Strengthening the AntiTumor NK Cell Function for the Treatment of Ovarian Cancer
Abstract
1. Overview on Epithelial Ovarian Cancer
2. Epithelial Ovarian Cancer: A Focus on Tumor Escape Mechanisms Impairing NK Cell Function
3. State-of-the-Art Therapies Targeting Ovarian Cancer
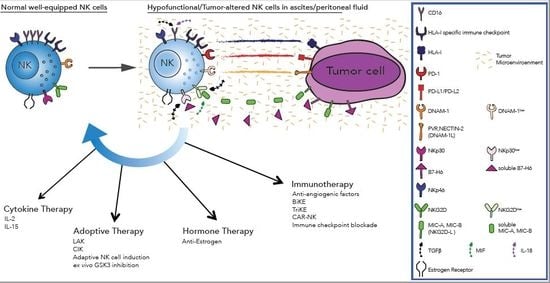
3.1. Cytokine Therapy
3.2. Adoptive Therapy of Immune Cells
3.3. Hormone Therapy in Ovarian Cancer
3.4. Immunotherapy
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P.; Kurzeder, C.; Mustea, A.; Wimberger, P.; Hauptmann, S.; Sehouli, J. The new WHO classification of ovarian, fallopian tube and primary peritoneal cancer and its clinical implications. Arch. Gynecol. Obstet. 2016, 293, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Sun, C.C.; Lu, K.H.; Coleman, R.L.; Sood, A.K.; Malpica, A.; Deavers, M.T.; Silva, E.G.; Bodurka, D.C. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet. Gynecol. 2006, 108, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ozols, R.F. Maintenance therapy in advanced ovarian cancer: Progression-free survival and clinical benefit. J. Clin. Oncol. 2003, 21, 2451–2453. [Google Scholar] [CrossRef] [PubMed]
- Gourley, C.; Farley, J.; Provencher, D.M.; Pignata, S.; Mileshkin, L.; Harter, P.; Maenpaa, J.; Kim, J.W.; Pujaide-Lauraine, E.; Glasspool, R.M.; et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian and primary peritoneal low-grade serous carcinomas. Int. J. Gynecol. Cancer 2014, 24, S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Baak, J.P.; Delemarre, J.F.; Langley, F.A.; Talerman, A. Grading ovarian tumors. Evaluation of decision making by different pathologists. Anal. Quant. Cytol. Histol. 1986, 8, 349–353. [Google Scholar] [PubMed]
- Stalsberg, H.; Abeler, V.; Blom, G.P.; Bostad, L.; Skarland, E.; Westgaard, G. Observer variation in histologic classification of malignant and borderline ovarian tumors. Hum. Pathol. 1988, 19, 1030–1035. [Google Scholar] [CrossRef]
- Bertelsen, K.; Holund, B.; Andersen, E. Reproducibility and prognostic value of histologic type and grade in early epithelial ovarian cancer. Int. J. Gynecol. Cancer 1993, 3, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Kamoi, S.; Amada, S.; Akiyama, F.; Silverberg, S.G. Toward the development of a universal grading system for ovarian epithelial carcinoma: Testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer 1998, 82, 893–901. [Google Scholar] [CrossRef]
- Ishioka, S.; Sagae, S.; Terasawa, K.; Sugimura, M.; Nishioka, Y.; Tsukada, K.; Kudo, R. Comparison of the usefulness between a new universal grading system for epithelial ovarian cancer and the FIGO grading system. Gynecol. Oncol. 2003, 89, 447–452. [Google Scholar] [CrossRef]
- Seidman, J.D.; Mehrotra, A. Benign ovarian serous tumors: A re-evaluation and proposed reclassification of serous “cystadenomas” and “cystadenofibromas”. Gynecol. Oncol. 2005, 96, 395–401. [Google Scholar] [CrossRef]
- Bonome, T.; Lee, J.Y.; Park, D.C.; Radonovich, M.; Pise-Masison, C.; Brady, J.; Gardner, G.J.; Hao, K.; Wong, W.H.; Barrett, J.C.; et al. Expression profiling of serous low malignant potential, low-grade and high-grade tumors of the ovary. Cancer Res. 2005, 65, 10602–10612. [Google Scholar] [CrossRef] [PubMed]
