Application of Data-Independent Acquisition Approach to Study the Proteome Change from Early to Later Phases of Tomato Pathogenesis Responses
Abstract
1. Introduction
2. Results and Discussion
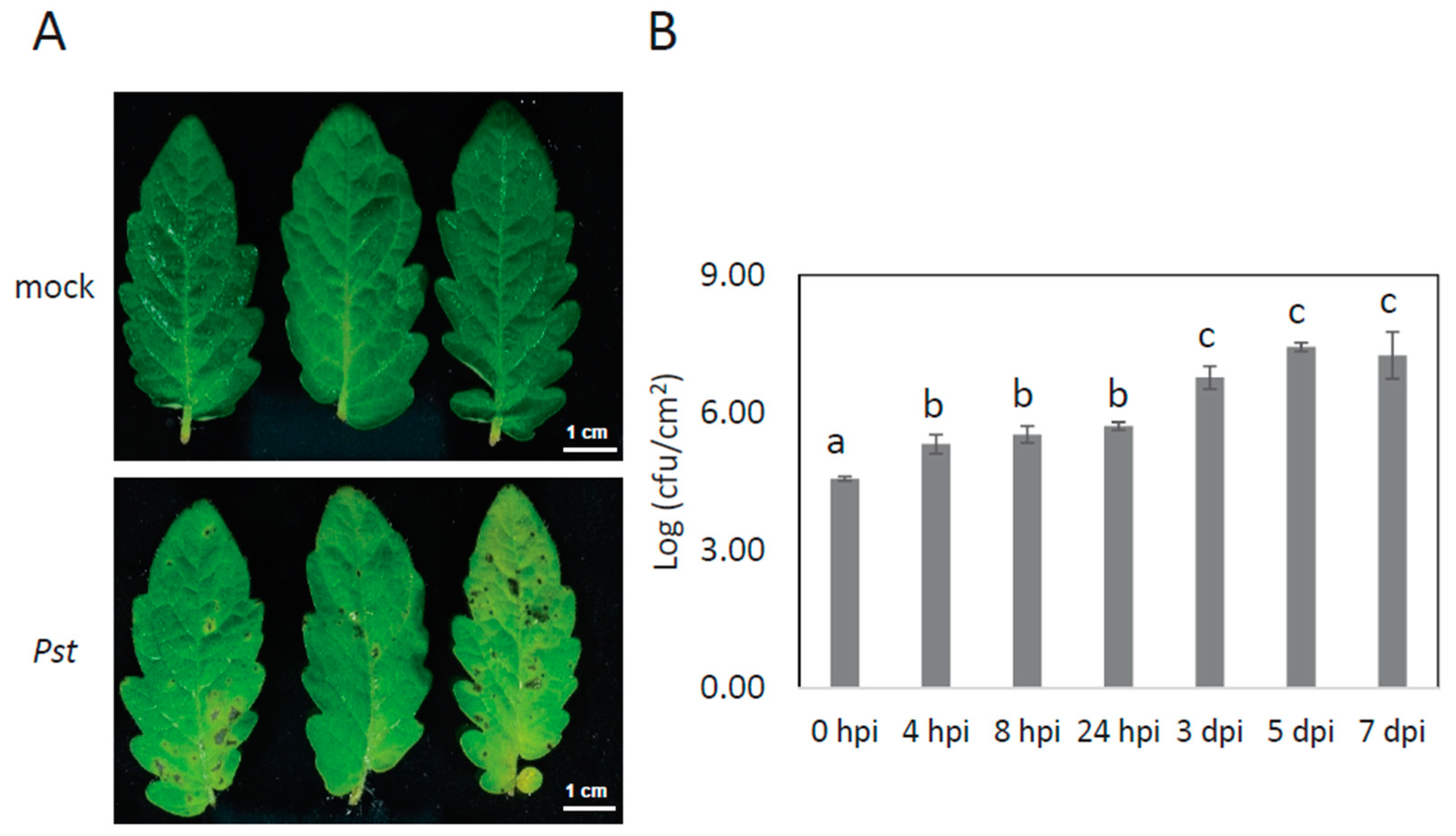
2.1. Experimental Design and the Identification and Quantification Result
2.2. Functional Classification of the Proteins Regulated by Pst DC3000
2.2.1. Function Categories of Proteins That are Significantly up- or down-Regulated during Pst DC3000 Inoculation
2.2.2. Proteins Related to the Key Mechanisms of Pathogenesis
2.3. Changes Associated with Defence and Immune Regulation
2.4. Changes Associated with the Reactive Oxygen Species (ROS) and Oxidation-Reduction Reactions
2.5. Changes Associated with Protein Folding, Transportation and Degradation
2.5.1. Protein Folding: Heat Shock Proteins and Chaperones
2.5.2. Protein Degradation
2.6. Changes Associated with Phytohormone Synthesis and Fatty Acid Metabolism
3. Materials and Methods
3.1. Plant Materials and Growth Condition
3.2. Pseudomonas Preparation and Inoculation Assays
3.3. Sample Preparation: Protein Extraction and Digestion
3.4. Liquid Chromatography-Mass Spectrometry Analysis
3.5. Data Analysis for LC-MS
3.6. Quantitation Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DIA | data-independent acquisition |
| DDA | data-dependent acquisition |
| PAMP | pathogen-associated molecular pattern |
| MAMP | microbial associated molecular pattern |
| DAMP | damaged-associated molecular pattern |
| ETS | effector-triggered susceptibility |
| PTI | PAMP-triggered immunity |
| ETI | effector-triggered immunity |
| HR | hypersensitive response |
| PCD | programmed cell death |
| SA | salicylic acid |
| JA | jasmonic acid |
| ABA | abscisic acid |
| ET | ethylene |
| Pst DC3000 | Pseudomonas syringae pv. tomato DC3000 |
| SAR | systemic acquired resistance |
References
- Underwood, W. The Plant Cell Wall: A Dynamic Barrier against Pathogen Invasion. Front. Plant Sci. 2012, 3, 1–6. [Google Scholar] [CrossRef]
- Takai, R.; Isogai, A.; Takayama, S.; Che, F.-S. Analysis of Flagellin Perception Mediated by Flg22 Receptor Osfls2 in Rice. Mol. Plant-Microbe Interact. 2008, 21, 1635–1642. [Google Scholar] [CrossRef]
- Pearce, G.; Strydom, D.; Johnson, S.; Ryan, C.A. A Polypeptide from Tomato Leaves Induces Wound-Inducible Proteinase Inhibitor Proteins. Science 1991, 253, 895–897. [Google Scholar] [CrossRef]
- Huffaker, A.; Pearce, G.; Ryan, C.A. An Endogenous Peptide Signal in Arabidopsis Activates Components of the Innate Immune Response. Proc. Natl. Acad. Sci. USA 2006, 103, 10098–10103. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Lee, C.-Y.; Cheng, K.-T.; Chang, W.-H.; Huang, R.-N.; Nam, H.G.; Chen, Y.-R. Quantitative Peptidomics Study Reveals That a Wound-Induced Peptide from PR-1 Regulates Immune Signaling in Tomato. Plant Cell 2014, 26, 4135–4148. [Google Scholar] [CrossRef]
- Tanaka, K.; Choi, J.; Cao, Y.; Stacey, G. Extracellular Atp Acts as a Damage-Associated Molecular Pattern (DAMP) Signal in Plants. Front. Plant Sci. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Choi, H.W.; Klessig, D.F. Damps, Mamps and Namps in Plant Innate Immunity. BMC Plant Biol. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Peng, Y.; Wersch, R.V.; Zhang, Y. Convergent and Divergent Signaling in PAMP-Triggered Immunity and Effector-Triggered Immunity. Mol. Plant Microbe Interact. 2018, 31, 403–409. [Google Scholar] [CrossRef]
- Dangl, J.L. Pivoting the Plant Immune System from Dissection to Deployment. Science 2013, 341, 745–751. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, Z.; Chen, W.; Glazebrook, J.; Chang, H.-S.; Han, B.; Zhu, T.; Zou, G.; Katagiri, F. Quantitative Nature of Arabidopsis Responses during Compatible and Incompatible Interactions with the Bacterial Pathogen Pseudomonas syringae. Plant Cell Online 2003, 15, 317–330. [Google Scholar] [CrossRef]
