Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice
Abstract
1. Introduction
2. Results
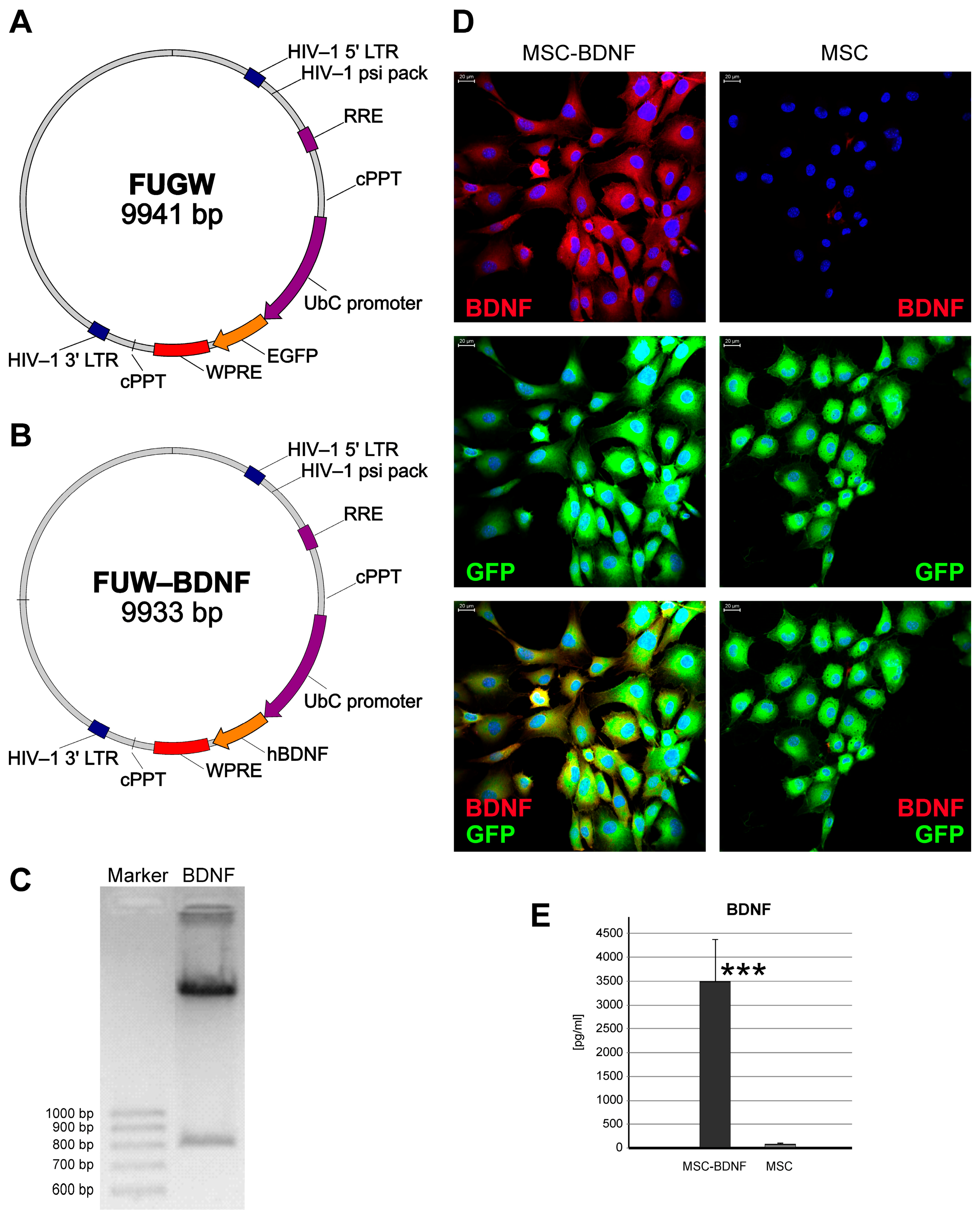
2.1. Evaluation of Ex Vivo Genetic Modification of MSCs for Effective BDNF Production
2.2. Homing, Migration, and Survival of Transplanted MSC within Injured Retina
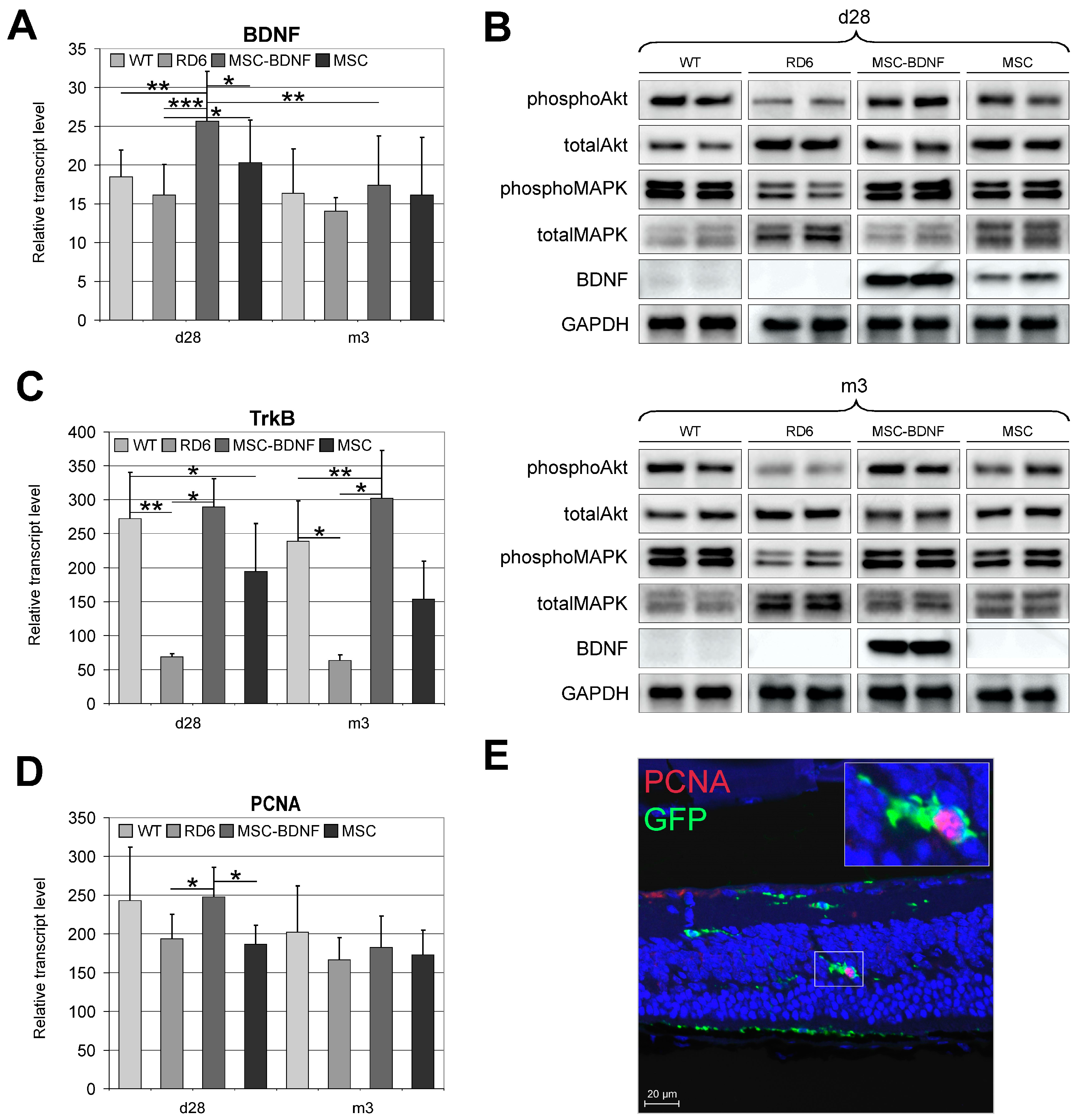
2.3. Long-Lasting BDNF Secretion by Engineered MSC and Its Biological Effects
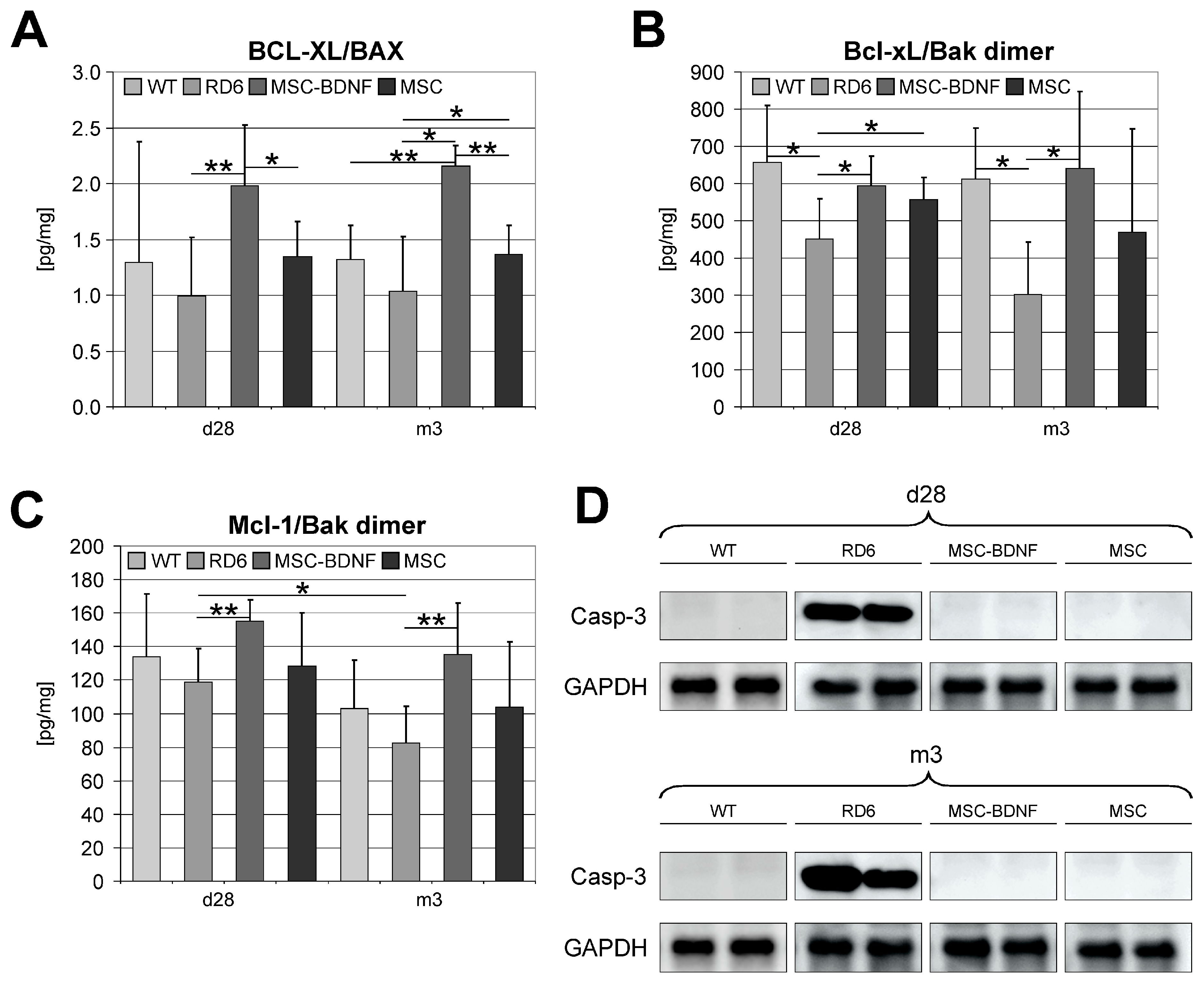
2.4. Induction of Anti-Apoptotic Signaling in Degenerated Retinas during Long-Term Observation
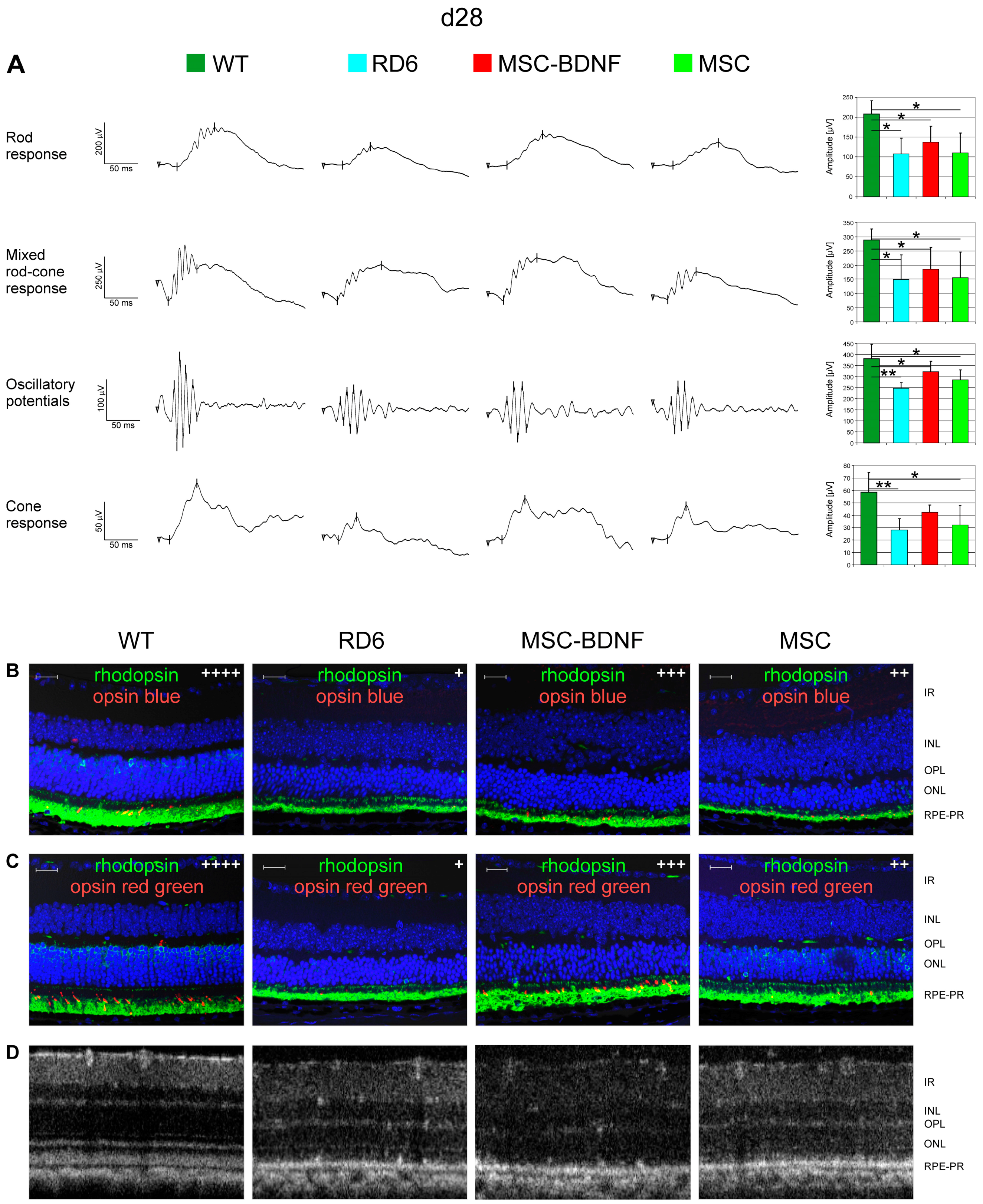
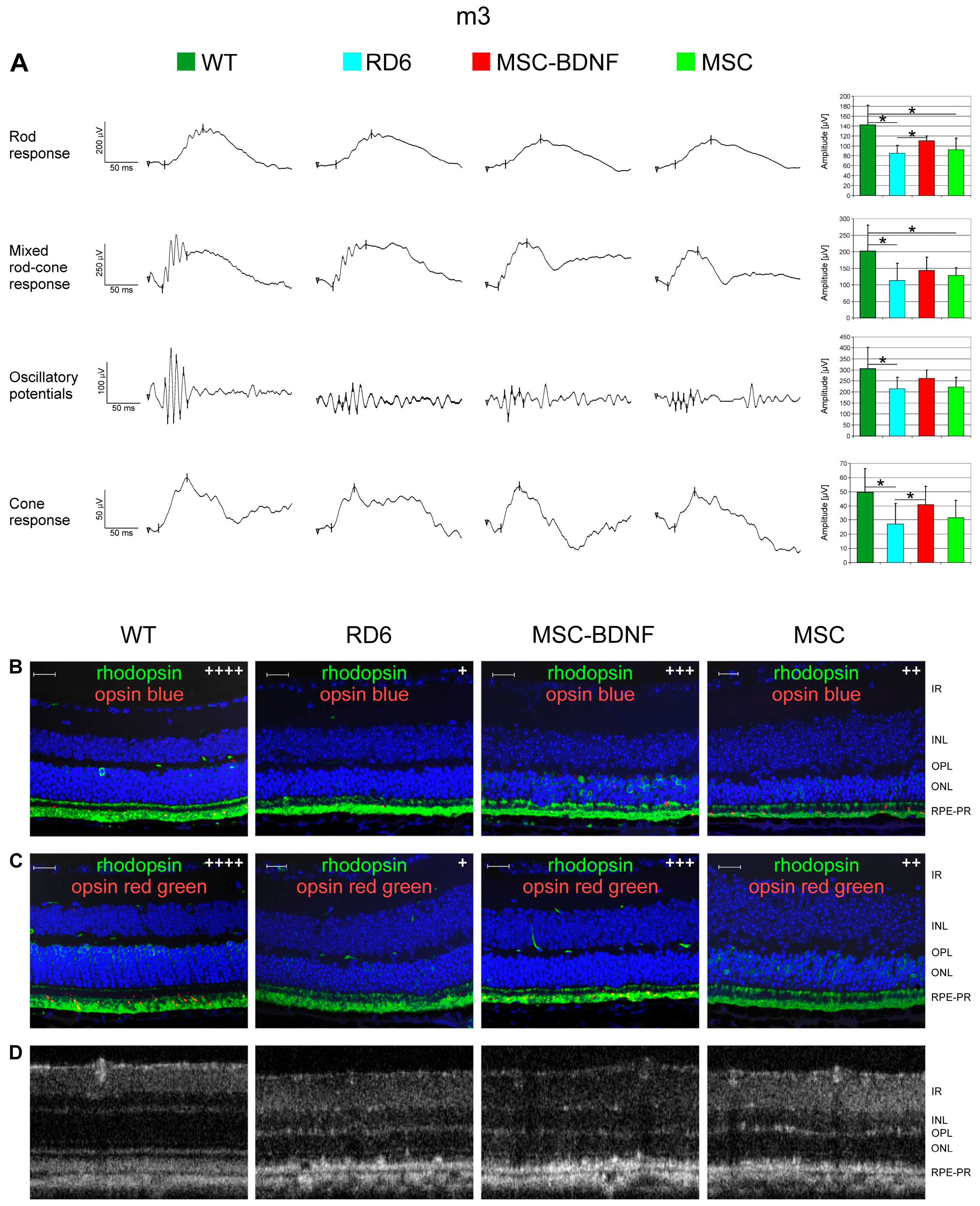
2.5. Neuroprotective Properties of Transplanted Cells after MSC-BDNF Treatment during Long-Term Observation
3. Discussion
4. Materials and Methods
4.1. Production of Recombinant Lentivirus Carrying the Human BDNF Gene
4.2. Isolation of MSCs
4.3. Lentiviral Transduction of Murine MSCs
4.4. Flow Cytometry of Murine MSCs
4.5. ELISA
4.6. Animals and Experimental Procedures
4.7. Immunofluorescence Analysis
4.8. qRT-PCR
4.9. Western Blot Analysis
4.10. Multiplex Immunoassay
4.11. Electroretinography
4.12. Spectral-Domain Optical Coherence Tomography (SD-OCT) Imaging
4.13. Statistical Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| qRT-PCR | Quantitative Reverse Transcription Polymerase Chain Reaction |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| Casp-3 | Caspase-3 |
| Bcl-xL | B-cell lymphoma-extra-large protein |
| Bcl-2 | B-cell lymphoma 2 protein |
| Mcl-1 | Induced myeloid leukemia cell differentiation protein |
| BDNF | Brain-derived Neurotrophic Factor |
| MFRP | Membrane-type Frizzled Related Protein |
| TrkA | Tyrosine Receptor Kinase A |