- Jazaeri, A.A.; Lu, K.; Schmandt, R.; Harris, C.P.; Rao, P.H.; Sotiriou, C.; Chandramouli, G.V.; Gershenson, D.M.; Liu, E.T. Molecular determinants of tumor differentiation in papillary serous ovarian carcinoma. Mol. Carcinog. 2003, 36, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Meinhold-Heerlein, I.; Bauerschlag, D.; Hilpert, F.; Dimitrov, P.; Sapinoso, L.M.; Orlowska-Volk, M.; Bauknecht, T.; Park, T.W.; Jonat, W.; Jacobsen, A.; et al. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene 2005, 24, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Bodurka, D.C.; Lu, K.H.; Nathan, L.C.; Milojevic, L.; Wong, K.K.; Malpica, A.; Sun, C.C. Impact of Age and Primary Disease Site on Outcome in Women With Low-Grade Serous Carcinoma of the Ovary or Peritoneum: Results of a Large Single-Institution Registry of a Rare Tumor. J. Clin. Oncol. 2015, 33, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Tsang, Y.T.; Deavers, M.T.; Mok, S.C.; Zu, Z.; Sun, C.; Malpica, A.; Wolf, J.K.; Lu, K.H.; Gershenson, D.M. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am. J. Pathol. 2010, 177, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Grisham, R.N.; Iyer, G.; Garg, K.; Delair, D.; Hyman, D.M.; Zhou, Q.; Iasonos, A.; Berger, M.F.; Dao, F.; Spriggs, D.R.; et al. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer 2013, 119, 548–554. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Coleman, R.L.; Markman, M. Ovarian cancer. Lancet 2009, 374, 1371–1382. [Google Scholar] [CrossRef]
- Colombo, N.; Peiretti, M.; Parma, G.; Lapresa, M.; Mancari, R.; Carinelli, S.; Sessa, C.; Castiglione, M.; Group, E.G.W. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v23–v30. [Google Scholar] [CrossRef]
- Schmeler, K.M.; Sun, C.C.; Bodurka, D.C.; Deavers, M.T.; Malpica, A.; Coleman, R.L.; Ramirez, P.T.; Gershenson, D.M. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol. Oncol. 2008, 108, 510–514. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Sun, C.C.; Bodurka, D.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Deavers, M.; Malpica, A.L.; Kavanagh, J.J. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol. Oncol. 2009, 114, 48–52. [Google Scholar] [CrossRef]
- Grabowski, J.P.; Harter, P.; Heitz, F.; Pujade-Lauraine, E.; Reuss, A.; Kristensen, G.; Ray-Coquard, I.; Heitz, J.; Traut, A.; Pfisterer, J.; et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol. Oncol. 2016, 140, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Kipps, E.; Tan, D.S.; Kaye, S.B. Meeting the challenge of ascites in ovarian cancer: New avenues for therapy and research. Nat. Rev. Cancer 2013, 13, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kulbe, H.; Chakravarty, P.; Leinster, D.A.; Charles, K.A.; Kwong, J.; Thompson, R.G.; Coward, J.I.; Schioppa, T.; Robinson, S.C.; Gallagher, W.M.; et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012, 72, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Tabellini, G.; Cantoni, C.; Patrizi, O.; Coltrini, D.; Rampinelli, F.; Matta, J.; Vivier, E.; Moretta, A.; Parolini, S.; et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma immune escape. Oncoimmunology 2015, 4, e1001224. [Google Scholar] [CrossRef]
- Peng, P.; Yan, Y.; Keng, S. Exosomes in the ascites of ovarian cancer patients: Origin and effects on antitumor immunity. Oncol. Rep. 2011, 25, 749–762. [Google Scholar] [PubMed]