- Navarro, L.; Zipfel, C.; Rowland, O.; Keller, I.; Robatzek, S.; Boller, T.; Jones, J.D.G. The Transcriptional Innate Immune Response to Flg22. Interplay and Overlap with Avr Gene-Dependent Defense Responses and Bacterial Pathogenesis. Plant Physiol. 2004, 135, 1113–1128. [Google Scholar] [CrossRef]
- Mukhtar, M.S.; McCormack, M.E.; Argueso, C.T.; Pajerowska-Mukhtar, K.M. Pathogen Tactics to Manipulate Plant Cell Death. Curr. Biol. 2016, 26, R608–R619. [Google Scholar] [CrossRef]
- Kim, Y.; Tsuda, K.; Igarashi, D.; Hillmer, R.A.; Sakakibara, H.; Myers, C.L.; Katagiri, F. Mechanisms Underlying Robustness and Tunability in a Plant Immune Signaling Network. Cell Host Microbe 2014, 15, 84–94. [Google Scholar] [CrossRef]
- Bozso, Z.; Ott, P.G.; Kaman-Toth, E.; Bognar, G.F.; Pogany, M.; Szatmari, A. Overlapping yet Response-Specific Transcriptome Alterations Characterize the Nature of Tobacco-Pseudomonas syringae Interactions. Front. Plant Sci. 2016, 7, 251. [Google Scholar] [CrossRef]
- Tsuda, K.; Sato, M.; Stoddard, T.; Glazebrook, J.; Katagiri, F. Network Properties of Robust Immunity in Plants. PLoS Genet. 2009, 5, e1000772. [Google Scholar] [CrossRef]
- Moore, J.; Penfold, C.A.; Jenkins, D.J.; Hill, C.; Baxter, L.; Kulasekaran, S.; Truman, W.; Littlejohn, G.; Prusinska, J.; Mead, A.; et al. Transcriptional Dynamics Driving MAMP-Triggered Immunity and Pathogen Effector-Mediated Immunosuppression in Arabidopsis Leaves Following Infection with Pseudomonas syringae pv. tomato DC3000. Plant Cell 2015, 27, 3038–3064. [Google Scholar]
- Parker, J.; Koh, J.; Yoo, M.J.; Zhu, N.; Feole, M.; Yi, S.; Chen, S. Quantitative Proteomics of Tomato Defense against Pseudomonas syringae Infection. Proteomics 2013, 13, 1934–1946. [Google Scholar] [CrossRef]
- Ow, S.Y.; Salim, M.; Noirel, J.; Evans, C.; Wright, P.C. Minimising Itraq Ratio Compression through Understanding LC-MS Elution Dependence and High-Resolution Hilic Fractionation. Proteomics 2011, 11, 2341–2346. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted Data Extraction of the MS/MS Spectra Generated by Data-Independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Mol. Cell. Proteom. 2012, 11, O111.016717. [Google Scholar] [CrossRef]
- Xin, X.F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What It Takes to Be a Pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Mysore, K.S.; Crasta, O.R.; Tuori, R.P.; Folkerts, O.; Swirsky, P.B.; Martin, G.B. Comprehensive Transcript Profiling of Pto- and Prf-Mediated Host Defense Responses to Infection by Pseudomonas syringae pv. tomato. Plant J. 2002, 32, 299–315. [Google Scholar] [CrossRef]
- Midoh, N.; Iwata, M. Cloning and Characterization of a Probenazole-Inducible Gene for an Intracellular Pathogenesis-Related Protein in Rice. Plant Cell Physiol. 1996, 37, 9–18. [Google Scholar] [CrossRef]
- Somssich, I.E.; Schmelzer, E.; Kawalleck, P.; Hahlbrock, K. Gene Structure and in Situ Transcript Localization of Pathogenesis-Related Protein 1 in Parsley. Mol. Gen. Genet. 1988, 213, 93–98. [Google Scholar] [CrossRef]
- Brunner, F.; Stintzi, A.; Fritig, B.; Legrand, M. Substrate Specificities of Tobacco Chitinases. Plant J. 1998, 14, 225–234. [Google Scholar] [CrossRef]
- Guevara-Morato, M.A.; de Lacoba, M.G.; Garcia-Luque, I.; Serra, M.T. Characterization of a Pathogenesis-Related Protein 4 (PR-4) Induced in Capsicum Chinense L3 Plants with Dual Rnase and Dnase Activities. J. Exp. Bot. 2010, 61, 3259–3271. [Google Scholar] [CrossRef]
- Caporale, C.; Di Berardino, I.; Leonardi, L.; Bertini, L.; Cascone, A.; Buonocore, V.; Caruso, C. Wheat Pathogenesis-Related Proteins of Class 4 Have Ribonuclease Activity. FEBS Lett. 2004, 575, 71–76. [Google Scholar] [CrossRef]
