3D-Printed Bioactive Calcium Silicate/Poly-ε-Caprolactone Bioscaffolds Modified with Biomimetic Extracellular Matrices for Bone Regeneration
Abstract
1. Introduction
2. Results and Discussion
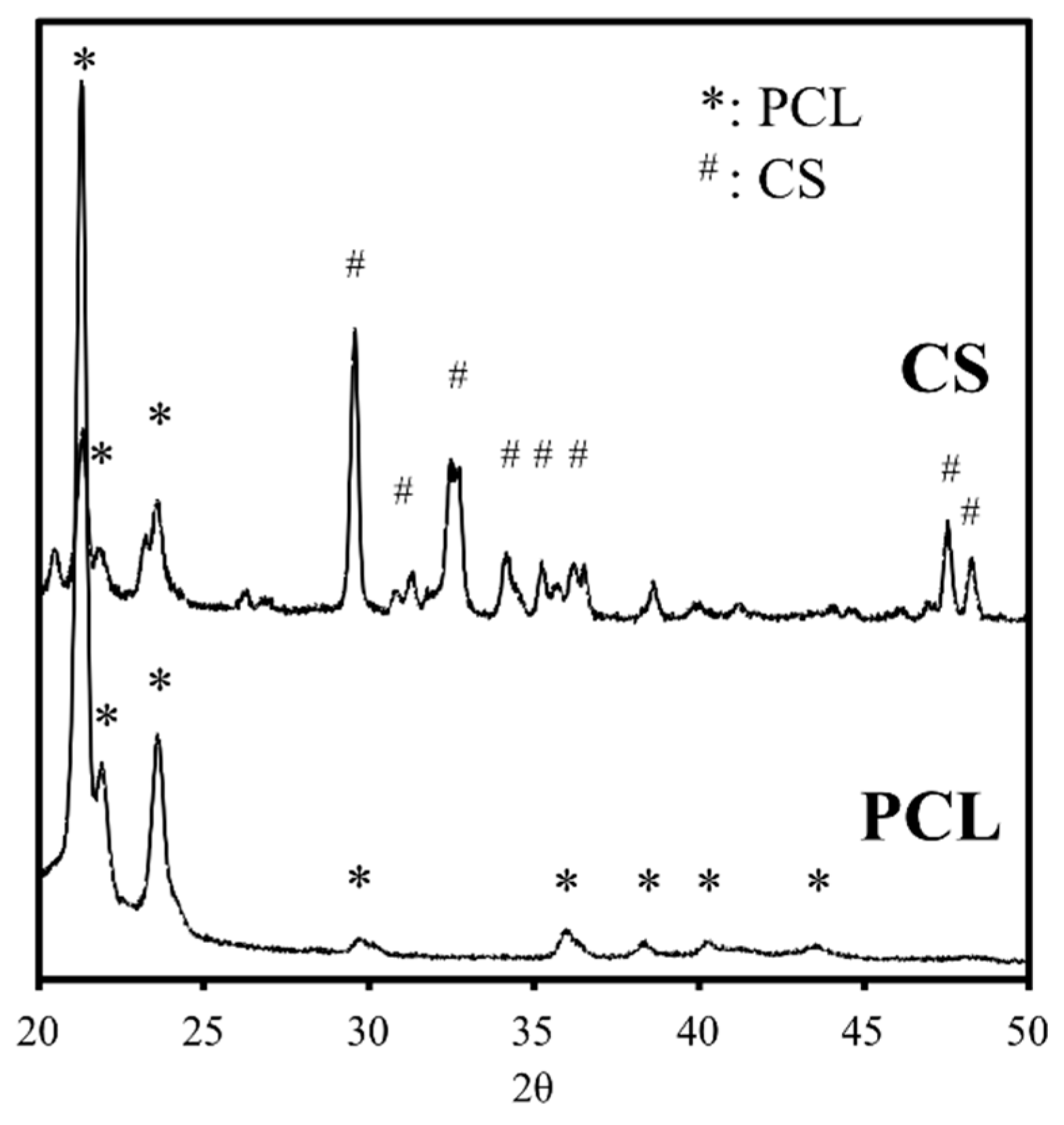
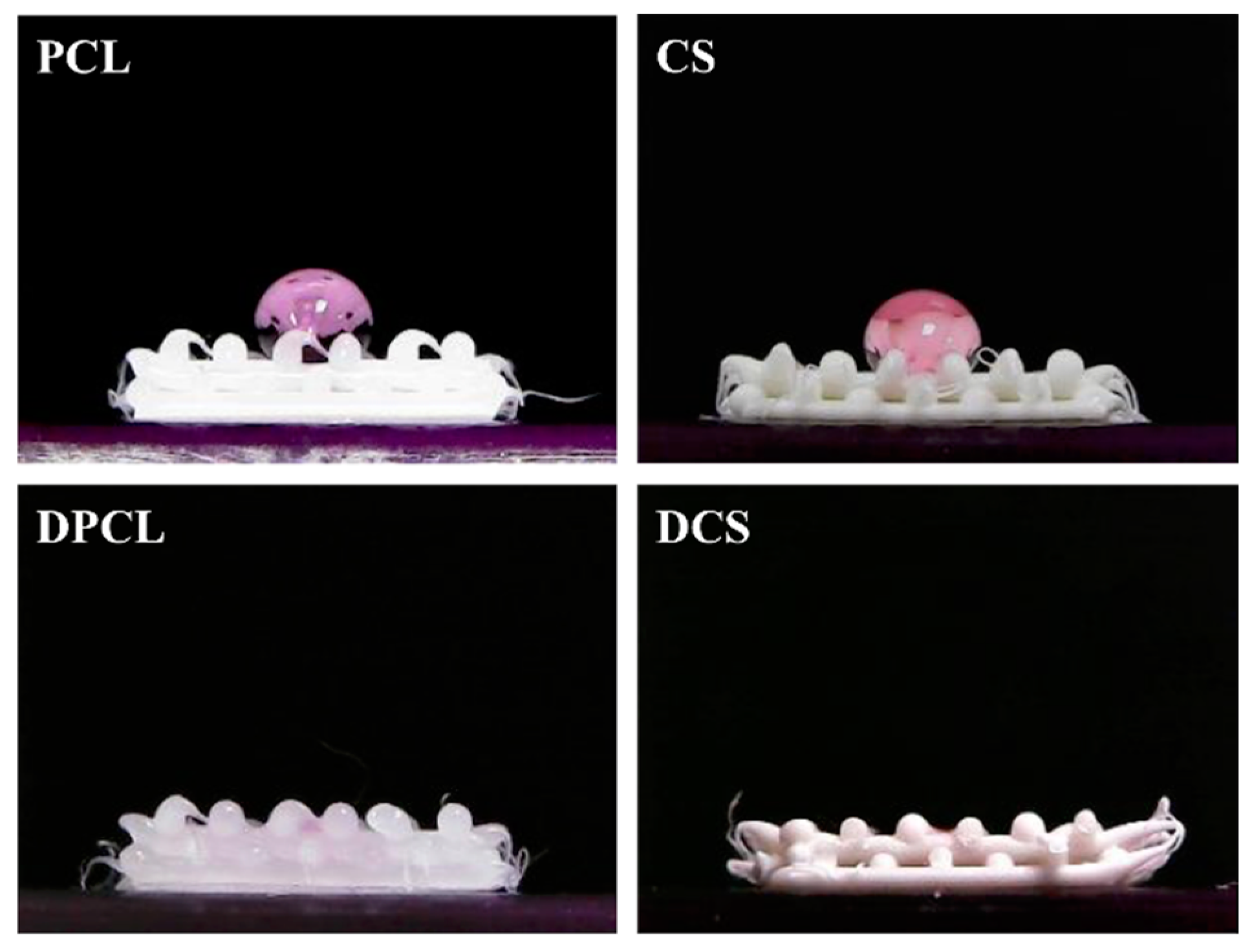
2.1. The Characterizations of CS/PCL Scaffold
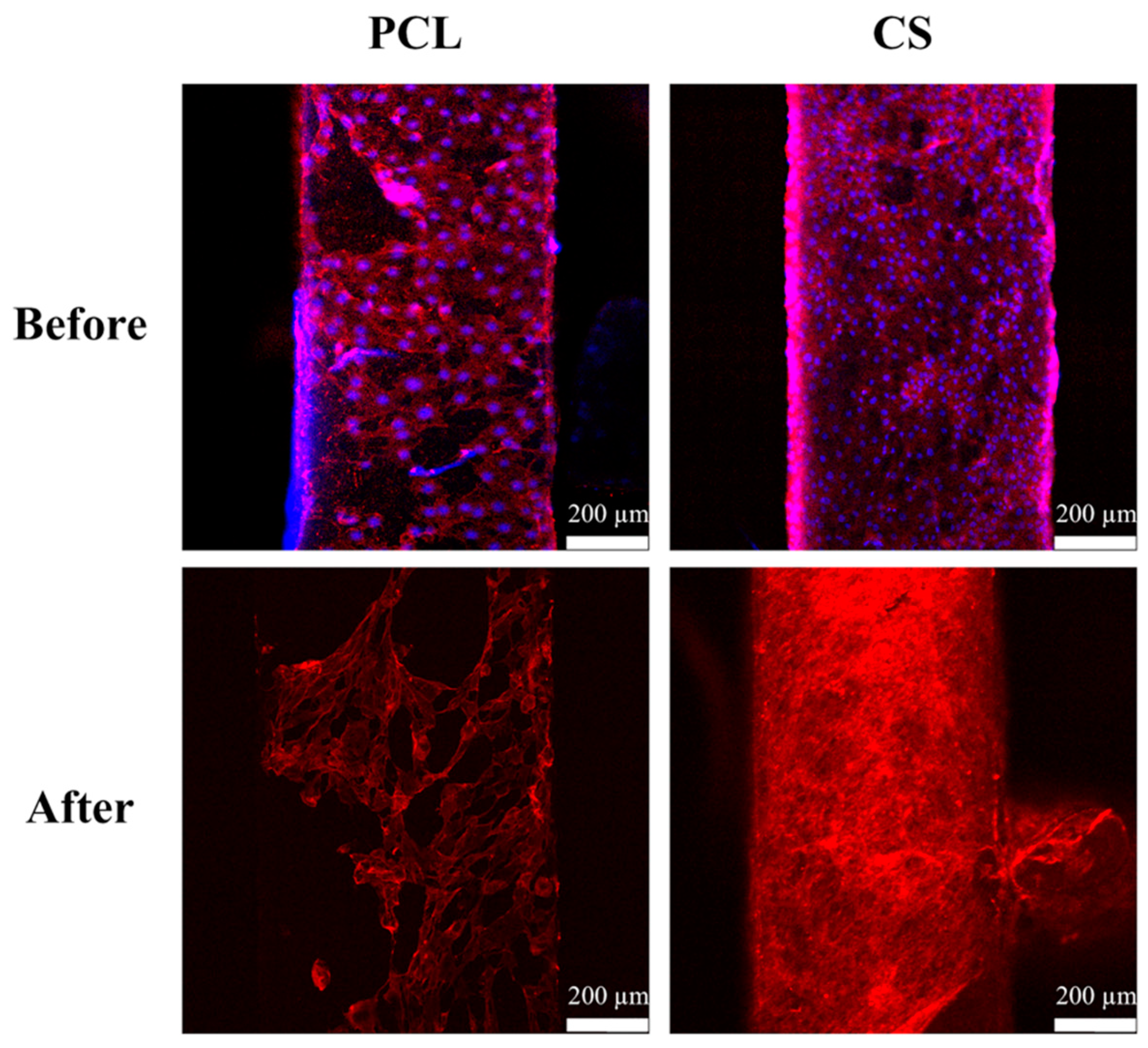
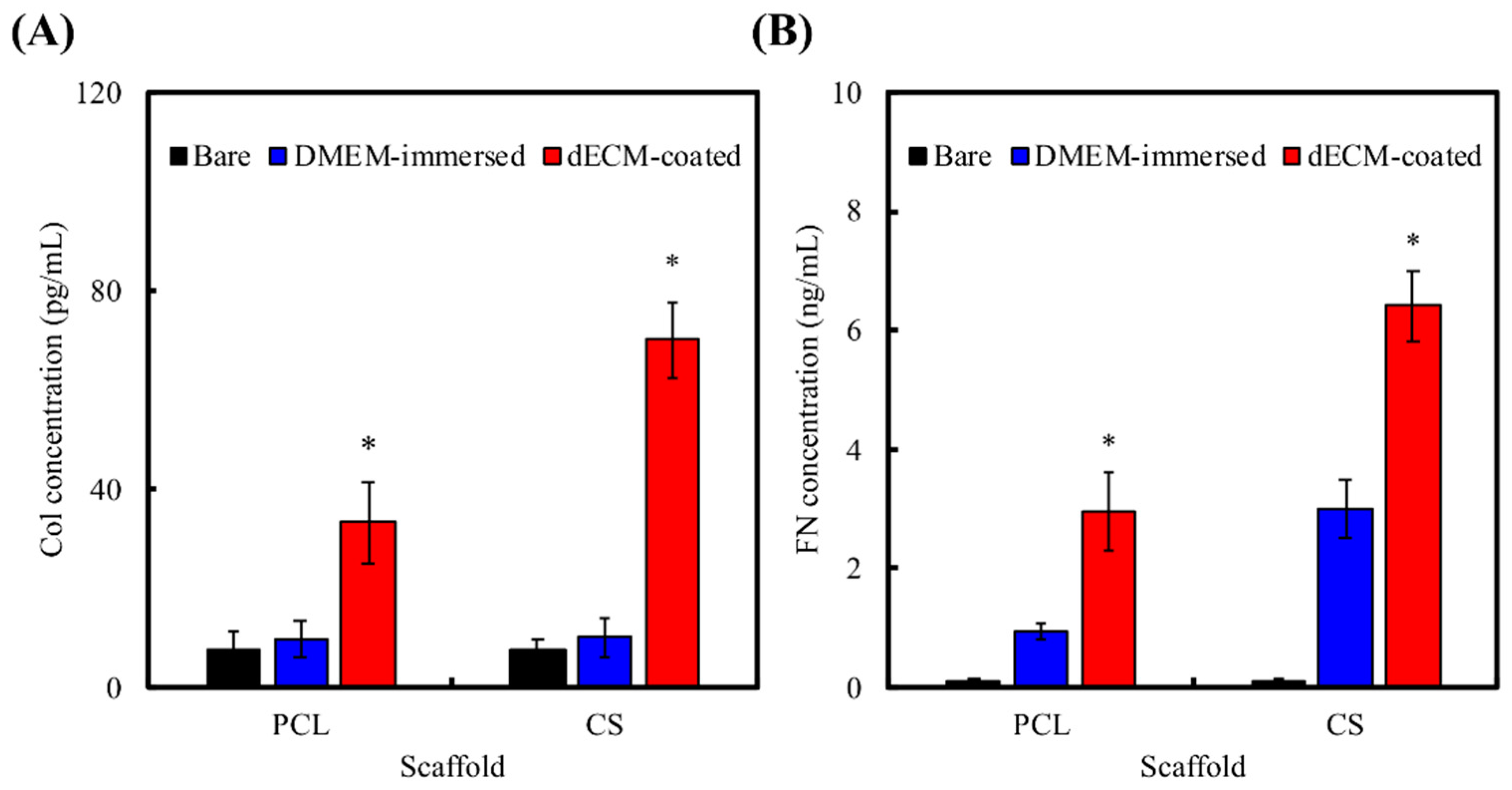
2.2. dECM Prepared from MG63
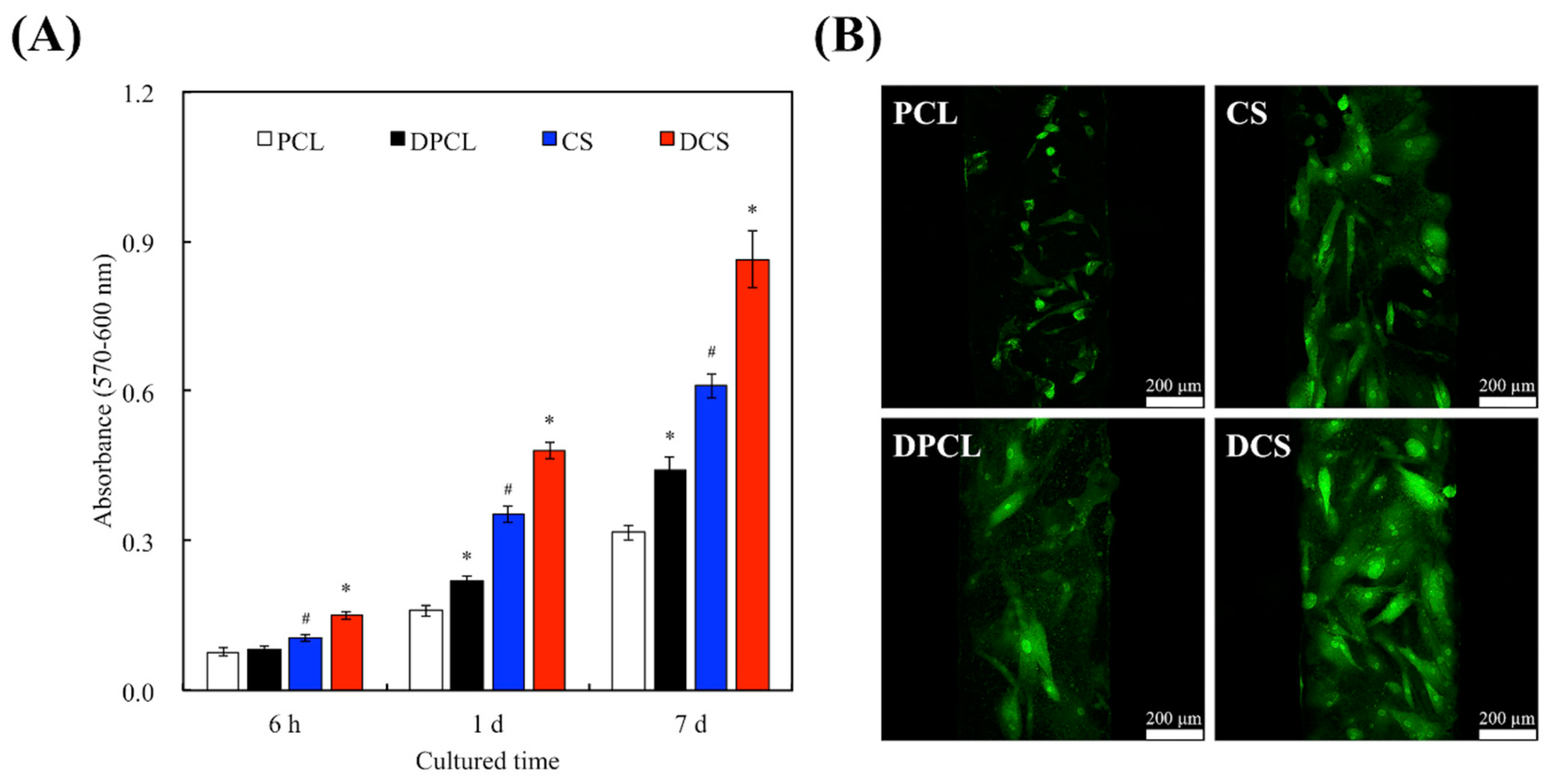
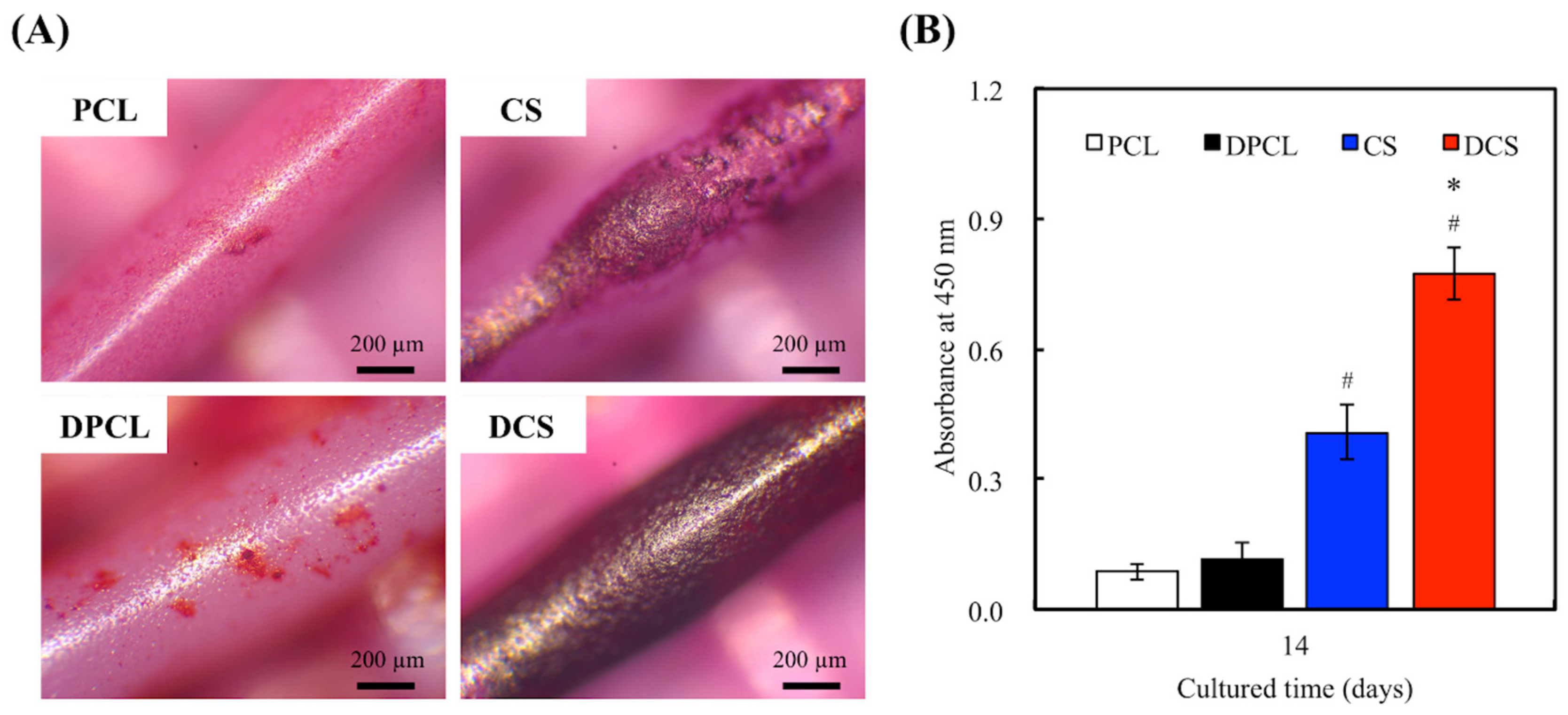
2.3. Cell Adhesion and Proliferation
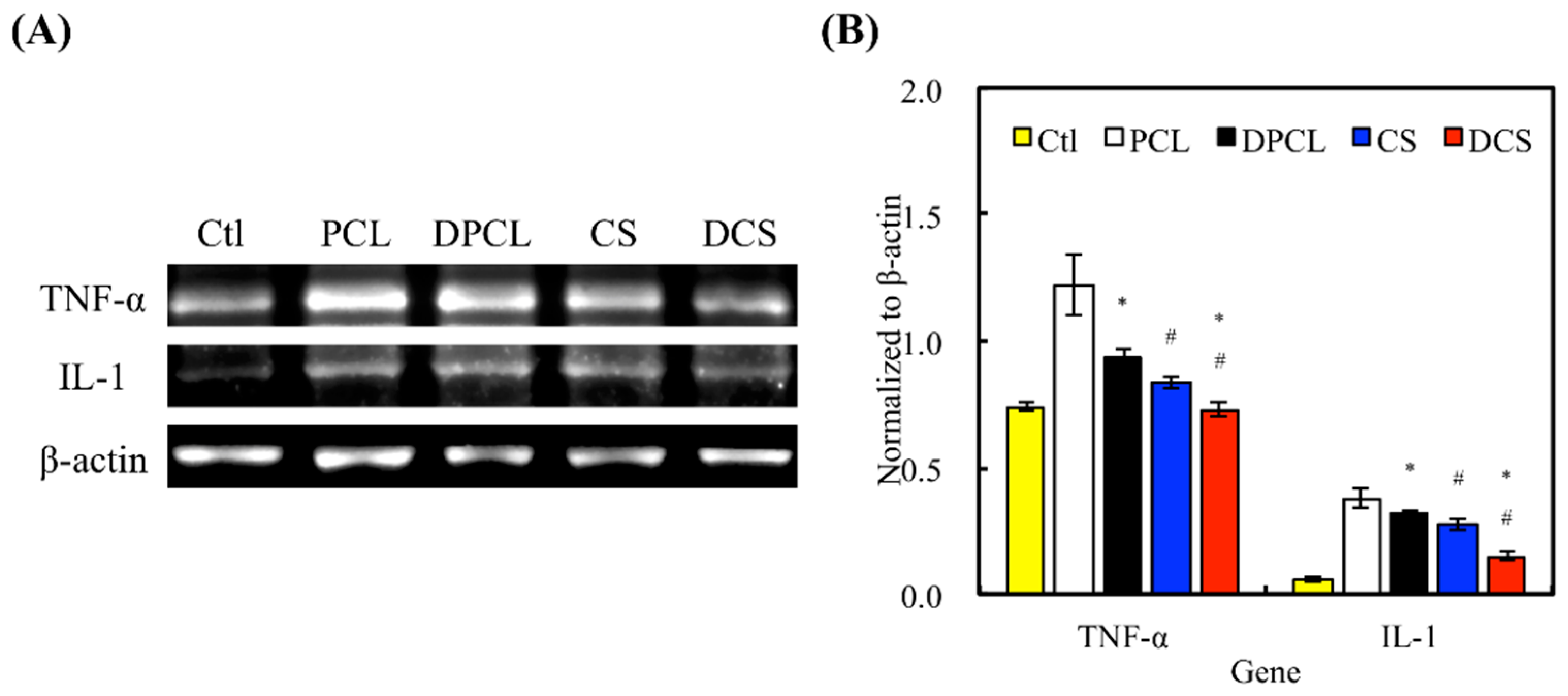
2.4. Inflammation Response
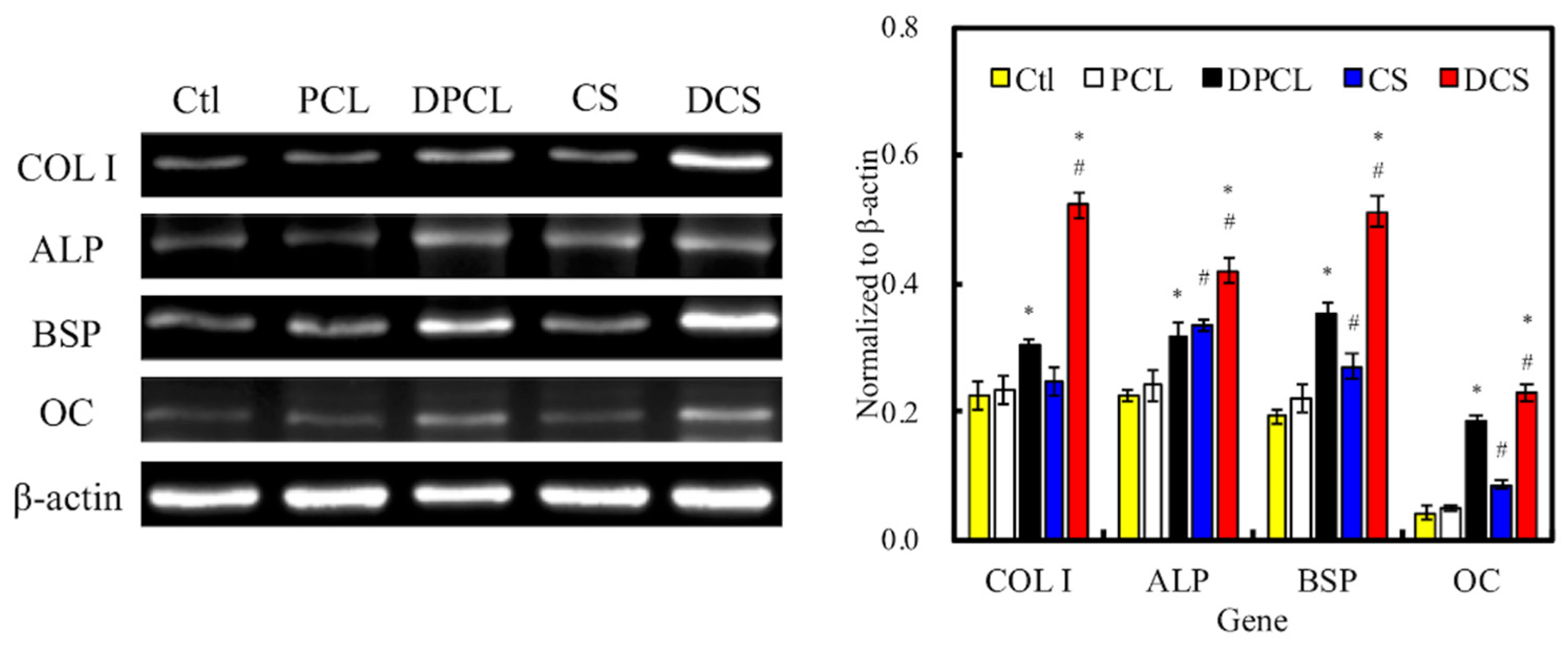
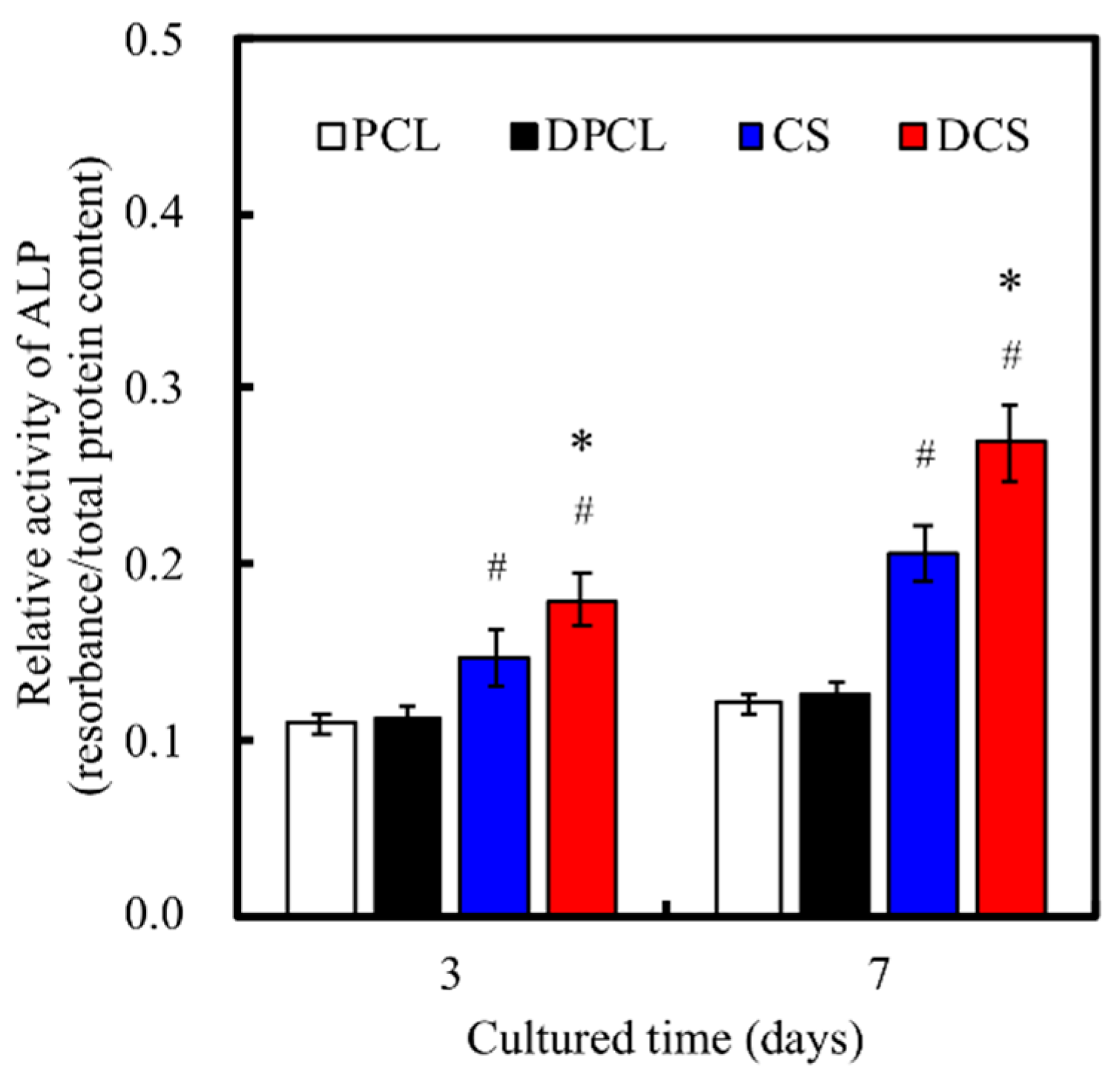
2.5. Osteogenesis and Mineralization
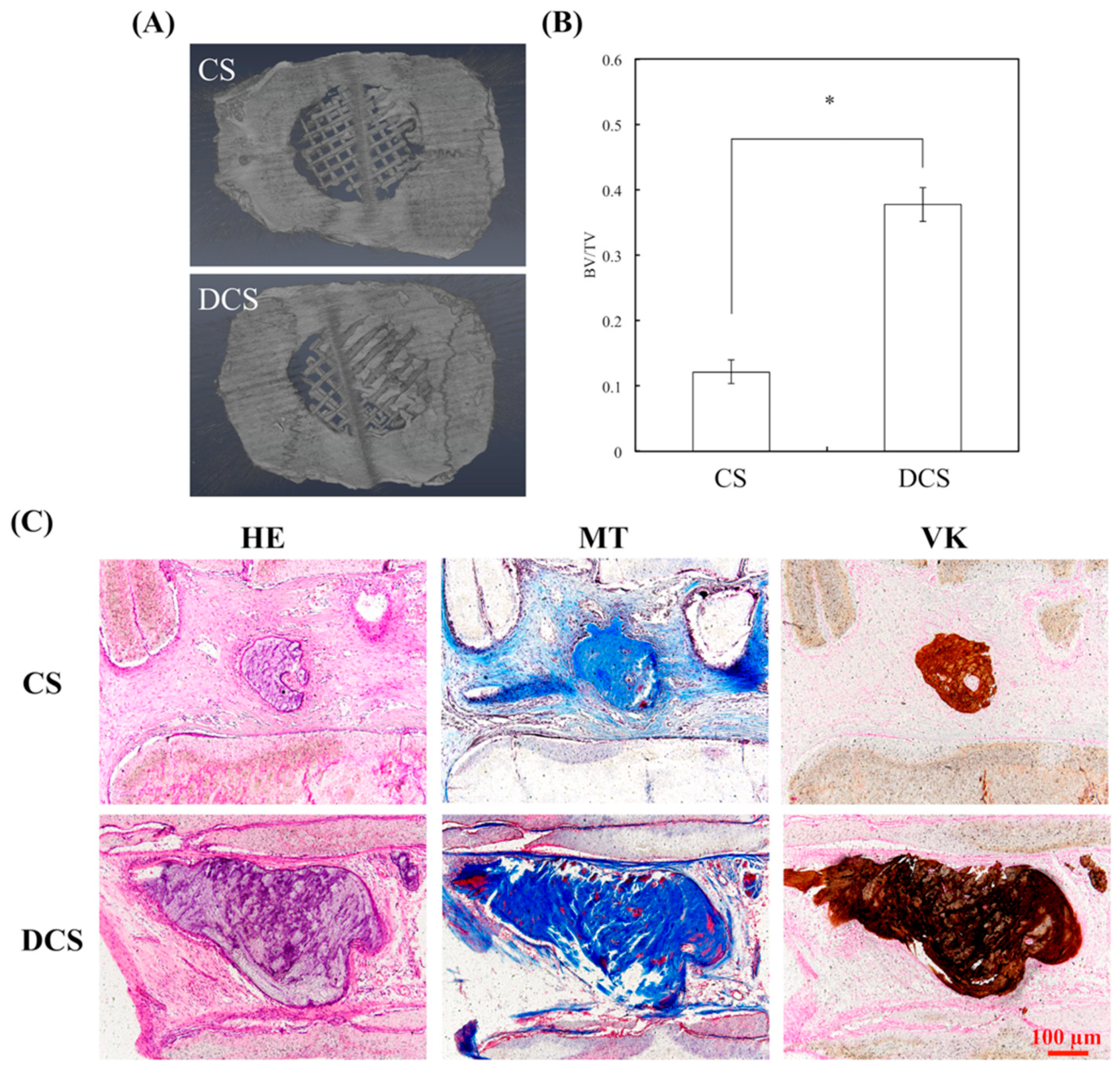
2.6. In Vivo Bone Formation in Calvarial Defects
3. Materials and Methods
3.1. CS Powder Preparation
3.2. Preparation of the CS/PCL Paste
3.3. Scaffold Fabrication
3.4. Phase Compositions and Morphology
3.5. Decellularized ECM Coating and Characterization
3.6. ELISA Analysis
3.7. Cell Adhesion and Proliferation
3.8. Reverse Transcription Polymerase Chain Reaction
3.9. Osteogenesis Assay
3.10. Alizarin Red S Stain
3.11. Implantation in Rat Calvaria Model
3.12. Micro-Computed Tomography
3.13. Histological Staining
3.14. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugiyama, K.; Okamura, A.; Kawazoe, N.; Tateishi, T.; Sato, S.; Chen, G. Coating of collagen on a poly(l-lactic acid) sponge surface for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 290–295. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J.H.; Shirahama, H.; Seo, J.; Glenn, J.S.; Cho, N.J. Extracellular matrix functionalization and Huh-7.5 cell coculture promote the hepatic differentiation of human adipose-derived mesenchymal stem cells in a 3D ICC hydrogel scaffold. ACS Biomater. Sci. Eng. 2016, 2, 2255–2265. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D bioprinting technologies for hard tissue and organ engineering. Materials 2016, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, C.; Sui, X.; Cai, N.; Zhang, J.; Zhu, Y.; Zhang, L. The hypothermic preservation of mammalian cells with assembling extracellular-matrix-mimetic microparticles. J. Mater. Chem. B 2017, 5, 1535–1541. [Google Scholar] [CrossRef]
- Lu, H.; Hoshiba, T.; Kawazoe, N.; Chen, G. Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials 2011, 32, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Sangkert, S.; Meesane, J.; Kamonmattayakul, S.; Chai, W.L. Modified silk fibroin scaffolds with collagen/decellularized pulp for bone tissue engineering in cleft palate: Morphological structures and biofunctionalities. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1138–1149. [Google Scholar] [CrossRef]
- Wilson, K.; Terlouw, A.; Roberts, K.; Wolchok, J.C. The characterization of decellularized human skeletal muscle as a blueprint for mimetic scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 125. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- Hwang, M.P.; Subbiah, R.; Kim, I.G.; Lee, K.E.; Park, J.; Kim, S.H.; Park, K. Approximating bone ECM: Crosslinking directs individual and coupled osteoblast/osteoclast behavior. Biomaterials 2016, 103, 22–32. [Google Scholar] [CrossRef]
- Chen, S.; Nakamoto, T.; Kawazoe, N.; Chen, G. Engineering multi-layered skeletal muscle tissue by using 3D microgrooved collagen scaffolds. Biomaterials 2015, 73, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 035002. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.P.K.; Burke, M.; Carter, B.M.; Davis, S.A.; Perriman, A.W. 3D bioprinting using a templated porous bioink. Adv. Healthc. Mater. 