Adipose Tissue-Derived Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat Model
Abstract
1. Introduction
2. Results
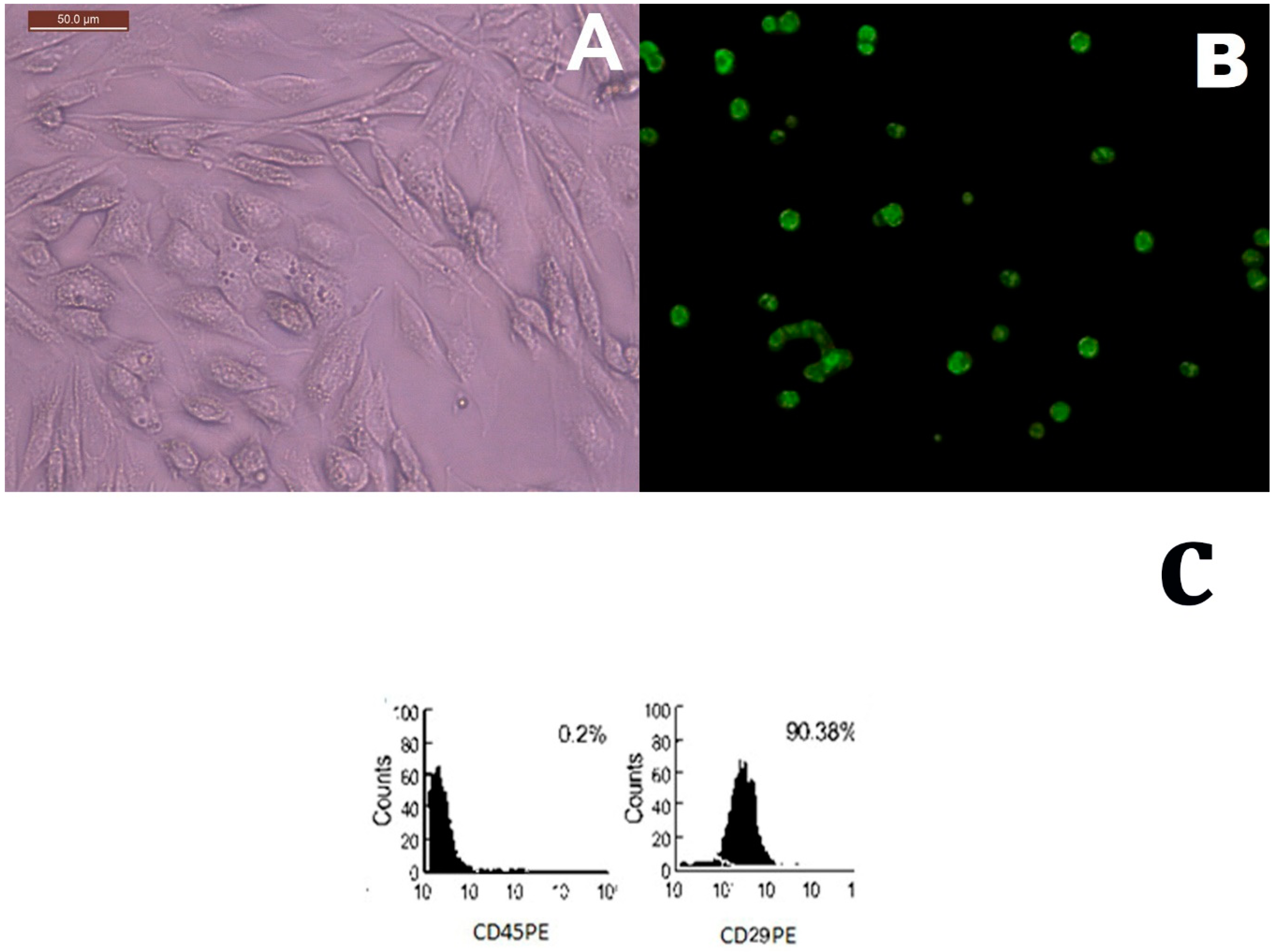
2.1. MSCs Identification in Culture and Tissue Tracking
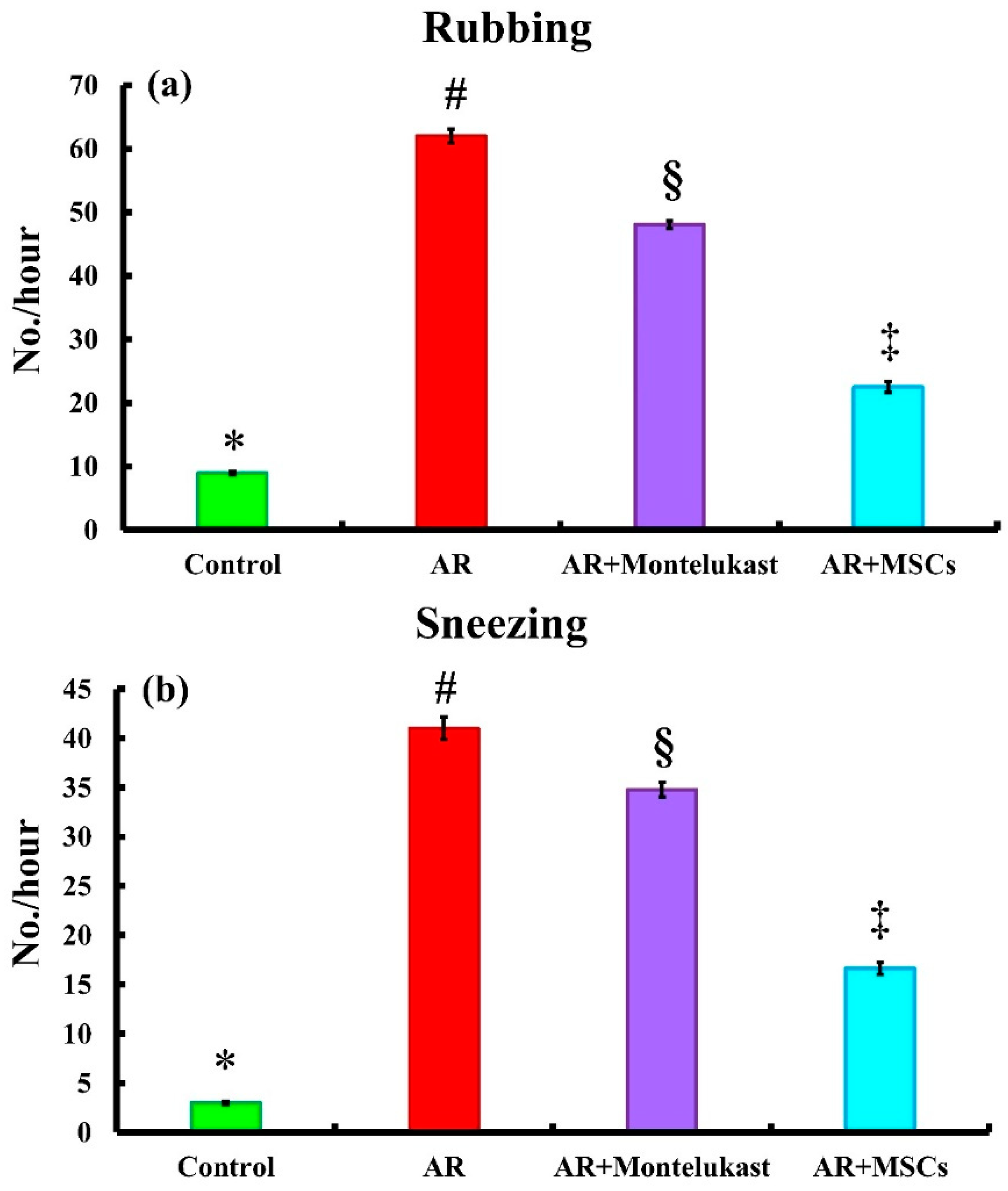
2.2. Symptom Score
2.3. Biochemical Results
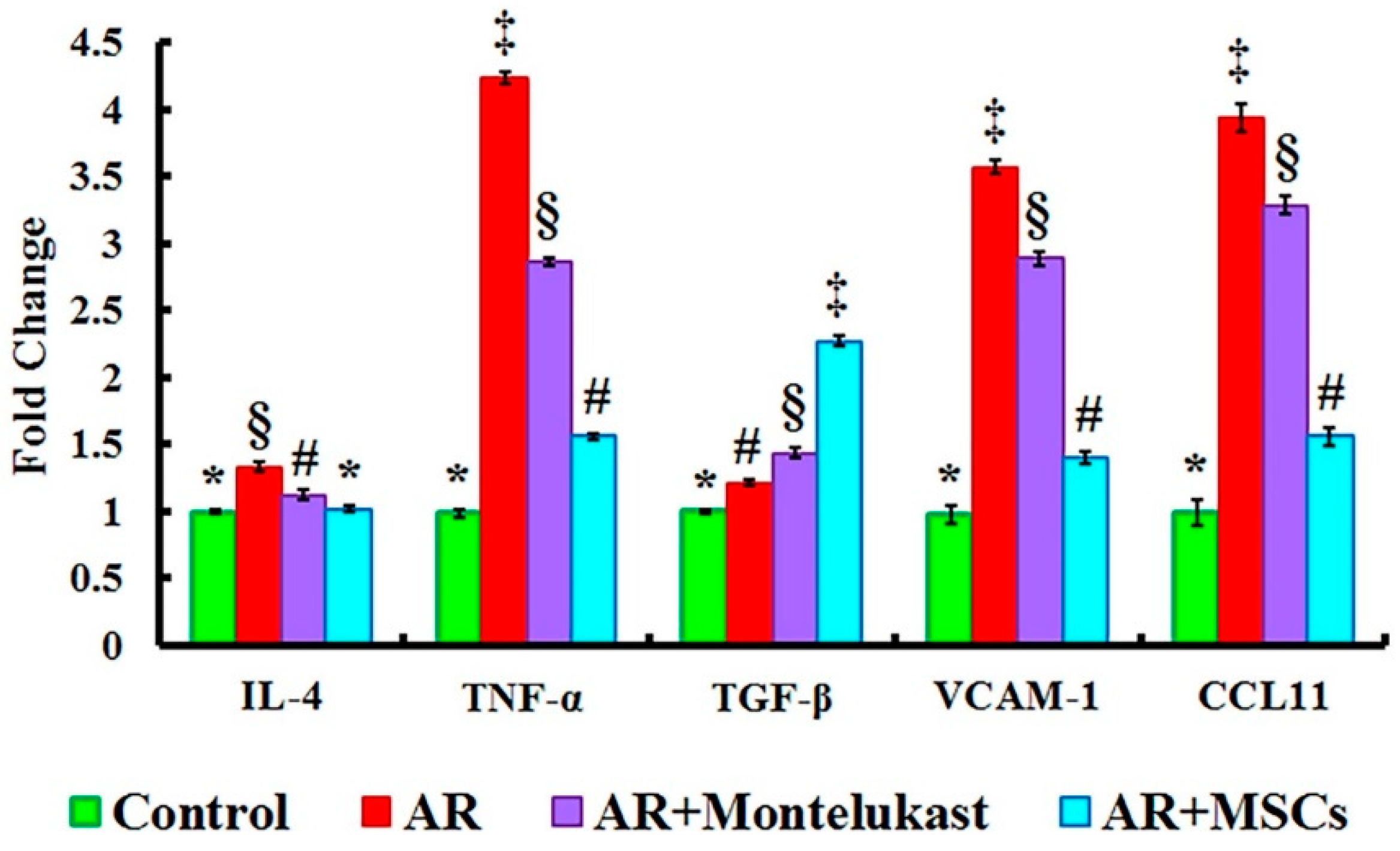
2.4. Gene Expression Results of IL-4, TNF-α, TGF-β, VCAM-1 and CCL11 Genes in All Experimental Groups
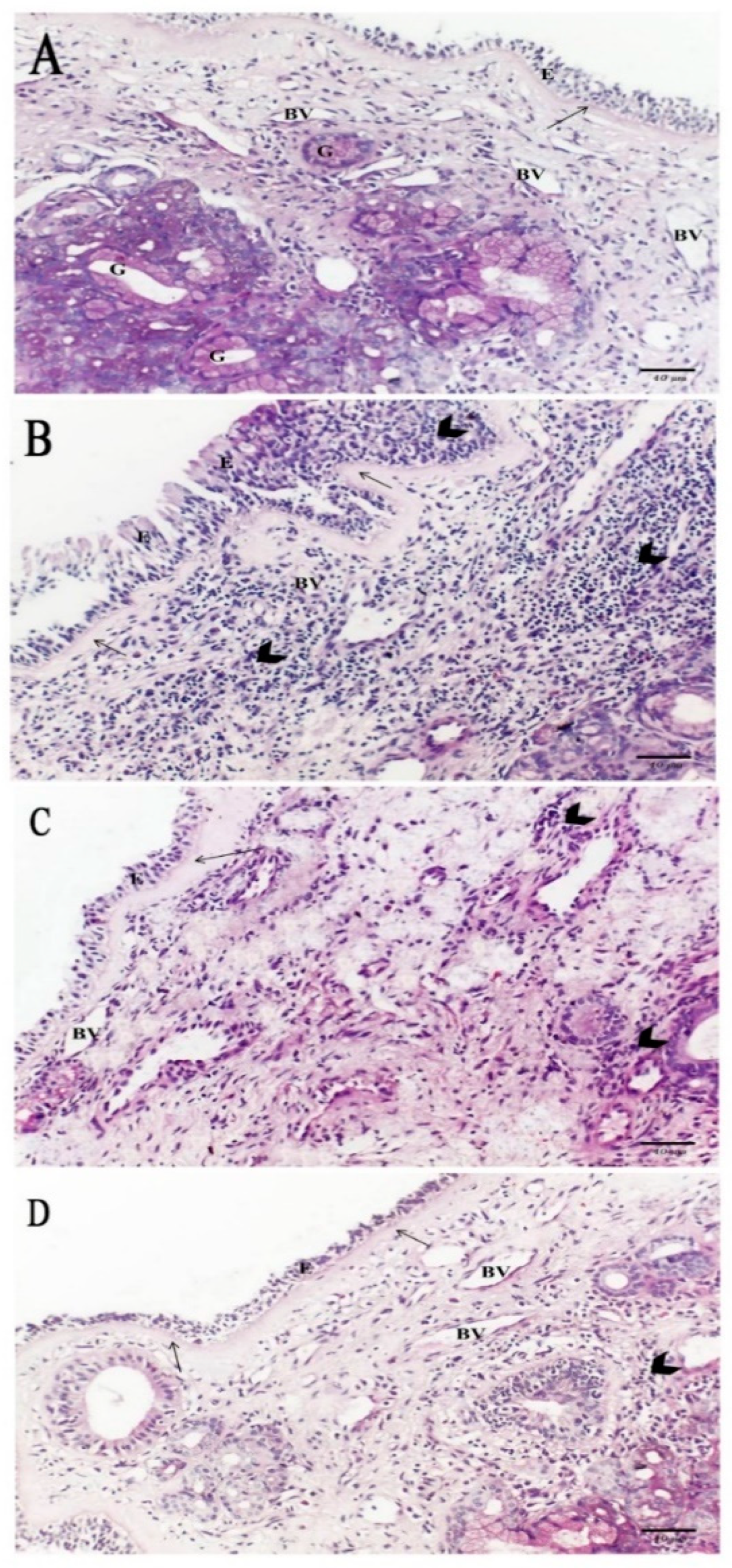
2.5. Histological Results
2.5.1. Hematoxylin and Eosin
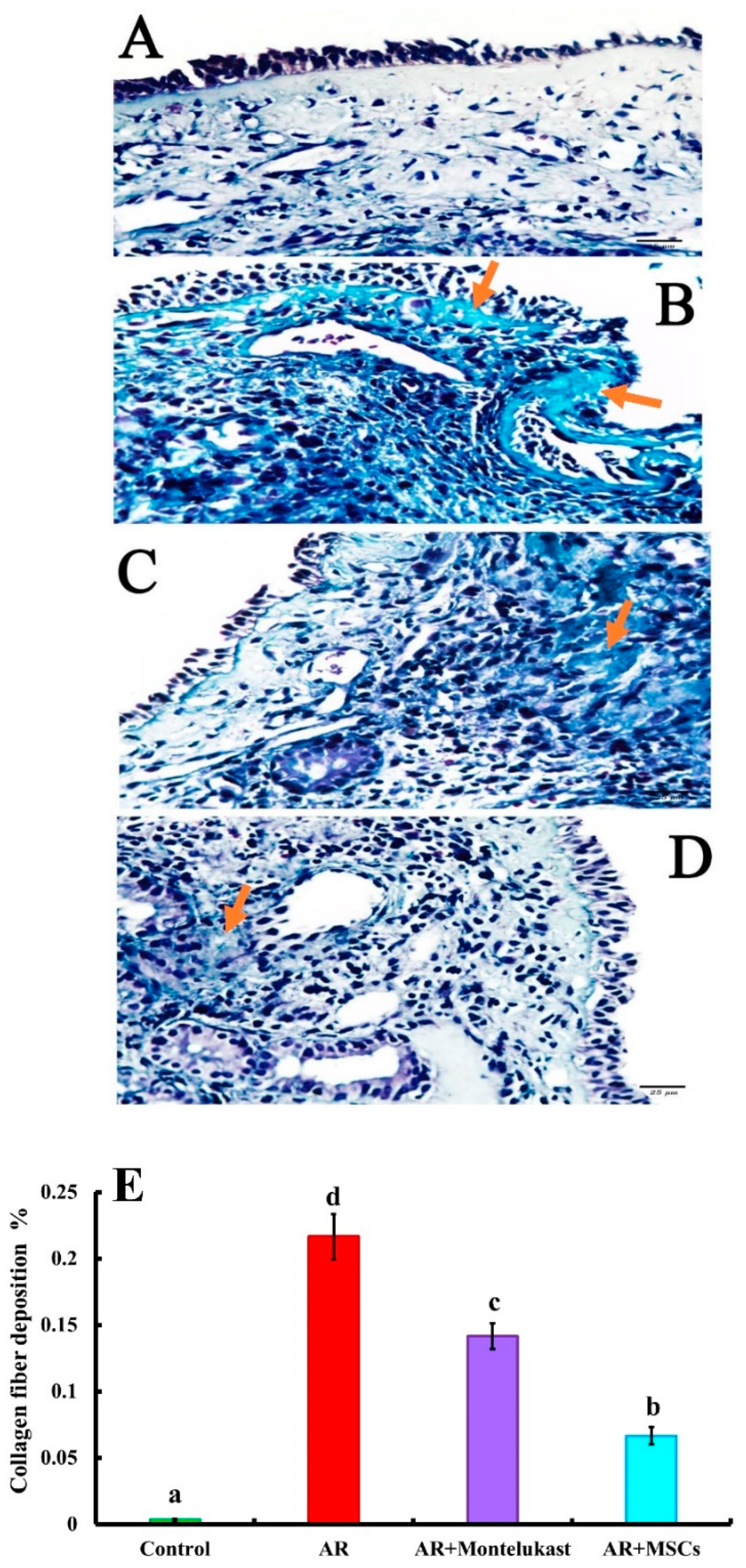
2.5.2. Masson’s Trichrome
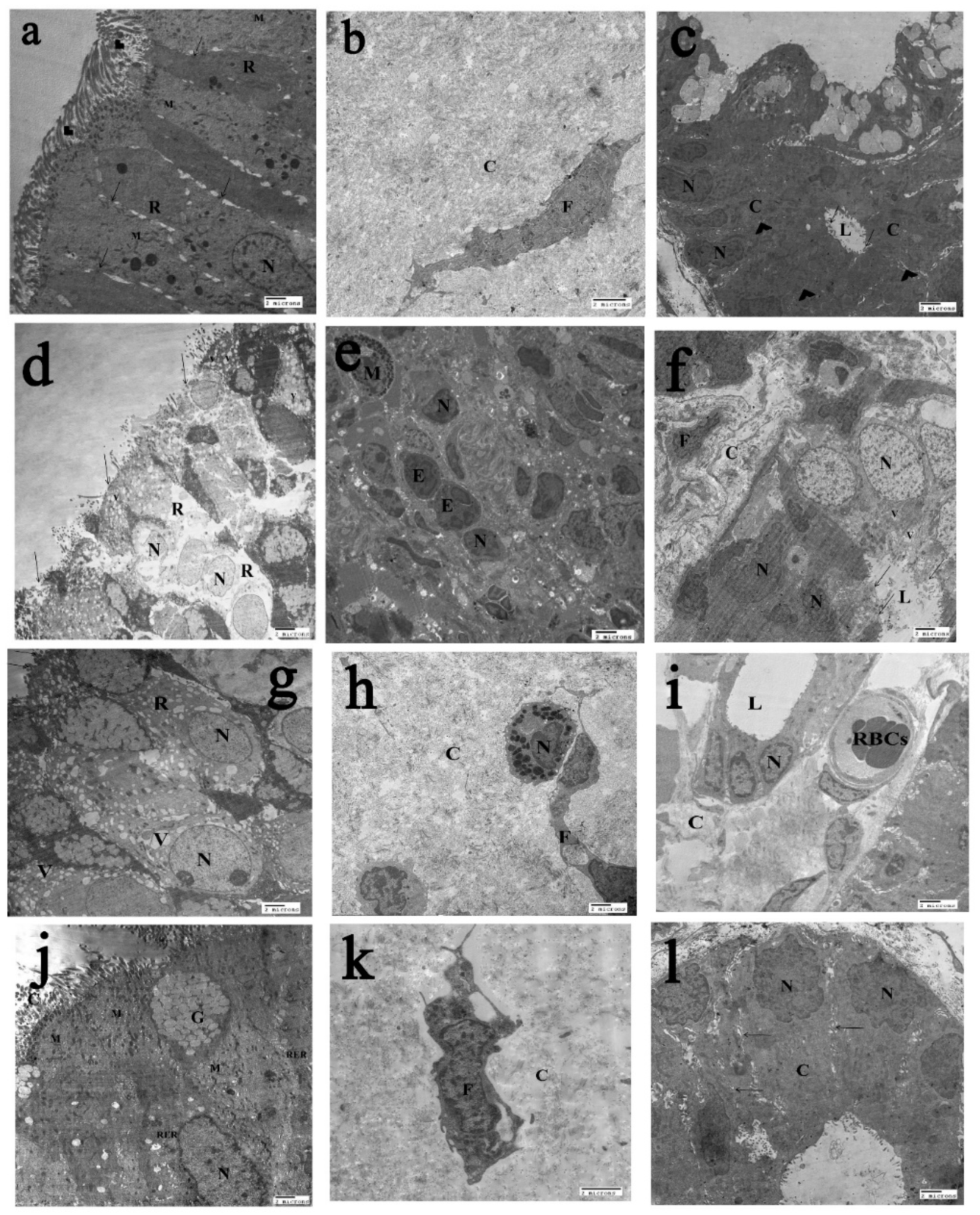
2.6. The Transmission Electron Microscopy (T.E.M) Results
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Ovalbumin-induced Allergic Rhinitis Rat Model
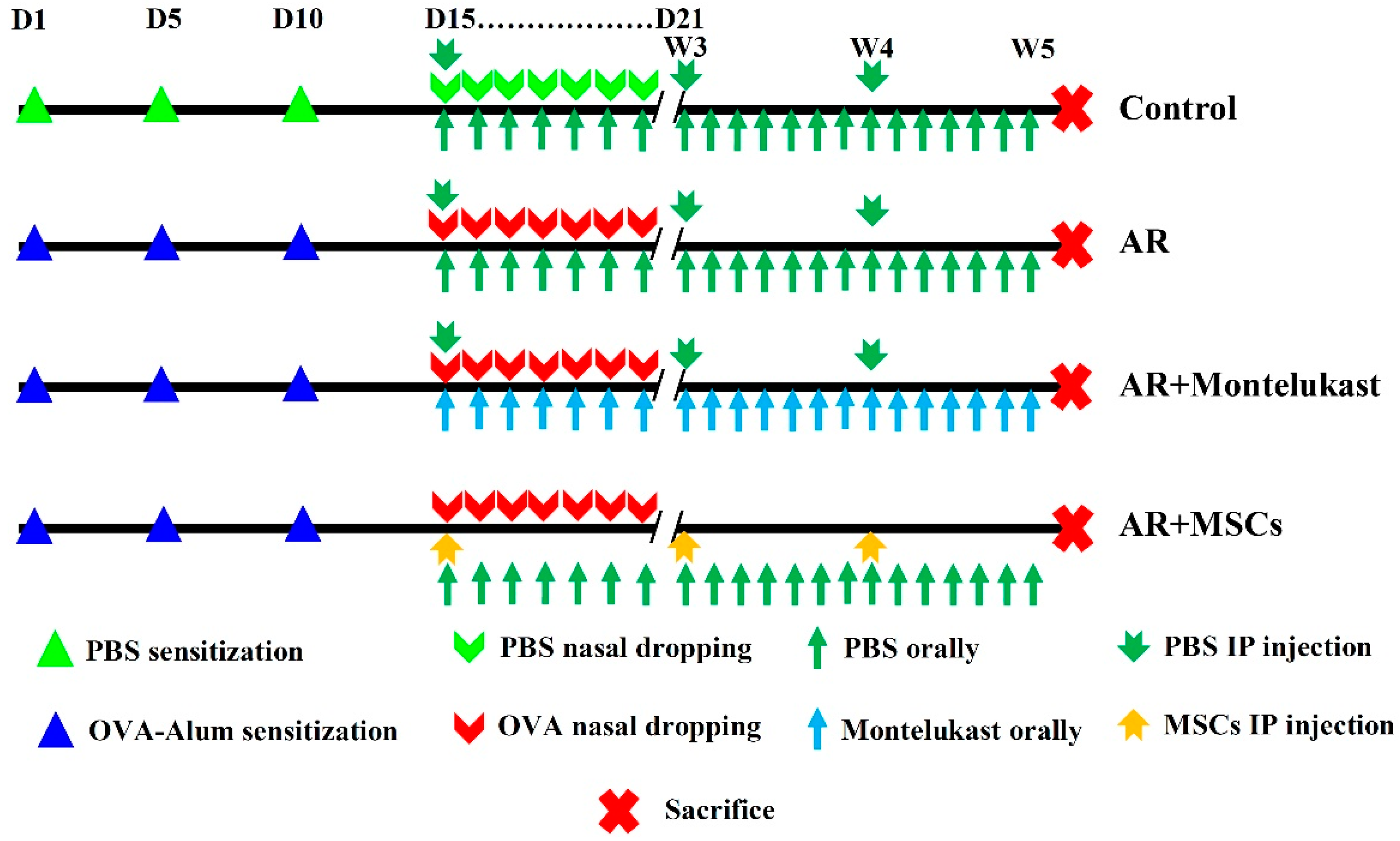
4.3. Experimental Design and Treatment Protocol
4.4. Sampling
4.5. MSCs Isolation, Culture, and Tracking
4.5.1. Adipose Derived Stem Cell Preparation
4.5.2. Labeling stem cells with Green Fluorescent Protein (GFP)
4.5.3. Immunophenotyping Characterization of Differentiated Stem Cells
4.5.4. Evaluation of Nasal Symptoms
4.5.5. Estimation of Serum OVA-Specific IgE, IgG1, IgG2a, PGE2 and Histamine
4.6. Determination of the Expressions of Interleukin (IL)-4, Transforming Growth Factor (TGF)-β, Tumor Necrosis Factor (TNF)-α, Vascular Cell Adhesion Molecule-1 (VCAM-1) and C-C Motif Chemokine Ligand 11 (CCL11) Genes by qPCR
4.6.1. Total RNA Extraction and Reverse Transcription
4.6.2. Quantitative real-time PCR
4.7. Histopathological Analysis
4.7.1. Light Microscopic Study
4.7.2. Morphometric Study
4.7.3. Transmission Electron Microscopic Study
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Allergic rhinitis |
| CCL11 | C-C Motif Chemokine Ligand 11 |
| ICAM | Intercellular adhesion molecules |
| Ig | Immunoglobulin |