- Hwang, I.S.; Choi, D.S.; Kim, N.H.; Kim, D.S.; Hwang, B.K. Pathogenesis-Related Protein 4b Interacts with Leucine-Rich Repeat Protein 1 to Suppress PR4b-Triggered Cell Death and Defense Response in Pepper. Plant J. 2014, 77, 521–533. [Google Scholar] [CrossRef]
- Despres, C.; Subramaniam, R.; Matton, D.P.; Brisson, N. The Activation of the Potato PR-Loa Gene Requires the Phosphorylation of the Nuclear Factor PBF-1. Plant Cell 1995, 7, 589–598. [Google Scholar] [CrossRef]
- Moiseyev, G.P.; Beintema, J.J.; Fedoreyeva, L.I.; Yakovlev, G.I. High Sequence Similarity between a Ribonuclease from Ginseng Calluses and Fungus-Elicited Proteins from Parsley Indicates That Intracellular Pathogenesis-Related Proteins Are Ribonucleases. Planta 1994, 193, 470–472. [Google Scholar] [CrossRef]
- Chiang, C.C.; Hadwiger, L.A. Cloning and Characterization of a Disease Resistance Response Gene in Pea Inducible by Fusarium Solani. Mol. Plant-Microbe Interact. 1990, 3, 78–85. [Google Scholar] [CrossRef]
- Warner, S.A.J.; Scott, R.; Draper, J. Characterisation of a Wound-Induced Transcript from the Monocot Asparagus That Shares Similarity with a Class of Intracellular Pathogenesis-Related (PR) Proteins. Plant Mol. Biol. 1992, 19, 555–561. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Ann. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Rochon, A.; Boyle, P.; Wignes, T.; Fobert, P.R.; Despres, C. The Coactivator Function of Arabidopsis NPR1 Requires the Core of Its Btb/Poz Domain and the Oxidation of C-Terminal Cysteines. Plant Cell 2006, 18, 3670–3685. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Palva, E.T. Kunitz Trypsin Inhibitor: An Antagonist of Cell Death Triggered by Phytopathogens and Fumonisin B1 in Arabidopsis. Mol. Plant 2008, 1, 482–495. [Google Scholar] [CrossRef]
- Zimmermann, P.; Hirsch-Hoffmann, M.; Hennig, L.; Gruissem, W. Genevestigator. Arabidopsis Microarray Database and Analysis Toolbox. Plant Physiol. 2004, 136, 2621–2632. [Google Scholar] [CrossRef]
- Chen, Z.; Silva, H.; Klessig, D.F. Active Oxygen Species in the Induction of Plant Systemic Acquired Resistance by Salicylic Acid. Science 1993, 262, 1883–1886. [Google Scholar] [CrossRef]
- Chen, H.; McCaig, B.C.; Melotto, M.; He, S.Y.; Howe, G.A. Regulation of Plant Arginase by Wounding, Jasmonate and the Phytotoxin Coronatine. J. Biol. Chem. 2004, 279, 45998–46007. [Google Scholar] [CrossRef]
- Delledonne, M. No News Is Good News for Plants. Curr. Opin. Plant Biol. 2005, 8, 390–396. [Google Scholar] [CrossRef]
- Huang, X.; Stettmaier, K.; Michel, C.; Hutzler, P.; Mueller, M.J.; Durner, J. Nitric Oxide Is Induced by Wounding and Influences Jasmonic Acid Signaling in Arabidopsis Thaliana. Planta 2004, 218, 938–946. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Paul Bolwell, G.J.P. Reactive Oxygen Species and Their Role in Plant Defence and Cell Wall Metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Butt, V.S.; Davies, D.R.; Zimmerlin, A. The Origin of the Oxidative Burst in Plants. Free Radic. Res. 1995, 23, 517–532. [Google Scholar] [CrossRef]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-Transferase Enzymes in Plant-Pathogen Interactions. Front. Plant Sci. 2018, 9, 1–19. [Google Scholar] [CrossRef]
- Jones, A.M.E.; Thomas, V.; Bennett, M.H.; Mansfield, J.; Grant, M. Modifications to the Arabidopsis Defense Proteome Occur Prior to Significant Transcriptional Change in Response to Inoculation with Pseudomonas syringae. Plant Physiol. 2006, 142, 1603–1620. [Google Scholar] [CrossRef]
- Serrato, A.J.; Fernández-Trijueque, J.; Barajas-López, J.-D.-D.; Chueca, A.; Sahrawy, M. Plastid Thioredoxins: A “One-for-All” Redox-Signaling System in Plants. Front. Plant Sci. 2013, 4, 463. [Google Scholar] [CrossRef]