| TrkB | Tyrosine Receptor Kinase B |
| TrkC | Tyrosine Receptor Kinase C |
| PCNA | Proliferating Cell Nuclear Antigene |
| DAPI | 4′,6-diamidino-2-phenylindole |
| Sca-1 | Stem cell antigen 1 |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| MSCs | Mesenchymal Stromal Cells |
| Bax | Bcl-2 associated X protein |
| Bak | Bcl-2 homologous antagonist killer |
| Akt | Protein kinase B |
| ERG | Electroretinography |
| GFP | Green Fluorescent Protein |
| PBS | Phosphate Buffered Saline |
| rd6 | Retinal degeneration 6 |
| OCT | Optical Coherence Tomography |
| RPE | Retinal Pigment Epithelium |
| RGC | Retinal Ganglion Cells |
| NGF | Neural Growth Factor |
| CNS | Central Nervous System |
| ALS | Amyotrophic Lateral Sclerosis |
| NT-3 | Neurotrophin-3 |
| NT-4/5 | Neurotrophin-4/5 |
| NTs | Neurotrophins |
| BM | Bone-Marrow |
| RT | Room Temperature |
| SD | Standard Deviation |
References
- Chichagova, V.; Hallam, D.; Collin, J.; Zerti, D.; Dorgau, B.; Felemban, M.; Lako, M.; Steel, D.H. Cellular regeneration strategies for macular degeneration: Past, present and future. Eye (London) 2018, 32, 946–971. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Moisseiev, E.; Bauer, G.; Anderson, J.D.; Grant, M.B.; Zam, A.; Zawadzki, R.J.; Werner, J.S.; Nolta, J.A. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog. Retin. Eye Res. 2017, 56, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.K.; Lu, B.; Girman, S.; Wang, S. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog. Retin. Eye Res. 2017, 58, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Pius-Sadowska, E.; Machalinski, B. BDNF—A key player in cardiovascular system. J. Mol. Cell Cardiol. 2017, 110, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Hetman, M.; Xia, Z. Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol. Exp. (Wars) 2000, 60, 531–545. [Google Scholar] [PubMed]