2016, 5, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Yu, J.; Ng, H.Y.; Lee, K.X.; Chen, C.C.; Chen, Y.S.; Shie, M.Y. The physicochemical properties of decellularized extracellular matrix-coated 3D printed poly(ε-caprolactone) nerve conduits for promoting Schwann cells proliferation and differentiation. Materials 2018, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Gerke, R.; Götz, H.; Stein, S.; Rommens, P.M. A new bone substitute developed from 3D-prints of polylactide (PLA) loaded with collagen I: An in vitro study. Int. J. Mol. Sci. 2017, 18, 2569. [Google Scholar] [CrossRef] [PubMed]
- Maietta, S.; Russo, T.; Santis, R.D.; Ronca, D.; Riccardi, F.; Catauro, M.; Martorelli, M.; Gloria, A. Further theoretical insight into the mechanical properties of polycaprolactone loaded with organic-inorganic hybrid fillers. Materials 2018, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Gloria, A.; Coelho, J.; Bartolo, P.; Ciurana, J. Three-dimensional printed bone scaffolds: The role of nano/micro-hydroxyapatite particles on the adhesion and differentiation of human mesenchymal stem cells. Proc. Inst. Mech. Eng. H 2017, 231, 555–564. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Schnettler, R.; Barbeck, M. Additive manufacturing for guided bone regeneration: A perspective for alveolar ridge augmentation. Int. J. Mol. Sci. 2018, 19, 3308. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hung, C.H.; Kuo, C.N.; Chen, C.Y.; Peng, Y.N.; Shie, M.Y. Improved bioactivity of 3D printed porous titanium alloy scaffold with chitosan/magnesium-calcium silicate composite for orthopaedic applications. Materials 2019, 12, 203. [Google Scholar] [CrossRef]
- Song, K.; Wang, Z.; Liu, R.; Chen, G.; Liu, L. Microfabrication-based three-dimensional (3-D) extracellular matrix microenvironments for cancer and other diseases. Int. J. Mol. Sci. 2018, 19, 935. [Google Scholar] [CrossRef]
- Kao, C.T.; Lin, C.C.; Chen, Y.W.; Yeh, C.H.; Fang, H.Y.; Shie, M.Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 165–173. [Google Scholar] [CrossRef]