| IL- | Interleukin- |
| MSCs | Mesenchymal stem cells |
| OVA | Ovalbumin |
| PGE2 | Prostaglandin E2 |
| TGF- | Transforming growth factor- |
| TNF- | Tumor necrosis factor- |
| VCAM | Vascular cell adhesion molecule |
References
- Singer, N.G.; Caplan, A.I. Mesenchymal stem cells: Mechanisms of inflammation. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Turker Sener, L.; Albeniz, I. Challenge of mesenchymal stem cells against diabetic foot ulcer. Curr. Stem Cell Res. Ther. 2015, 10, 530–534. [Google Scholar] [CrossRef]
- Yang, F.Y.; Chen, R.; Zhang, X.; Huang, B.; Tsang, L.L.; Li, X.; Jiang, X. Preconditioning Enhances the Therapeutic Effects of Mesenchymal Stem Cells on Colitis Through PGE2-Mediated T-Cell Modulation. Cell Transplant. 2018, 27, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- Gaber, T.; Schönbeck, K.; Hoff, H.; Tran, C.; Strehl, C.; Lang, A.; Ohrndorf, S.; Pfeiffenberger, M.; Röhner, E.; Matziolis, G. CTLA-4 Mediates Inhibitory Function of Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2018, 19, 2312. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Pistoia, V.; Moretta, L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007, 28, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, T.L.; Koloze, M.; Lennon, D.P.; Zuchowski, B.; Yang, S.E.; Caplan, A.I. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L760–L770. [Google Scholar] [CrossRef]
- Fu, Q.; Chow, Y.; Sun, S.; Zeng, Q.; Li, H.; Shi, J.; Sun, Y.; Wen, W.; Tse, H.; Lian, Q. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy 2012, 67, 1215–1222. [Google Scholar] [CrossRef]
- Fan, X.-L.; Zeng, Q.-X.; Li, X.; Li, C.-L.; Xu, Z.-B.; Deng, X.-Q.; Shi, J.; Chen, D.; Zheng, S.G.; Fu, Q.-L. Induced pluripotent stem cell-derived mesenchymal stem cells activate quiescent T cells and elevate regulatory T cell response via NF-κB in allergic rhinitis patients. Stem Cell Res. Ther. 2018, 9, 170. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Lin, H.; Zhao, K.; Zheng, C. Nasal mucosa derived-mesenchymal stem cells from mice reduce inflammation via modulating immune responses. PLoS ONE 2015, 10, e0118849. [Google Scholar] [CrossRef]