- Seitz, R.; Hackl, S.; Seibuchner, T.; Tamm, E.R.; Ohlmann, A. Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the Wnt/beta-catenin signaling pathway and the induction of neuroprotective growth factors in Muller cells. J. Neurosci. 2010, 30, 5998–6010. [Google Scholar] [CrossRef]
- Bennett, J.L.; Zeiler, S.R.; Jones, K.R. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest. Ophthalmol. Vis. Sci. 1999, 40, 2996–3005. [Google Scholar]
- Zigova, T.; Pencea, V.; Wiegand, S.J.; Luskin, M.B. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol. Cell Neurosci. 1998, 11, 234–245. [Google Scholar] [CrossRef]
- Peinado-Ramon, P.; Salvador, M.; Villegas-Perez, M.P.; Vidal-Sanz, M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest. Ophthalmol. Vis. Sci 1996, 37, 489–500. [Google Scholar]
- Di Polo, A.; Aigner, L.J.; Dunn, R.J.; Bray, G.M.; Aguayo, A.J. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc. Natl. Acad. Sci. USA 1998, 95, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.B.; McKernan, D.P.; O’Brien, C.J.; Cotter, T.G. Retinal ganglion cells: Dying to survive. Int. J. Dev. Biol. 2006, 50, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, Y.; Wang, Y.; Zhang, D.; Shen, B.; Luo, M.; Gu, P. Progress of stem/progenitor cell-based therapy for retinal degeneration. J. Transl. Med. 2017, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: Preliminary phase 1 clinical trial findings. Invest. Ophthalmol. Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Tabata, Y.; Gao, J.Q. Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J. Control. Release 2012, 162, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Cislo-Pakuluk, A.; Marycz, K. A Promising Tool in Retina Regeneration: Current Perspectives and Challenges When Using Mesenchymal Progenitor Stem Cells in Veterinary and Human Ophthalmological Applications. Stem Cell Rev. 2017, 13, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.S.; Kumar, S.; Mok, P.L. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Holan, V.; Hermankova, B.; Kossl, J. Perspectives of Stem Cell-Based Therapy for Age-Related Retinal Degenerative Diseases. Cell Transplant. 2017, 26, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Meyerrose, T.; Olson, S.; Pontow, S.; Kalomoiris, S.; Jung, Y.; Annett, G.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv. Drug Deliv. Rev. 2010, 62, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- McGinley, L.; McMahon, J.; Strappe, P.; Barry, F.; Murphy, M.; O’Toole, D.; O’Brien, T. Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Res. Ther. 2011, 2, 12. [Google Scholar] [CrossRef]
- Machalińska, A.; Kawa, M.; Pius-Sadowska, E.; Stępniewski, J.; Nowak, W.; Rogińska, D.; Kaczyńska, K.; Baumert, B.; Wiszniewska, B.; Józkowicz, A.; et al. Long-term neuroprotective effects of NT-4-engineered mesenchymal stem cells injected intravitreally in a mouse model of acute retinal injury. Invest. Ophthalmol. Vis. Sci. 2013, 54, 8292–8305. [Google Scholar] [CrossRef] [PubMed]