- Yu, Y.; Moncal, K.K.; Li, J.; Peng, W.; Rivero, I.; Martin, J.A.; Ozbolat, I.T. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci. Rep. 2016, 6, 395. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.W.; Lee, K.-W.; Park, J.H.; Lee, J.; Jung, C.R.; Yu, J.; Kim, H.Y.; Kim, D.H. 3D bioprinted artificial trachea with epithelial cells and chondrogenic-differentiated bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 1624. [Google Scholar] [CrossRef] [PubMed]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.-C.; Al-Zeer, M.A.; Kurtz, A.; Hocke, A.C.; et al. Generation of a 3D liver model comprising human extracellular matrix in an alginate/gelatin-based bioink by extrusion bioprinting for infection and transduction studies. Int. J. Mol. Sci. 2018, 19, 3129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Chen, Y.W.; Wang, K.; Shie, M.Y. Enhanced adhesion and differentiation of human mesenchymal stem cell inside apatite-mineralized/poly(dopamine)-coated poly(ε-caprolactone) scaffolds by stereolithography. J. Mater. Chem. B 2016, 4, 6307–6315. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Q.; Gao, X.; Dong, Y. Characteristics and effects on dental pulp cells of a polycaprolactone/submicron bioactive glass composite scaffold. J. Endod. 2016, 42, 1070–1075. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Fang, H.Y.; Hsu, T.T.; Lin, C.Y.; Shie, M.Y. The characteristics of Mineral Trioxide Aggregate/polycaprolactone 3-dimensional scaffold with osteogenesis properties for tissue regeneration. J. Endod. 2017, 43, 923–929. [Google Scholar] [CrossRef]
- Shim, J.H.; Won, J.Y.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Bae, E.B.; et al. Effects of 3D-printed polycaprolactone/β-tricalcium phosphate membranes on guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yeh, C.H.; Shie, M.Y. Stimulatory effects of the fast setting and degradable Ca–Si–Mg cement on both cementogenesis and angiogenesis differentiation of human periodontal ligament cells. J. Mater. Chem. B 2015, 3, 7099–7108. [Google Scholar] [CrossRef]