- Dubreuil-Maurizi, C.; Vitecek, J.; Marty, L.; Branciard, L.; Frettinger, P.; Wendehenne, D.; Meyer, A.J.; Mauch, F.; Poinssot, B. Glutathione Deficiency of the Arabidopsis Mutant Pad2-1 Affects Oxidative Stress-Related Events, Defense Gene Expression and the Hypersensitive Response. Plant Physiol. 2011, 157, 2000–2012. [Google Scholar] [CrossRef]
- Mattoo, R.U.H.; Goloubinoff, P. Molecular Chaperones Are Nanomachines That Catalytically Unfold Misfolded and Alternatively Folded Proteins. Cell. Mol. Life Sci. 2014, 71, 3311–3325. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakanishi, H.; Bower, J.; Yoder, D.W.; Osteryoung, K.W.; Miyagishima, S.Y. Plastid Chaperonin Proteins Cpn60 Alpha and Cpn60 Beta Are Required for Plastid Division in Arabidopsis Thaliana. BMC Plant Biol. 2009, 9, 38. [Google Scholar] [CrossRef]
- Oh, S.E.; Yeung, C.; Babaei-Rad, R.; Zhao, R. Cosuppression of the Chloroplast Localized Molecular Chaperone Hsp90.5 Impairs Plant Development and Chloroplast Biogenesis in Arabidopsis. BMC Res. Notes 2014, 7, 643. [Google Scholar] [CrossRef]
- Schulze-Lefert, P. Plant Immunity: The Origami of Receptor Activation. Curr. Biol. 2004, 14, R22–R24. [Google Scholar] [CrossRef]
- Hubert, D.A. Cytosolic Hsp90 Associates with and Modulates the Arabidopsis RPM1 Disease Resistance Protein. EMBO J. 2003, 22, 5679–5689. [Google Scholar] [CrossRef]
- Liu, Y.; Burch-Smith, T.; Schiff, M.; Feng, S.; Dinesh-Kumar, S.P. Molecular Chaperone Hsp90 Associates with Resistance Protein N and Its Signaling Proteins SGT1 and Rar1 to Modulate an Innate Immune Response in Plants. J. Biol. Chem. 2004, 279, 2101–2108. [Google Scholar] [CrossRef]
- Shirasu, K. Complex Formation, Promiscuity and Multi-Functionality: Protein Interactions in Disease-Resistance Pathways. Trends Plant Sci. 2003, 8, 252–258. [Google Scholar] [CrossRef]
- Kadota, Y.; Shirasu, K. The Hsp90 Complex of Plants. Biochim. Biophys. Acta 2012, 1823, 689–697. [Google Scholar] [CrossRef]
- Thomas, E.L.; van der Hoorn, R.A.L. Ten Prominent Host Proteases in Plant-Pathogen Interactions. Int. J. Mol. Sci. 2018, 19, 639. [Google Scholar] [CrossRef]
- Shompole, S.; Jasmer, D.P. Cathepsin B-Like Cysteine Proteases Confer Intestinal Cysteine Protease Activity in Haemonchus Contortus. J. Biol. Chem. 2001, 276, 2928–2934. [Google Scholar] [CrossRef]
- Zhao, M.; Ji, H.M.; Gao, Y.; Cao, X.X.; Mao, H.Y.; Ouyang, S.Q.; Liu, P. An Integrated Analysis of Mrna and Srna Transcriptional Profiles in Tomato Root: Insights on Tomato Wilt Disease. PLoS ONE 2018, 13, 1–18. [Google Scholar] [CrossRef]
- Bernoux, M.; Timmers, T.; Jauneau, A.; Briere, C.; de Wit, P.J.G.M.; Marco, Y.; Deslandes, L. RD19, an Arabidopsis Cysteine Protease Required for RRS1-R-Mediated Resistance, Is Relocalized to the Nucleus by the Ralstonia Solanacearum Popp2 Effector. Plant Cell Online 2008, 20, 2252–2264. [Google Scholar] [CrossRef]
- Koizumi, M.; Yamaguchi-Shinozaki, K.; Tsuji, H.; Shinozaki, K. Structure and Expression of Two Genes That Encode Distinct Drought-Inducible Cysteine Proteinases in Arabidopsis Thaliana. Gene 1993, 129, 175–182. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by Small-Molecule Hormones in Plant Immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Brooks, D.M.; Hernandez-Guzman, G.; Kloek, A.P.; Alarcon-Chaidez, F.; Sreedharan, A.; Rangaswamy, V.; Penaloza-Vazquez, A.; Bender, C.L.; Kunkel, B.N. Identification and Characterization of a Well-Defined Series of Coronatine Biosynthetic Mutants of Pseudomonas syringae Pv. Tomato DC3000. Mol. Plant Microbe Interact. 2004, 17, 162–174. [Google Scholar] [CrossRef]