- Wyse, R.D.; Dunbar, G.L.; Rossignol, J. Use of genetically modified mesenchymal stem cells to treat neurodegenerative diseases. Int. J. Mol. Sci. 2014, 15, 1719–1745. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Kameya, S.; Hawes, N.L.; Chang, B.; Heckenlively, J.R.; Naggert, J.K.; Nishina, P.M. Mfrp, a gene encoding a frizzled related protein, is mutated in the mouse retinal degeneration 6. Hum. Mol. Genet. 2002, 11, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Hawes, N.L.; Chang, B.; Hageman, G.S.; Nusinowitz, S.; Nishina, P.M.; Schneider, B.S.; Smith, R.S.; Roderick, T.H.; Davisson, M.T.; Heckenlively, J.R. Retinal degeneration 6 (rd6): A new mouse model for human retinitis punctata albescens. Invest. Ophthalmol. Vis. Sci. 2000, 41, 3149–3157. [Google Scholar] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M. Cell-Based Therapy for Degenerative Retinal Disease. Trends Mol. Med. 2016, 22, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Machalinska, A.; Baumert, B.; Kuprjanowicz, L.; Wiszniewska, B.; Karczewicz, D.; Machalinski, B. Potential application of adult stem cells in retinal repair--challenge for regenerative medicine. Curr. Eye Res. 2009, 34, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Machalińska, A.; Rogińska, D.; Pius-Sadowska, E.; Kawa, M.P.; Paczkowska, E.; Rudnicki, M.; Lejkowska, R.; Baumert, B.; Wiszniewska, B.; Machaliński, B. Neuroprotective and antiapoptotic activity of lineage-negative bone marrow cells after intravitreal injection in a mouse model of acute retinal injury. Stem Cells Int. 2015, 2015, 620364. [Google Scholar] [CrossRef]
- Sobus, A.; Baumert, B.; Litwinska, Z.; Golab-Janowska, M.; Stepniewski, J.; Kotowski, M.; Pius-Sadowska, E.; Kawa, M.P.; Grodecka-Szwajkiewicz, D.; Peregud-Pogorzelski, J.; et al. Safety and Feasibility of Lin- Cells Administration to ALS Patients: A Novel View on Humoral Factors and miRNA Profiles. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Czarzasta, J.; Habich, A.; Siwek, T.; Czaplinski, A.; Maksymowicz, W.; Wojtkiewicz, J. Stem cells for ALS: An overview of possible therapeutic approaches. Int. J. Dev. Neurosci. 2017, 57, 46–55. [Google Scholar] [CrossRef]