- Latifi, A.; Luwor, R.B.; Bilandzic, M.; Nazaretian, S.; Stenvers, K.; Pyman, J.; Zhu, H.; Thompson, E.W.; Quinn, M.A.; Findlay, J.K.; et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: Molecular phenotype of chemoresistant ovarian tumors. PLoS ONE 2012, 7, e46858. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.C.; Goode, E.L.; Hartmann, L.C.; Kalli, K.R.; Knutson, K.L. Immunity and immune suppression in human ovarian cancer. Immunotherapy 2011, 3, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Worzfeld, T.; Pogge von Strandmann, E.; Huber, M.; Adhikary, T.; Wagner, U.; Reinartz, S.; Muller, R. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front. Oncol. 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Norell, H.; Bryceson, Y.T.; Poschke, I.; Schedvins, K.; Ljunggren, H.G.; Kiessling, R.; Malmberg, K.J. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J. Immunol. 2009, 183, 4921–4930. [Google Scholar] [CrossRef] [PubMed]
- Vyas, M.; Reinartz, S.; Hoffmann, N.; Reiners, K.S.; Lieber, S.; Jansen, J.M.; Wagner, U.; Muller, R.; von Strandmann, E.P. Soluble NKG2D ligands in the ovarian cancer microenvironment are associated with an adverse clinical outcome and decreased memory effector T cells independent of NKG2D downregulation. Oncoimmunology 2017, 6, e1339854. [Google Scholar] [CrossRef] [PubMed]
- Marcenaro, E.; Dondero, A.; Moretta, A. Multi-directional crossregulation of NK cell function during innate immune responses. Transpl. Immunol. 2006, 17, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Marcenaro, E.; Ferranti, B.; Moretta, A. NK-DC interaction: On the usefulness of auto-aggression. Autoimmun. Rev. 2005, 4, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Carlomagno, S.; Pesce, S.; Moretta, A.; Vitale, M.; Marcenaro, E. TLR/NCR/KIR: Which One to Use and When? Front. Immunol. 2014, 5, 105. [Google Scholar] [CrossRef]
- Lai, P.; Rabinowich, H.; Crowley-Nowick, P.A.; Bell, M.C.; Mantovani, G.; Whiteside, T.L. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin. Cancer Res. 1996, 2, 161–173. [Google Scholar] [PubMed]
- Lukesova, S.; Vroblova, V.; Tosner, J.; Kopecky, J.; Sedlakova, I.; Cermakova, E.; Vokurkova, D.; Kopecky, O. Comparative study of various subpopulations of cytotoxic cells in blood and ascites from patients with ovarian carcinoma. Contemp. Oncol. 2015, 19, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Mailliard, R.B.; Alber, S.M.; Shen, H.; Watkins, S.C.; Kirkwood, J.M.; Herberman, R.B.; Kalinski, P. IL-18-induced CD83+CCR7+ NK helper cells. J. Exp. Med. 2005, 202, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Otegbeye, F.; Ojo, E.; Moreton, S.; Mackowski, N.; Lee, D.A.; de Lima, M.; Wald, D.N. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS ONE 2018, 13, e0191358. [Google Scholar]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Krockenberger, M.; Dombrowski, Y.; Weidler, C.; Ossadnik, M.; Honig, A.; Hausler, S.; Voigt, H.; Becker, J.C.; Leng, L.; Steinle, A.; et al. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J. Immunol. 2008, 180, 7338–7348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Y.; Chen, L.; Xu, B.; Wu, C.; Jiang, J. B7-H6 expression correlates with cancer progression and patient’s survival in human ovarian cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9428–9433. [Google Scholar] [PubMed]
- Zhang, M.; Yang, J.; Hua, W.; Li, Z.; Xu, Z.; Qian, Q. Monitoring checkpoint inhibitors: Predictive biomarkers in immunotherapy. Front. Med. 2019, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Wu, T.C.; Monie, A.; Roden, R. Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol. Rev. 2008, 222, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Neal, R.D.; Kehoe, S. Diagnosis of ovarian cancer. BMJ 2015, 351, h4443. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M., Jr.; Chi, D.S. Surgical management of recurrent ovarian cancer. Semin. Oncol. 2009, 36, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K. Treatment for recurrent ovarian cancer-at first relapse. J. Oncol. 2010, 2010, 497429. [Google Scholar] [CrossRef] [PubMed]