- Han, D.; Wang, C.; Lou, W.; Gu, Y.; Wang, Y.; Zhang, L. Allergen-specific IL-10-secreting type IT regulatory cells, but not CD4+ CD25+ Foxp3+ T cells, are decreased in peripheral blood of patients with persistent allergic rhinitis. Clin. Immunol. 2010, 136, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Stuck, B.A.; Czajkowski, J.; Hagner, A.-E.; Klimek, L.; Verse, T.; Hörmann, K.; Maurer, J.T. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: A controlled clinical trial. J. Allergy Clin. Immunol. 2004, 113, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Zuberbier, T. Undertreatment of rhinitis symptoms in Europe: Findings from a cross-sectional questionnaire survey. Allergy 2007, 62, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Ciprandi, G.; Marseglia, A.; Castagnoli, R.; Barberi, S.; Caimmi, S.; Marseglia, G.L. Current recommendations and emerging options for the treatment of allergic rhinitis. Expert Rev. Clin. Immunol. 2014, 10, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Samivel, R.; Kim, E.H.; Chung, Y.-J.; Mo, J.-H. Immunomodulatory effect of tonsil-derived mesenchymal stem cells in a mouse model of allergic rhinitis. Am. J. Rhinol. Allergy 2015, 29, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J.; Sutton, B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef]
- Takhar, P.; Corrigan, C.J.; Smurthwaite, L.; O’connor, B.J.; Durham, S.R.; Lee, T.H.; Gould, H.J. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 2007, 119, 213–218. [Google Scholar] [CrossRef]
- Eifan, A.; Durham, S. Pathogenesis of rhinitis. Clin. Exp. Allergy 2016, 46, 1139–1151. [Google Scholar] [CrossRef]