- Shie, M.Y.; Chiang, W.H.; Chen, I.W.P.; Liu, W.Y.; Chen, Y.W. Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate–graphene composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 726–735. [Google Scholar] [CrossRef]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pei, P.; Zhu, M.; Du, X.; Xin, C.; Zhao, S.; Li, X.; Zhu, Y. Three dimensional printing of calcium sulfate and mesoporous bioactive glass scaffolds for improving bone regeneration in vitro and in vivo. Sci. Rep. 2017, 7, 42556. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, A.; Shao, H.; Yang, X.; Ma, C.; Yan, S.; Liu, Y.; He, Y.; Gou, Z. Systematical evaluation of mechanically strong 3D printed diluted magnesium doping wollastonite scaffolds on osteogenic capacity in rabbit calvarial defects. Sci. Rep. 2016, 6, 34029. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Ding, S.J. Integrin binding and MAPK signal pathways in primary cell responses to surface chemistry of calcium silicate cements. Biomaterials 2013, 34, 6589–6606. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Chiu, Y.C.; Shen, Y.F.; Wu, Y.H.; Shie, M.Y. Bioactive calcium silicate/poly-ε-caprolactone composite scaffolds 3D printed under mild conditions for bone tissue engineering. J. Mater. Sci. Mater. Med. 2018, 29, 11. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. 3D printed tricalcium phosphate scaffolds: Effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater. Sci. 2013, 1, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Ho, C.C.; Huang, T.H.; Hsu, T.T.; Shie, M.Y. The ionic products from mineral trioxide aggregate–induced odontogenic differentiation of dental pulp cells via activation of the Wnt/β-catenin signaling pathway. J. Endod. 2016, 42, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, L.; Chen, X.; Liu, T.; Pan, G.; Cui, W.; Li, M.; Luo, Z.P.; Pei, M.; Yang, H.; et al. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Kawazoe, N.; Chen, G. Influence of surfaces modified with biomimetic extracellular matrices on adhesion and proliferation of mesenchymal stem cells and osteosarcoma cells. Colloids Surf. B 2015, 126, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Y.; Chen, Y.W.; Kao, C.T.; Hsu, T.T.; Huang, T.H.; Shie, M.Y. Human dental pulp cells responses to apatite precipitation from dicalcium silicates. Materials 2015, 8, 4491–4504. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.Y.; Lin, H.Y.; Chen, Y.W.; Lin, C.Y.; Hsu, T.T.; Kao, C.T. Laser sintered magnesium-calcium silicate/poly-ε-caprolactone scaffold for bone tissue engineering. Materials 2017, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Fu, S.J. Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.T.; Hsu, T.T.; Huang, T.H.; Wu, Y.T.; Chen, Y.W.; Shie, M.Y. The synergistic effect on osteogenic differentiation of human mesenchymal stem cell by diode laser-treated stimulating human umbilical vein endothelial cell. Laser Phys. Lett. 2016, 13, 025604. [Google Scholar] [CrossRef]
- Steeves, A.J.; Atwal, A.; Schock, S.C.; Variola, F. Evaluation of the direct effects of poly(dopamine) on the in vitro response of human osteoblastic cells. J. Mater. Chem. B 2016, 4, 3145–3156. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Chen, Y.C.; Cai, H.; Yang, X.; Zhu, X.; Fan, Y.; Zhang, X. Roles of calcium phosphate-mediated integrin expression and MAPK signaling pathways in the osteoblastic differentiation of mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 2280–2289. [Google Scholar] [CrossRef]
