc (%) |
| 5 | 5.07 | 6.24 | 8.60 | 11.53 | 15.29 |
| 15 | 12.85 | 16.26 | 17.51 | 20.85 | 36.79 |
| 30 | 27.34 | 30.63 | 33.89 | 36.75 | 45.44 |
| 45 | 41.84 | 43.90 | 48.48 | 53.92 | 63.93 |
| 60 | 54.13 | 56.36 | 63.90 | 65.68 | 78.65 |
| 75 | 65.75 | 72.77 | 80.85 | 82.47 | 88.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.-P.; Yang, Y.-L.; Cheng, X.-X.; Li, W.-R.; Cai, J.-Y. Synthesis, Spectroscopic Study and Radical Scavenging Activity of Kaempferol Derivatives: Enhanced Water Solubility and Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 975. https://doi.org/10.3390/ijms20040975
Deng S-P, Yang Y-L, Cheng X-X, Li W-R, Cai J-Y. Synthesis, Spectroscopic Study and Radical Scavenging Activity of Kaempferol Derivatives: Enhanced Water Solubility and Antioxidant Activity. International Journal of Molecular Sciences. 2019; 20(4):975. https://doi.org/10.3390/ijms20040975
Chicago/Turabian StyleDeng, Sui-Ping, Yi-Li Yang, Xing-Xing Cheng, Wen-Rong Li, and Ji-Ye Cai. 2019. "Synthesis, Spectroscopic Study and Radical Scavenging Activity of Kaempferol Derivatives: Enhanced Water Solubility and Antioxidant Activity" International Journal of Molecular Sciences 20, no. 4: 975. https://doi.org/10.3390/ijms20040975