- Min, Y.-G. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol. Res. 2010, 2, 65–76. [Google Scholar] [CrossRef]
- Mandhane, S.N.; Shah, J.H.; Thennati, R. Allergic rhinitis: An update on disease, present treatments and future prospects. Int. Immunopharmacol. 2011, 11, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Peters-Golden, M.; Henderson, W.R., Jr. The role of leukotrienes in allergic rhinitis. Ann. Allergy Asthma Immunol. 2005, 94, 609–618. [Google Scholar] [CrossRef]
- Elnabtity, M.H.; Singh, R.F.; Ansong, M.A.; Craig, T.J. Leukotriene modifiers in the management of asthma. J. Am. Osteopath. Assoc. 1999, 99 (Suppl. 7), S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, H.; Himi, T. Role of cysteinyl leukotrienes in allergic rhinitis. In Excellence in Otolaryngology; Karger Publishers: Basel, Switzerland, 2016; Volume 77, pp. 40–45. [Google Scholar]
- Scadding, G.K. Allergic rhinitis: Background, symptoms, diagnosis and treatment options. Nurs. Times 2009, 105, 24–27. [Google Scholar]
- Ricketti, P.A.; Alandijani, S.; Lin, C.H.; Casale, T.B. Investigational new drugs for allergic rhinitis. Expert Opin. Investig. Drugs 2017, 26, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, I. Immune modulation by mesenchymal stem cells. Exp. Cell Res. 2006, 312, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-S.; Park, M.-K.; Kang, S.; Park, H.-Y.; Hong, S.-L.; Park, H.-K.; Yu, H.-S.; Roh, H.-J. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediat. Inflamm. 2014, 2014, 436476. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Deng, M.X.; He, J.; Zeng, Q.X.; Wen, W.; Wong, D.S.; Tse, H.F.; Xu, G.; Lian, Q.; Shi, J. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 2012, 30, 2692–2699. [Google Scholar] [CrossRef]