- Wu, B.C.; Kao, C.T.; Huang, T.H.; Hung, C.J.; Shie, M.Y.; Chung, H.Y. Effect of verapamil, a calcium channel blocker, on the odontogenic activity of human dental pulp cells cultured with silicate-based materials. J. Endod. 2014, 40, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Scaffold design and manufacturing: From concept to clinic. Adv. Mater. 2009, 21, 3330–3342. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Portolés, P.; Montes-Casado, M.; Izquierdo-Barba, I.; Vallet-Regí, M.; Portolés, M.T. Effects of 3D nanocomposite bioceramic scaffolds on the immune response. J. Mater. Chem. B 2014, 2, 3469–3479. [Google Scholar] [CrossRef]
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Declercq, H.; Martens, L.; de Coster, P. Gene expression profiling and molecular signaling of various cells in response to tricalcium silicate cements: A systematic review. J. Endod. 2016, 42, 1713–1725. [Google Scholar] [CrossRef]
- Lai, W.Y.; Kao, C.T.; Hung, C.J.; Huang, T.H.; Shie, M.Y. An evaluation of the inflammatory response of lipopolysaccharide-treated primary dental pulp cells with regard to calcium silicate-based cements. Int. J. Oral Sci. 2014, 6, 94–98. [Google Scholar] [CrossRef]
- Park, H.C.; Quan, H.; Zhu, T.; Kim, Y.; Kim, B.; Yang, H.C. The effects of M1 and M2 macrophages on odontogenic differentiation of human dental pulp cells. J. Endod. 2017, 43, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Song, T.H.; Rijal, G.; Jang, J.; Kim, S.W.; Cho, D.W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 2015, 37, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Raja, N.; Yun, H.S. A simultaneous 3D printing process for the fabrication of bioceramic and cell-laden hydrogel core/shell scaffolds with potential application in bone tissue regeneration. J. Mater. Chem. B 2016, 4, 4707–4716. [Google Scholar] [CrossRef]
- Huang, M.H.; Shen, Y.F.; Hsu, T.T.; Huang, T.H.; Shie, M.Y. Physical characteristics, antimicrobial and odontogenesis potentials of calcium silicate cement containing hinokitiol. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence |
|---|---|
| TNF-α | Forward: 5′-CCCAGGCAGTCAGATCATCTTC-3′ |