- Uppendahl, L.D.; Dahl, C.M.; Miller, J.S.; Felices, M.; Geller, M.A. Natural Killer Cell-Based Immunotherapy in Gynecologic Malignancy: A Review. Front. Immunol. 2017, 8, 1825. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.S. Recent immunologic advances affecting the management of ovarian cancer. Clin. Obstet. Gynecol. 1985, 28, 853–871. [Google Scholar] [CrossRef]
- Freedman, R.S.; Edwards, C.L.; Bowen, J.M.; Lotzova, E.; Katz, R.; Lewis, E.; Atkinson, N.; Carsetti, R. Viral oncolysates in patients with advanced ovarian cancer. Gynecol. Oncol. 1988, 29, 337–347. [Google Scholar] [CrossRef]
- Berek, J.S.; Bast, R.C., Jr.; Lichtenstein, A.; Hacker, N.F.; Spina, C.A.; Lagasse, L.D.; Knapp, R.C.; Zighelboim, J. Lymphocyte cytotoxicity in the peritoneal cavity and blood of patients with ovarian cancer. Obstet. Gynecol. 1984, 64, 708–714. [Google Scholar]
- Recchia, F.; Saggio, G.; Cesta, A.; Candeloro, G.; Nuzzo, A.; Lombardo, M.; Carta, G.; Rea, S. Interleukin-2 and 13-cis retinoic acid as maintenance therapy in advanced ovarian cancer. Int. J. Oncol. 2005, 27, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Mantia-Smaldone, G.M.; Corr, B.; Chu, C.S. Immunotherapy in ovarian cancer. Hum. Vaccin Immunother. 2012, 8, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Oyer, J.L.; Igarashi, R.Y.; Gitto, S.B.; Copik, A.J.; Altomare, D.A. Antiovarian tumor response of donor peripheral blood mononuclear cells is due to infiltrating cytotoxic NK cells. Oncotarget 2016, 7, 7318–7328. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.F.; Yoshida, A.; Cardozo, D.M.; Jales, R.M.; Paust, S.; Derchain, S.; Guimaraes, F. Natural Killer Cells Response to IL-2 Stimulation Is Distinct between Ascites with the Presence or Absence of Malignant Cells in Ovarian Cancer Patients. Int. J. Mol. Sci. 2017, 18, 856. [Google Scholar] [CrossRef]
- Pillet, A.H.; Bugault, F.; Theze, J.; Chakrabarti, L.A.; Rose, T. A programmed switch from IL-15- to IL-2-dependent activation in human NK cells. J. Immunol. 2009, 182, 6267–6277. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; Debacker, V.; de Smedt, M.; Plum, J. Differential effects of interleukin-15 and interleukin-2 on differentiation of bipotential T/natural killer progenitor cells. J. Exp. Med. 1996, 184, 325–336. [Google Scholar] [CrossRef]
- Childs, R.W.; Carlsten, M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: The force awakens. Nat. Rev. Drug Discov. 2015, 14, 487–498. [Google Scholar] [CrossRef]
- Felices, M.; Chu, S.; Kodal, B.; Bendzick, L.; Ryan, C.; Lenvik, A.J.; Boylan, K.L.M.; Wong, H.C.; Skubitz, A.P.N.; Miller, J.S.; et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol. Oncol. 2017, 145, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Hoogstad-van Evert, J.S.; Maas, R.J.; van der Meer, J.; Cany, J.; van der Steen, S.; Jansen, J.H.; Miller, J.S.; Bekkers, R.; Hobo, W.; et al. Peritoneal NK cells are responsive to IL-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget 2018, 9, 34810–34820. [Google Scholar] [PubMed]
- Kamada, M.; Sakamoto, Y.; Furumoto, H.; Mori, K.; Daitoh, T.; Irahara, M.; Aono, T.; Nii, A.; Yanagawa, H.; Sone, S.; et al. Treatment of malignant ascites with allogeneic and autologous lymphokine-activated killer cells. Gynecol. Oncol. 1989, 34, 34–37. [Google Scholar] [CrossRef]