- Desai, M.B.; Gavrilova, T.; Liu, J.; Patel, S.A.; Kartan, S.; Greco, S.J.; Capitle, E.; Rameshwar, P. Pollen-induced antigen presentation by mesenchymal stem cells and T cells from allergic rhinitis. Clin. Transl. Immunol. 2013, 2, e7. [Google Scholar] [CrossRef]
- Goodwin, M.; Sueblinvong, V.; Eisenhauer, P.; Ziats, N.P.; LeClair, L.; Poynter, M.E.; Steele, C.; Rincon, M.; Weiss, D.J. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 2011, 29, 1137–1148. [Google Scholar] [CrossRef]
- Cho, K.S.; Park, H.K.; Park, H.Y.; Jung, J.S.; Jeon, S.G.; Kim, Y.K.; Roh, H.J. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells 2009, 27, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.G. Pathophysiology, diagnosis, and treatment of allergic rhinitis. Korean J. Otorhinolaryngol. Head Neck Surg. 2013, 56, 256–265. [Google Scholar] [CrossRef]
- Li, C.; Fu, Y.; Wang, Y.; Kong, Y.; Li, M.; Ma, D.; Zhai, W.; Wang, H.; Lin, Y.; Liu, S. Mesenchymal stromal cells ameliorate acute allergic rhinitis in rats. Cell Biochem. Funct. 2017, 35, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.B.; Pratt, E.L.; Hanks, C.; McCann, J.; Craig, T.J. Allergic rhinitis and its effect on sleep, fatigue, and daytime somnolence. Ann. Allergy Asthma Immunol. 2006, 97, 579–587. [Google Scholar] [CrossRef]
- Huber, V.C.; McKeon, R.M.; Brackin, M.N.; Miller, L.A.; Keating, R.; Brown, S.A.; Makarova, N.; Perez, D.R.; MacDonald, G.H.; McCullers, J.A. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccine Immunol. 2006, 13, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Saito, K.; Takemura, M.; Sekikawa, K.; Fujii, H.; Yamada, Y.; Wada, H.; Mizuta, K.; Seishima, M.; Ito, Y. TNF-α contributes to the development of allergic rhinitis in mice. J. Allergy Clin. Immunol. 2003, 112, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Qiu, Q.; Lu, H.; Lu, C.; Chen, S.; Han, H. Variations in TGF-beta, IL-10, and IL-17 after specific immunotherapy and correlations with symptoms in patients with allergic rhinitis. J. Investig. Allergol. Clin. Immunol. 2012, 22, 311–312. [Google Scholar]