- Lech, W.; Figiel-Dabrowska, A.; Sarnowska, A.; Drela, K.; Obtulowicz, P.; Noszczyk, B.H.; Buzanska, L.; Domanska-Janik, K. Phenotypic, Functional, and Safety Control at Preimplantation Phase of MSC-Based Therapy. Stem Cells Int. 2016, 2016, 2514917. [Google Scholar] [CrossRef] [PubMed]
- Drela, K.; Lech, W.; Figiel-Dabrowska, A.; Zychowicz, M.; Mikula, M.; Sarnowska, A.; Domanska-Janik, K. Enhanced neuro-therapeutic potential of Wharton’s Jelly-derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy 2016, 18, 497–509. [Google Scholar] [CrossRef]
- Drela, K.; Siedlecka, P.; Sarnowska, A.; Domanska-Janik, K. Human mesenchymal stem cells in the treatment of neurological diseases. Acta Neurobiol. Exp. (Wars) 2013, 73, 38–56. [Google Scholar] [PubMed]
- Singer, N.G.; Caplan, A.I. Mesenchymal stem cells: Mechanisms of inflammation. Annu. Rev. Pathol. 2011, 6, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Tzameret, A.; Sher, I.; Belkin, M.; Treves, A.J.; Meir, A.; Nagler, A.; Levkovitch-Verbin, H.; Barshack, I.; Rosner, M.; Rotenstreich, Y. Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Exp. Eye Res. 2014, 118, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, S.; Absenger, Y.; Klein, H.; Addicks, K.; Schraermeyer, U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 414–422. [Google Scholar] [CrossRef]