- Desclos-Theveniau, M.; Arnaud, D.; Huang, T.Y.; Lin, G.J.; Chen, W.Y.; Lin, Y.C.; Zimmerli, L. The Arabidopsis Lectin Receptor Kinase Lecrk-V.5 Represses Stomatal Immunity Induced by Pseudomonas syringae Pv. Tomato DC3000. PLoS Pathog. 2012, 8, e1002513. [Google Scholar] [CrossRef]
- Chakravarthy, S.; Butcher, B.G.; Liu, Y.; D’Amico, K.; Coster, M.; Filiatrault, M.J. Virulence of Pseudomonas syringae Pv. Tomato DC3000 Is Influenced by the Catabolite Repression Control Protein Crc. Mol. Plant Microbe Interact. 2017, 30, 283–294. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two Simple Media for the Demonstration of Pyocyanin and Fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
- Betancourt, L.H.; Sanchez, A.; Pla, I.; Kuras, M.; Zhou, Q.; Andersson, R.; Marko-Varga, G. Quantitative Assessment of Urea in-Solution Lys-C/Trypsin Digestions Reveals Superior Performance at Room Temperature over Traditional Proteolysis at 37 °C. J. Proteome Res. 2018, 17, 2556–2561. [Google Scholar] [CrossRef]
- Mertins, P.; Tang, L.C.; Krug, K.; Clark, D.J.; Gritsenko, M.A.; Chen, L.; Clauser, K.R.; Clauss, T.R.; Shah, P.; Gillette, M.A.; et al. Reproducible Workflow for Multiplexed Deep-Scale Proteome and Phosphoproteome Analysis of Tumor Tissues by Liquid Chromatography–Mass Spectrometry. Nat. Protoc. 2018, 13, 1632–1661. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, W.Y.; Tseng, M.C.; Chen, Y.R. Tunnel Frit: A Nonmetallic in-Capillary Frit for Nanoflow Ultra High-Performance Liquid Chromatography-Mass Spectrometryapplications. Anal. Chem. 2012, 84, 297–303. [Google Scholar] [CrossRef]
- Scheltema, R.A.; Hauschild, J.P.; Lange, O.; Hornburg, D.; Denisov, E.; Damoc, E.; Kuehn, A.; Makarov, A.; Mann, M. The Q Exactive HF, a Benchtop Mass Spectrometer with a Pre-Filter, High-Performance Quadrupole and an Ultra-High-Field Orbitrap Analyzer. Mol. Cell Proteom. 2014, 13, 3698–3708. [Google Scholar] [CrossRef]
- Bruderer, R.; Bernhardt, O.M.; Gandhi, T.; Xuan, Y.; Sondermann, J.; Schmidt, M.; Gomez-Varela, D.; Reiter, L. Optimization of Experimental Parameters in Data-Independent Mass Spectrometry Significantly Increases Depth and Reproducibility of Results. Mol. Cell Proteom. 2017, 16, 2296–2309. [Google Scholar] [CrossRef]
- Craig, R.; Beavis, R.C. Tandem: Matching Proteins with Tandem Mass Spectra. Bioinformatics 2004, 20, 1466–1467. [Google Scholar] [CrossRef]
- Eng, J.K.; Jahan, T.A.; Hoopmann, M.R. Comet: An Open-Source MS/MS Sequence Database Search Tool. Proteomics 2013, 13, 22–24. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Mendoza, L.; Shteynberg, D.; Slagel, J.; Sun, Z.; Moritz, R.L. Trans-Proteomic Pipeline, a Standardized Data Processing Pipeline for Large-Scale Reproducible Proteomics Informatics. Proteom. Clin. Appl. 2015, 9, 745–754. [Google Scholar] [CrossRef]
- Röst, H.L.; Sachsenberg, T.; Aiche, S.; Bielow, C.; Weisser, H.; Aicheler, F.; Andreotti, S.; Ehrlich, H.C.; Gutenbrunner, P.; Kenar, E.; et al. OpenMS: A Flexible Open-Source Software Platform for Mass Spectrometry Data Analysis. Nat. Methods 2016, 13, 741–748. [Google Scholar] [CrossRef]
- Röst, H.L.; Rosenberger, G.; Navarro, P.; Gillet, L.; Miladinović, S.M.; Schubert, O.T.; Wolski, W.; Collins, B.C.; Malmström, J.; Malmström, L.; et al. OpenSWATH Enables Automated, Targeted Analysis of Data- Independent Acquisition MS Data. Nat. Biotechnol. 2014, 32, 219–223. [Google Scholar] [CrossRef]
- Lambert, J.P.; Ivosev, G.; Couzens, A.L.; Larsen, B.; Taipale, M.; Lin, Z.Y.; Zhong, Q.; Lindquist, S.; Vidal, M.; Aebersold, R.; et al. Mapping Differential Interactomes by Affinity Purification Coupled with Data-Independent Mass Spectrometry Acquisition. Nat. Methods 2013, 10, 1239–1245. [Google Scholar] [CrossRef]