- Lloyd, C. Chemokines in allergic lung inflammation. Immunology 2002, 105, 144–154. [Google Scholar] [CrossRef]
- Salib, R.; Lau, L.; Howarth, P. Nasal lavage fluid concentrations of eotaxin-1 (CCL11) in naturally occurring allergic rhinitis: Relationship to disease activity, nasal luminal eosinophil influx, and plasma protein exudation. Clin. Exp. Allergy 2005, 35, 995–1002. [Google Scholar] [CrossRef]
- Lee, B.-J.; Naclerio, R.M.; Bochner, B.S.; Taylor, R.M.; Lim, M.C.; Baroody, F.M. Nasal challenge with allergen upregulates the local expression of vascular endothelial adhesion molecules. J. Allergy Clin. Immunol. 1994, 94, 1006–1016. [Google Scholar] [CrossRef]
- Bozkurt, M.K.; Tülekb, B.; Bozkurt, B.; Akyürek, N.; Mehmet, Ö.; Kiyici, A. Comparison of the efficacy of prednisolone, montelukast, and omalizumab in an experimental allergic rhinitis model. Turk. J. Med. Sci. 2014, 44, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Knipping, S.; Holzhausen, H.; Riederer, A.; Schrom, T. Allergic and idiopathic rhinitis: An ultrastructural study. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Toppozada, H.H.; Talaat, M.A. The allergic nasal mucosa following vidian neurectomy. ORL 1976, 38, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Morikawa, H.; Howie, K.; Crawford, L.; Baatjes, A.J.; Denburg, E.; Cyr, M.M.; Denburg, J.A. Effects of a cysteinyl leukotriene receptor antagonist on eosinophil recruitment in experimental allergic rhinitis. Immunology 2004, 113, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Chik, S.; Chan, A.; Li, Z.; Tsang, K.; Li, W. Anti-inflammatory effects of high-dose montelukast in an animal model of acute asthma. Clin. Exp. Allergy 2003, 33, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Muller, W. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef]