- Mittica, G.; Capellero, S.; Genta, S.; Cagnazzo, C.; Aglietta, M.; Sangiolo, D.; Valabrega, G. Adoptive immunotherapy against ovarian cancer. J. Ovarian Res. 2016, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Urba, W.J.; Clark, J.W.; Steis, R.G.; Bookman, M.A.; Smith, J.W., 2nd; Beckner, S.; Maluish, A.E.; Rossio, J.L.; Rager, H.; Ortaldo, J.R.; et al. Intraperitoneal lymphokine-activated killer cell/interleukin-2 therapy in patients with intra-abdominal cancer: Immunologic considerations. J. Natl. Cancer Inst. 1989, 81, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Steis, R.G.; Urba, W.J.; VanderMolen, L.A.; Bookman, M.A.; Smith, J.W., 2nd; Clark, J.W.; Miller, R.L.; Crum, E.D.; Beckner, S.K.; McKnight, J.E.; et al. Intraperitoneal lymphokine-activated killer-cell and interleukin-2 therapy for malignancies limited to the peritoneal cavity. J. Clin. Oncol. 1990, 8, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.A.; Belinson, J.L.; Moore, A.L.; Dorighi, J.A.; Grant, B.W.; Haugh, L.D.; Roberts, J.D.; Albertini, R.J.; Branda, R.F. Phase I trial of intraperitoneal recombinant interleukin-2/lymphokine-activated killer cells in patients with ovarian cancer. Cancer Res. 1990, 50, 6302–6310. [Google Scholar] [PubMed]
- Schmidt-Wolf, I.G.; Negrin, R.S.; Kiem, H.P.; Blume, K.G.; Weissman, I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991, 174, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kang, J.S.; Lim, J.; Park, S.K.; Lee, K.; Yoon, Y.D.; Lee, C.W.; Lee, K.H.; Han, G.; Yang, K.H.; et al. Inhibition of human ovarian tumor growth by cytokine-induced killer cells. Arch. Pharm. Res. 2007, 30, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.; Cao, S.; Zhang, X.; Yu, J.; Qi, J.; An, X.; Yu, W.; Ren, X.; Hao, X. Maintenance therapy with autologous cytokine-induced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. J. Immunother. 2014, 37, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Uppendahl, L.D.; Felices, M.; Bendzick, L.; Ryan, C.; Kodal, B.; Hinderlie, P.; Boylan, K.L.M.; Skubitz, A.P.N.; Miller, J.S.; Geller, M.A. Cytokine-induced memory-like natural killer cells have enhanced function, proliferation and in vivo expansion against ovarian cancer cells. Gynecol. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Valamehr, B.; Bjordahl, R.; Zhang, B.; Rezner, B.; Rogers, P.; Gaidarova, S.; Moreno, S.; Tuininga, K.; Dougherty, P.; et al. GSK3 Inhibition Drives Maturation of NK Cells and Enhances Their Antitumor Activity. Cancer Res. 2017, 77, 5664–5675. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.S. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood 2009, 114, 3513–3523. [Google Scholar] [CrossRef] [PubMed]
- Woll, P.S.; Grzywacz, B.; Tian, X.; Marcus, R.K.; Knorr, D.A.; Verneris, M.R.; Kaufman, D.S. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood 2009, 113, 6094–6101. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Sheridan, T.B.; Mabuchi, S.; Yoshino, K.; Hasegawa, K.; Studeman, K.D.; Im, D.D.; Rosenshein, N.B.; Roman, L.D.; Sood, A.K. Estrogen receptor expression and increased risk of lymphovascular space invasion in high-grade serous ovarian carcinoma. Gynecol. Oncol. 2014, 133, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kato, K.; Kuboyama, A.; Inoue, T.; Tanaka, Y.; Kuhara, A.; Kinoshita, K.; Takeda, S.; Wake, N. Induction of senescence by progesterone receptor-B activation in response to cAMP in ovarian cancer cells. Gynecol. Oncol. 2009, 113, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Sieh, W.; Kobel, M.; Longacre, T.A.; Bowtell, D.D.; deFazio, A.; Goodman, M.T.; Hogdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet. Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef]