- Mi, H.; Huang, X.; Muruganujan, A.; Tang, H.; Mills, C.; Kang, D.; Thomas, P.D. Panther Version 11: Expanded Annotation Data from Gene Ontology and Reactome Pathways and Data Analysis Tool Enhancements. Nucleic Acids Res. 2017, 45, 183–189. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. Large-Scale Gene Function Analysis with the Panther Classification System. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using David Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Vizcaino, J.A.; Cote, R.G.; Csordas, A.; Dianes, J.A.; Fabregat, A.; Foster, J.M.; Griss, J.; Alpi, E.; Birim, M.; Contell, J.; et al. The Proteomics Identifications (PRIDE) Database and Associated Tools: Status in 2013. Nucleic Acids Res. 2013, 41, D1063–D1069. [Google Scholar] [CrossRef]


| Protein Description | Gene Accession | 4 hpi | 8 hpi | 24 hpi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| log2 Ratio a | p-Value b | log2 Ratio a | p-Value b | log2 Ratio a | p-Value b | ||||||
| Defence | |||||||||||
| Pathogenesis-related protein 4 (PR-4) | Solyc01g097240 | −0.183 | 0.496 | 0.885 | 0.049 | NQ | NQ | ||||
| PIN-I protein (PR-6) | Solyc09g083440 | −0.023 | 0.971 | −1.163 | 0.052 | 1.484 | 0.004 | ||||
| Wound-induced proteinase inhibitor 1 | Solyc09g084465 | NQ | NQ | NQ | NQ | 1.183 | 0.019 | ||||
| Major allergen Pru ar 1 (PR-STH 2) | Solyc09g090970 | 0.239 | 0.038 | 0.827 | 0.003 | 1.106 | 0.036 | ||||
| Biotin-binding protein | Solyc09g065540 | 0.067 | 0.695 | 0.093 | 0.794 | 0.615 | 0.048 | ||||
| Chitinase Z15140 | Solyc10g055810 | −0.195 | 0.487 | 0.869 | 0.006 | 0.101 | 0.907 | ||||
| Beta-1,3-glucanase (PR-2) | Solyc01g008620 | 0.123 | 0.669 | 0.291 | 0.290 | 1.111 | 0.008 | ||||
| Immune Regulation | |||||||||||
| Pathogenesis-related protein 1 (PR-1) | Solyc00g174340 | −0.182 | 0.153 | 0.949 | 0.052 | 3.059 | 0.001 | ||||
| 1-aminocyclopropane-1-carboxylate oxidase 1 | Solyc07g049530 | 0.132 | 0.705 | NQ | NQ | 2.831 | 0.004 | ||||
| Arginase 2 (ARG2) | Solyc01g091170 | 0.407 | 0.356 | 0.289 | 0.466 | 2.691 | 0.011 | ||||
| Cathepsin D Inhibitor | Solyc03g098780 | NQ | NQ | NQ | NQ | 1.947 | 0.040 | ||||
| Activator of 90 kDa heat shock ATPase | Solyc10g078930 | 0.204 | 0.442 | 0.544 | 0.065 | 0.936 | 0.005 | ||||
| FKBP-like peptidyl-prolyl cis-trans isomerase family protein | Solyc09g008650 | 0.084 | 0.827 | 0.851 | 0.006 | 0.925 | 0.035 | ||||
| Kunitz trypsin inhibitor | Solyc03g098730 | −0.355 | 0.187 | NQ | NQ | 0.714 | 0.046 | ||||
| Zeaxanthin epoxidase (ZEP) | Solyc02g090890 | 0.112 | 0.353 | −0.799 | 0.067 | −0.711 | 0.047 | ||||
| ROS/Redox | |||||||||||
| Glutathione S-transferase/peroxidase | Solyc07g056480 | 0.041 | 0.814 | NQ | NQ | 1.870 | 0.005 | ||||
| Peroxidase | Solyc09g072700 | −0.345 | 0.089 | −0.500 | 0.336 | −0.814 | 0.029 | ||||
| Thioredoxin reductase | Solyc02g082250 | 0.064 | 0.930 | 0.813 | 0.022 | NQ | NQ | ||||
| Peroxidase | Solyc04g071900 | NQ | NQ | 0.906 | 0.016 | NQ | NQ | ||||
| Glutathione Reductase (GR) | Solyc09g091840 | 0.476 | 0.086 | 0.368 | 0.290 | 0.902 | 0.010 | ||||
| Glutathione S-transferase-like protein | Solyc09g011570 | 0.158 | 0.430 | 0.765 | 0.038 | 0.812 | 0.001 | ||||
| Glutathione S-transferase | Solyc06g009040 | 0.201 | 0.401 | 0.865 | 0.049 | 0.429 | 0.210 | ||||
| Protein Description | Gene Accession | 4 hpi | 8 hpi | 24 hpi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| log2 Ratio a | p-Value b | log2 Ratio a | p-Value b | log2 Ratio a | p-Value b | ||||||
| Protein Translation, Folding, Degradation and Transportation | |||||||||||
| Importin subunit alpha | Solyc01g060470 | −0.122 | 0.473 | 0.185 | 0.449 | 1.138 | 0.016 | ||||
| Protein transport protein sec23, putative | Solyc05g053830 | −0.023 | 0.894 | 0.262 | 0.271 | 1.012 | 0.016 | ||||