- Çobanoğlu, B.; Toskala, E.; Ural, A.; Cingi, C. Role of leukotriene antagonists and antihistamines in the treatment of allergic rhinitis. Curr. Allergy Asthma Rep. 2013, 13, 203–208. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, Y.; Liang, H.; Jiang, X. Bone marrow-derived mesenchymal stem cells reduce immune reaction in a mouse model of allergic rhinitis. Am. J. Transl. Res. 2016, 8, 5628–5636. [Google Scholar]
- Haddad, R.; Saldanha-Araujo, F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far? BioMed Res. Int. 2014, 2014, 216806. [Google Scholar] [CrossRef]
- Zhao, Q.; Ren, H.; Han, Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J. Cell. Immunother. 2016, 2, 3–20. [Google Scholar] [CrossRef]
- Thakare, V.N.; Osama, M.; Naik, S.R. Therapeutic potential of curcumin in experimentally induced allergic rhinitis in guinea pigs. Int. Immunopharmacol. 2013, 17, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, A.; Harrell, C.R.; Fellabaum, C.; Volarevic, V. Mesenchymal Stem Cells as New Therapeutic Agents for the Treatment of Primary Biliary Cholangitis. Anal. Cell. Pathol. 2017, 2017, 7492836. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Niki, H.; Hosokawa, S.; Nagaike, K.; Tagawa, T. A new immunofluorostaining method using red fluorescence of PerCP on formalin-fixed paraffin-embedded tissues. J. Immunol. Methods 2004, 293, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sabry, D.; Noh, O.; Samir, M. Comparative evaluation for potential differentiation of endothelial progenitor cells and mesenchymal stem cells into endothelial-like cells. Int. J. Stem Cells 2016, 9, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaffl, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Asp. Med. 2006, 27, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Zhao, X.; Yaster, M.; Tao, Y.X. Expression and distribution of mTOR, p70S6K, 4E-BP1, and their phosphorylated counterparts in rat dorsal root ganglion and spinal cord dorsal horn. Brain Res. 2010, 1336, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.A. Principles and Techniques of Electron Microscopy. Biological Applications; Edward Arnold: London, UK, 1981. [Google Scholar]
- Wiame, I.; Remy, S.; Swennen, R.; Sagi, L. Irreversible heat inactivation of DNase I without RNA degradation. BioTechniques 2000, 29, 252–256. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahim, N.; Mandour, Y.M.H.; Farid, A.S.; Nafie, E.; Mohamed, A.Z.; Safwat, M.; Taha, R.; Sabry, D.; Sorour, S.M.; Refae, A. Adipose Tissue-Derived Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat Model. Int. J. Mol. Sci. 2019, 20, 873. https://doi.org/10.3390/ijms20040873
Ebrahim N, Mandour YMH, Farid AS, Nafie E, Mohamed AZ, Safwat M, Taha R, Sabry D, Sorour SM, Refae A. Adipose Tissue-Derived Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat Model. International Journal of Molecular Sciences. 2019; 20(4):873. https://doi.org/10.3390/ijms20040873
Chicago/Turabian StyleEbrahim, Nesrine, Yasser Mohammad Hassan Mandour, Ayman Samir Farid, Ebtesam Nafie, Amira Zaky Mohamed, Miriam Safwat, Radwa Taha, Dina Sabry, Safwa M. Sorour, and Ahmed Refae. 2019. "Adipose Tissue-Derived Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat Model" International Journal of Molecular Sciences 20, no. 4: 873. https://doi.org/10.3390/ijms20040873
APA StyleEbrahim, N., Mandour, Y. M. H., Farid, A. S., Nafie, E., Mohamed, A. Z., Safwat, M., Taha, R., Sabry, D., Sorour, S. M., & Refae, A. (2019). Adipose Tissue-Derived Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat Model. International Journal of Molecular Sciences, 20(4), 873. https://doi.org/10.3390/ijms20040873