- Paleari, L.; Gandini, S.; Provinciali, N.; Puntoni, M.; Colombo, N.; DeCensi, A. Clinical benefit and risk of death with endocrine therapy in ovarian cancer: A comprehensive review and meta-analysis. Gynecol. Oncol. 2017, 146, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Bodurka, D.C.; Coleman, R.L.; Lu, K.H.; Malpica, A.; Sun, C.C. Hormonal Maintenance Therapy for Women With Low-Grade Serous Cancer of the Ovary or Peritoneum. J. Clin. Oncol. 2017, 35, 1103–1111. [Google Scholar] [CrossRef]
- Fader, A.N.; Bergstrom, J.; Jernigan, A.; Tanner, E.J., 3rd; Roche, K.L.; Stone, R.L.; Levinson, K.L.; Ricci, S.; Wethingon, S.; Wang, T.L.; et al. Primary cytoreductive surgery and adjuvant hormonal monotherapy in women with advanced low-grade serous ovarian carcinoma: Reducing overtreatment without compromising survival? Gynecol. Oncol. 2017, 147, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Curran, E.M.; Berghaus, L.J.; Vernetti, N.J.; Saporita, A.J.; Lubahn, D.B.; Estes, D.M. Natural killer cells express estrogen receptor-alpha and estrogen receptor-beta and can respond to estrogen via a non-estrogen receptor-alpha-mediated pathway. Cell Immunol. 2001, 214, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Baral, E.; Nagy, E.; Berczi, I. Modulation of natural killer cell-mediated cytotoxicity by tamoxifen and estradiol. Cancer 1995, 75, 591–599. [Google Scholar] [CrossRef]
- Gauchez, A.S.; Riondel, J.; Fernandes-Carlos, T.; Jacrot, M.; Guiraud, P.; Coudray, C.; Calop, J.; Favier, A. Effect of oestrone on the natural killer (NK) cell activity, antioxidant status and tumour growth in athymic mice xenografted with human tumours. Anticancer Res. 1996, 16, 853–859. [Google Scholar] [PubMed]
- Fernandes-Carlos, T.; Riondel, J.; Glise, D.; Guiraud, P.; Favier, A. Modulation of natural killer cell functional activity in athymic mice by beta-carotene, oestrone and their association. Anticancer Res. 1997, 17, 2523–2527. [Google Scholar] [PubMed]
- Gleason, M.K.; Verneris, M.R.; Todhunter, D.A.; Zhang, B.; McCullar, V.; Zhou, S.X.; Panoskaltsis-Mortari, A.; Weiner, L.M.; Vallera, D.A.; Miller, J.S. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol. Cancer Ther. 2012, 11, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Vallera, D.A.; Zhang, B.; Gleason, M.K.; Oh, S.; Weiner, L.M.; Kaufman, D.S.; McCullar, V.; Miller, J.S.; Verneris, M.R. Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. Cancer Biother. Radiopharm. 2013, 28, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, J.U.; Felices, M.; Todhunter, D.; Taras, E.; Miller, J.S.; Vallera, D.A. Tetraspecific scFv construct provides NK cell mediated ADCC and self-sustaining stimuli via insertion of IL-15 as a crosslinker. Oncotarget 2016, 7, 73830–73844. [Google Scholar] [CrossRef] [PubMed]
- Davis, Z.B.; Vallera, D.A.; Miller, J.S.; Felices, M. Natural killer cells unleashed: Checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated antitumor immunotherapy. Semin. Immunol. 2017, 31, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet. Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999-2007 by country and age: Results of EUROCARE--5-a population-based study. Lancet. Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Oliver, K.E.; McGuire, W.P. Ovarian cancer and antiangiogenic therapy: Caveat emptor. J. Clin. Oncol. 2014, 32, 3353–3356. [Google Scholar] [CrossRef] [PubMed]