| Golgin candidate 6 | Solyc08g081410 | −0.087 | 0.655 | −0.095 | 0.612 | 0.680 | 0.040 | ||||
| Cathepsin B-like cysteine protease | Solyc02g069110 | NQ | NQ | NQ | NQ | 1.802 | 0.000 | ||||
| T-complex protein 1 subunit beta | Solyc11g069000 | −0.487 | 0.460 | 0.101 | 0.597 | 1.352 | 0.017 | ||||
| 26S proteasome non-ATPase regulatory subunit 3 | Solyc01g111700 | 0.116 | 0.811 | 0.300 | 0.034 | 0.960 | 0.013 | ||||
| DNA damage-inducible protein 1 | Solyc10g005890 | −0.612 | 0.038 | −0.417 | 0.298 | −0.021 | 0.862 | ||||
| Pre-pro-cysteine proteinase | Solyc04g080960 | 0.313 | 0.193 | −0.634 | 0.025 | −0.388 | 0.316 | ||||
| Chaperonin 60 alpha subunit | Solyc06g075010 | −0.128 | 0.579 | −0.080 | 0.607 | 1.506 | 0.012 | ||||
| Heat shock 70 kDa protein, putative | Solyc07g043560 | 0.233 | 0.194 | 0.251 | 0.298 | 1.223 | 0.002 | ||||
| Peptidyl-prolyl cis-trans isomerase | Solyc06g076970 | 0.211 | 0.697 | 0.759 | 0.500 | 1.212 | 0.033 | ||||
| Heat shock protein 90 | Solyc06g036290 | 1.140 | 0.050 | 1.475 | 0.109 | 0.841 | 0.050 | ||||
| Calnexin | Solyc03g118040 | 0.672 | 0.028 | 0.256 | 0.576 | −0.067 | 0.881 | ||||
| Protein Description | Gene Accession | 4 hpi | 8 hpi | 24 hpi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| log2 Ratio a | p-Value b | log2 Ratio a | p-Value b | log2 Ratio a | p-Value b | ||||||
| Metabolism-Primary-Carbohydrate Metabolisms-Glycolysis & TCA | |||||||||||
| Glucose-6-phosphate 1-dehydrogenase | Solyc02g093830 | 0.289 | 0.111 | 0.752 | 0.146 | 0.955 | 0.013 | ||||
| Pyruvate kinase family protein | Solyc10g083720 | −0.104 | 0.326 | 0.316 | 0.208 | 0.915 | 0.001 | ||||
| Glyceraldehyde 3-phosphate dehydrogenase | Solyc05g014470 | 0.219 | 0.501 | 0.155 | 0.400 | 0.773 | 0.022 | ||||
| Glyceraldehyde-3-phosphate dehydrogenase | Solyc04g009030 | 0.258 | 0.027 | 0.196 | 0.208 | 0.663 | 0.004 | ||||
| 2-oxoglutarate dehydrogenase E1 component family protein | Solyc05g054640 | −0.193 | 0.360 | 0.490 | 0.007 | 0.654 | 0.033 | ||||
| Fructokinase 2 | Solyc06g073190 | 0.030 | 0.805 | 0.254 | 0.149 | 0.624 | 0.019 | ||||
| Metabolism-Primary-Carbohydrate metabolism-PPP | |||||||||||
| 6-phosphogluconate dehydrogenase, decarboxylating | Solyc04g005160 | −0.372 | 0.392 | 0.919 | 0.016 | 1.221 | 0.006 | ||||
| Transaldolase | Solyc00g006800 | −0.183 | 0.302 | 0.159 | 0.641 | 0.604 | 0.039 | ||||
| Metabolism-Primary-Carbon fixation | |||||||||||
| Photosystem II oxygen-evolving complex protein 3 | Solyc02g079950 | −0.739 | 0.057 | −0.694 | 0.028 | −0.250 | 0.157 | ||||
| Chlororespiratory reduction31 | Solyc08g082400 | −0.529 | 0.136 | −1.402 | 0.033 | −1.397 | 0.042 | ||||
| ATP-dependent zinc metalloprotease FTSH protein | Solyc07g055320 | 0.118 | 0.646 | −1.340 | 0.029 | −2.270 | 0.008 | ||||
| Protein CURVATURE THYLAKOID 1A, chloroplastic | Solyc10g011770 | −0.072 | 0.814 | −1.558 | 0.040 | −2.373 | 0.007 | ||||
| Cytochrome b6-f complex iron-sulphur subunit | Solyc12g005630 | −0.087 | 0.801 | −1.758 | 0.039 | −2.812 | 0.002 | ||||
| Chlorophyll a-b binding protein, chloroplastic | Solyc07g063600 | −0.353 | 0.601 | −1.891 | 0.011 | −2.993 | 0.047 | ||||
| Photosystem I reaction centre subunit III | Solyc02g069450 | 0.220 | 0.625 | −1.496 | 0.079 | −3.137 | 0.026 | ||||
| Chlorophyll a/b-binding protein | Solyc03g005760 | −0.540 | 0.493 | −1.788 | 0.024 | −3.159 | 0.024 | ||||
| Metabolism-Primary-Carbohydrate Metabolism-Others | |||||||||||
| Sucrose synthase | Solyc07g042550 | 0.250 | 0.297 | 1.258 | 0.031 | 1.542 | 0.031 | ||||
| Beta-fructofuranosidase | Solyc04g081440 | 0.261 | 0.367 | NQ | NQ | 0.818 | 0.005 | ||||
| xyloglucan endotransglucosylase-hydrolase 7 | Solyc02g091920 | 0.066 | 0.888 | 0.590 | 0.022 | 0.573 | 0.323 | ||||