| Reverse: 5′-AGCTGCCCCTCAGCTTGA-3′ | |
| IL-1 | Forward: 5′-AGTCAGCTCTCTCCTTTCAGG-3′ |
| Reverse: 5′-CTTGCCCCCTTTGAATAAAT-3′ | |
| Col I | Forward: 5′-CCATGTGAAATTGTCTCCCA-3′ |
| Reverse: 5′-GGGGCAAGACAGTGATTGAA-3′ | |
| ALP | Forward: 5′-TAGTTCCTGGTACCTCTGCTCC-3′ |
| Reverse: 5′-CAGTTTCCTTTCCTGAATACCG-3′ | |
| BSP | Forward: 5′- TCACCTGTGCCATACCAGTTAA-3′ |
| Reverse: 5′- TGAGATGGGTCAGGGTTTAGC-3′ | |
| OC | Forward: 5′- GTGCAGAGTCCAGCAAAGGT-3′ |
| Reverse: 5′- CGATAGGCCTCCTGAAAGC-3′ | |
| β-actin | Forward: 5′-AGAGCTACGAGCTGCCTGAC-3′ |
| Reverse: 5′-AGCACTGTGTTGGCGTACAG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-H.A.; Chiu, Y.-C.; Lin, Y.-H.; Ho, C.-C.; Shie, M.-Y.; Chen, Y.-W. 3D-Printed Bioactive Calcium Silicate/Poly-ε-Caprolactone Bioscaffolds Modified with Biomimetic Extracellular Matrices for Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 942. https://doi.org/10.3390/ijms20040942
Wu Y-HA, Chiu Y-C, Lin Y-H, Ho C-C, Shie M-Y, Chen Y-W. 3D-Printed Bioactive Calcium Silicate/Poly-ε-Caprolactone Bioscaffolds Modified with Biomimetic Extracellular Matrices for Bone Regeneration. International Journal of Molecular Sciences. 2019; 20(4):942. https://doi.org/10.3390/ijms20040942
Chicago/Turabian StyleWu, Yuan-Haw Andrew, Yung-Cheng Chiu, Yen-Hong Lin, Chia-Che Ho, Ming-You Shie, and Yi-Wen Chen. 2019. "3D-Printed Bioactive Calcium Silicate/Poly-ε-Caprolactone Bioscaffolds Modified with Biomimetic Extracellular Matrices for Bone Regeneration" International Journal of Molecular Sciences 20, no. 4: 942. https://doi.org/10.3390/ijms20040942
APA StyleWu, Y.-H. A., Chiu, Y.-C., Lin, Y.-H., Ho, C.-C., Shie, M.-Y., & Chen, Y.-W. (2019). 3D-Printed Bioactive Calcium Silicate/Poly-ε-Caprolactone Bioscaffolds Modified with Biomimetic Extracellular Matrices for Bone Regeneration. International Journal of Molecular Sciences, 20(4), 942. https://doi.org/10.3390/ijms20040942