- Johnson, T.V.; Bull, N.D.; Hunt, D.P.; Marina, N.; Tomarev, S.I.; Martin, K.R. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2051–2059. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Dardik, R.; Vander, S.; Melamed, S. Mechanism of retinal ganglion cells death in secondary degeneration of the optic nerve. Exp. Eye Res. 2010, 91, 127–134. [Google Scholar] [CrossRef]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest. Ophthalmol. Vis. Sci. 2013, 54, 7544–7556. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.S.; Shin, J.M.; Chun, M.H.; Oh, S.J. Nestin expressing progenitor cells during establishment of the neural retina and its vasculature. Anat. Cell Biol. 2012, 45, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Messias, A.; Gurgel, V.P.; Simoes, B.P.; Scott, I.U.; Jorge, R. Improvement of ischaemic macular oedema after intravitreal injection of autologous bone marrow-derived haematopoietic stem cells. Acta Ophthalmol. 2015, 93, e174–e176. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S.; Malkin, A. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: A preliminary report. Neural Regen. Res. 2015, 10, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.M.; Adamson, L.; Blits, B.; Bunge, M.B.; Grozdanic, S.D.; Sakaguchi, D.S. Brain-derived neurotrophic factor released from engineered mesenchymal stem cells attenuates glutamate- and hydrogen peroxide-mediated death of staurosporine-differentiated RGC-5 cells. Exp. Eye Res. 2009, 89, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Chen, G.; Lv, L.; Li, L.; Wei, D.; Gu, P.; Gao, J.; Miao, Y.; Hu, W. The effect of lentivirus-mediated TH and GDNF genetic engineering mesenchymal stem cells on Parkinson’s disease rat model. Neurol Sci. 2011, 32, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Ji, F.; Liu, B.; Wang, F.; Dong, F.; Zhu, Y. Improvement