| Starch synthase, chloroplastic/amyloplastic | Solyc03g083095 | −0.134 | 0.339 | −0.601 | 0.013 | −0.231 | 0.588 | ||||
| Protein Description | Gene Accession | 4 hpi | 8 hpi | 24 hpi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| log2 Ratio a | p-Value b | log2 Ratio a | p-Value b | log2 Ratio a | p-Value b | ||||||
| Metabolism-Primary-Oxidative phosphorylation | |||||||||||
| ATP synthase subunit alpha, chloroplastic | Solyc06g072540 | −0.244 | 0.124 | −0.708 | 0.011 | −0.879 | 0.001 | ||||
| ATP synthase delta-subunit protein | Solyc12g056830 | −0.546 | 0.011 | −1.014 | 0.018 | −1.061 | 0.020 | ||||
| Metabolism-Primary-Amino Acid & Polyamine Metabolism | |||||||||||
| S-adenosylmethionine synthase 2 (SAM2) | Solyc12g099000 | 0.045 | 0.924 | −0.345 | 0.564 | 1.194 | 0.006 | ||||
| Chorismate synthase 1 precursor | Solyc04g049350 | 0.151 | 0.259 | 0.335 | 0.072 | 0.830 | 0.030 | ||||
| S-adenosylmethionine synthase 1 (SAM1) | Solyc01g101060 | 0.224 | 0.091 | 0.190 | 0.034 | 0.681 | 0.012 | ||||
| S-adenosylmethionine synthase | Solyc10g083970 | NQ | NQ | NQ | NQ | 0.630 | 0.007 | ||||
| Methylthioribose kinase | Solyc01g107550 | 0.125 | 0.156 | 0.333 | 0.169 | 0.626 | 0.005 | ||||
| Dehydroquinate dehydratase/shikimate: NADP oxidoreductase | Solyc01g067750 | −0.191 | 0.086 | 0.090 | 0.755 | 0.598 | 0.014 | ||||
| Ornithine decarboxylase | Solyc04g082030 | 0.153 | 0.211 | 0.728 | 0.293 | 1.673 | 0.037 | ||||
| Spermidine synthase | Solyc05g005710 | −0.494 | 0.048 | −0.020 | 0.955 | 1.007 | 0.014 | ||||
| Metabolism Primary-Fatty Acid/Lipids | |||||||||||
| Fatty acid beta-oxidation multifunctional protein | Solyc12g007170 | 0.340 | 0.573 | 0.663 | 0.204 | 2.662 | 0.004 | ||||
| Acetoacetyl-CoA thiolase | Solyc07g045350 | 0.210 | 0.020 | 0.849 | 0.050 | 2.195 | 0.030 | ||||
| 12-oxophytodienoate reductase 3 | Solyc07g007870 | −0.045 | 0.860 | 0.347 | 0.198 | 1.468 | 0.023 | ||||
| 4-coumarate-CoA ligase | Solyc03g117870 | 0.004 | 0.987 | 0.382 | 0.233 | 1.282 | 0.005 | ||||
| ATP-citrate synthase, putative | Solyc05g005160 | 0.091 | 0.649 | 0.587 | 0.045 | 0.998 | 0.117 | ||||
| Lipoxygenase | Solyc01g006560 | −0.437 | 0.184 | NQ | NQ | 0.767 | 0.038 | ||||
| Metabolism-Secondary | |||||||||||
| 5-enolpyruvylshikimate-3-phosphate synthase | Solyc01g091190 | 0.166 | 0.744 | 0.351 | 0.242 | 1.442 | 0.043 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, K.-T.; Wang, K.-H.; Chang, W.-H.; Yang, J.-C.; Yeh, C.-F.; Cheng, K.-T.; Hung, S.-C.; Chen, Y.-R. Application of Data-Independent Acquisition Approach to Study the Proteome Change from Early to Later Phases of Tomato Pathogenesis Responses. Int. J. Mol. Sci. 2019, 20, 863. https://doi.org/10.3390/ijms20040863
Fan K-T, Wang K-H, Chang W-H, Yang J-C, Yeh C-F, Cheng K-T, Hung S-C, Chen Y-R. Application of Data-Independent Acquisition Approach to Study the Proteome Change from Early to Later Phases of Tomato Pathogenesis Responses. International Journal of Molecular Sciences. 2019; 20(4):863. https://doi.org/10.3390/ijms20040863
Chicago/Turabian StyleFan, Kai-Ting, Kuo-Hsin Wang, Wei-Hung Chang, Jhih-Ci Yang, Ching-Fang Yeh, Kai-Tan Cheng, Sheng-Chi Hung, and Yet-Ran Chen. 2019. "Application of Data-Independent Acquisition Approach to Study the Proteome Change from Early to Later Phases of Tomato Pathogenesis Responses" International Journal of Molecular Sciences 20, no. 4: 863. https://doi.org/10.3390/ijms20040863
APA StyleFan, K.-T., Wang, K.-H., Chang, W.-H., Yang, J.-C., Yeh, C.-F., Cheng, K.-T., Hung, S.-C., & Chen, Y.-R. (2019). Application of Data-Independent Acquisition Approach to Study the Proteome Change from Early to Later Phases of Tomato Pathogenesis Responses. International Journal of Molecular Sciences, 20(4), 863. https://doi.org/10.3390/ijms20040863