- Klapdor, R.; Wang, S.; Hacker, U.; Buning, H.; Morgan, M.; Dork, T.; Hillemanns, P.; Schambach, A. Improved Killing of Ovarian Cancer Stem Cells by Combining a Novel Chimeric Antigen Receptor-Based Immunotherapy and Chemotherapy. Hum. Gene. Ther. 2017, 28, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, D.L.; Kaufman, D.S. Utilizing chimeric antigen receptors to direct natural killer cell activity. Front. Immunol. 2015, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Kim, M.; Lee, K.H.; Eom, K.Y.; Kjeldsen, M.K.; Mirza, M.R.; Kim, J.W. Major clinical research advances in gynecologic cancer in 2017. J. Gynecol. Oncol. 2018, 29, e31. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Matsumura, N.; Abiko, K.; Baba, T.; Konishi, I. PD-1/PD-L1 blockade in cancer treatment: Perspectives and issues. Int. J. Clin. Oncol. 2016, 21, 462–473. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Matsuo, K.; Spragg, S.E.; Ciccone, M.A.; Blake, E.A.; Ricker, C.; Pham, H.Q.; Roman, L.D. Nivolumab use for BRCA gene mutation carriers with recurrent epithelial ovarian cancer: A case series. Gynecol. Oncol. Rep. 2018, 25, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Braly, P.; Nicodemus, C.F.; Chu, C.; Collins, Y.; Edwards, R.; Gordon, A.; McGuire, W.; Schoonmaker, C.; Whiteside, T.; Smith, L.M.; et al. The Immune adjuvant properties of front-line carboplatin-paclitaxel: A randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J. Immunother. 2009, 32, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.R.; Liu, H.; Irwanto, A.; Fu, X.A.; Li, Y.; Yu, G.Q.; Yu, Y.X.; Chen, M.F.; Low, H.Q.; Li, J.H.; et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N. Engl. J. Med. 2013, 369, 1620–1628. [Google Scholar] [PubMed]
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. AntiNKG2A mAb Is a Checkpoint Inhibitor that Promotes Antitumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greppi, M.; Tabellini, G.; Patrizi, O.; Candiani, S.; Decensi, A.; Parolini, S.; Sivori, S.; Pesce, S.; Paleari, L.; Marcenaro, E. Strengthening the AntiTumor NK Cell Function for the Treatment of Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 890. https://doi.org/10.3390/ijms20040890
Greppi M, Tabellini G, Patrizi O, Candiani S, Decensi A, Parolini S, Sivori S, Pesce S, Paleari L, Marcenaro E. Strengthening the AntiTumor NK Cell Function for the Treatment of Ovarian Cancer. International Journal of Molecular Sciences. 2019; 20(4):890. https://doi.org/10.3390/ijms20040890
Chicago/Turabian StyleGreppi, Marco, Giovanna Tabellini, Ornella Patrizi, Simona Candiani, Andrea Decensi, Silvia Parolini, Simona Sivori, Silvia Pesce, Laura Paleari, and Emanuela Marcenaro. 2019. "Strengthening the AntiTumor NK Cell Function for the Treatment of Ovarian Cancer" International Journal of Molecular Sciences 20, no. 4: 890. https://doi.org/10.3390/ijms20040890
APA StyleGreppi, M., Tabellini, G., Patrizi, O., Candiani, S., Decensi, A., Parolini, S., Sivori, S., Pesce, S., Paleari, L., & Marcenaro, E. (2019). Strengthening the AntiTumor NK Cell Function for the Treatment of Ovarian Cancer. International Journal of Molecular Sciences, 20(4), 890. https://doi.org/10.3390/ijms20040890