of deficits by transplantation of lentiviral vector-modified human amniotic mesenchymal cells after cerebral ischemia in rats. Brain Res. 2012, 1448, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Anderson, J.D.; Yu, A.S.; Annett, G.; Fink, K.D.; Nolta, J.A. Engineered BDNF producing cells as a potential treatment for neurologic disease. Expert Opin. Biol. Ther. 2016, 16, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Canals, J.M.; Pineda, J.R.; Torres-Peraza, J.F.; Bosch, M.; Martin-Ibanez, R.; Munoz, M.T.; Mengod, G.; Ernfors, P.; Alberch, J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J. Neurosci. 2004, 24, 7727–7739. [Google Scholar] [CrossRef]
- Vicario-Abejon, C.; Owens, D.; McKay, R.; Segal, M. Role of neurotrophins in central synapse formation and stabilization. Nat. Rev. Neurosci. 2002, 3, 965–974. [Google Scholar] [CrossRef]
- Zhang, X.; Poo, M.M. Localized synaptic potentiation by BDNF requires local protein synthesis in the developing axon. Neuron 2002, 36, 675–688. [Google Scholar] [CrossRef]
- Murer, M.G.; Yan, Q.; Raisman-Vozari, R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 2001, 63, 71–124. [Google Scholar] [CrossRef]
- Paczkowska, E.; Luczkowska, K.; Piecyk, K.; Roginska, D.; Pius-Sadowska, E.; Ustianowski, P.; Cecerska, E.; Dolegowska, B.; Celewicz, Z.; Machalinski, B. The influence of BDNF on human umbilical cord blood stem/progenitor cells: Implications for stem cell-based therapy of neurodegenerative disorders. Acta Neurobiol. Exp. (Wars) 2015, 75, 172–191. [Google Scholar] [PubMed]
- Paczkowska, E.; Kaczynska, K.; Pius-Sadowska, E.; Roginska, D.; Kawa, M.; Ustianowski, P.; Safranow, K.; Celewicz, Z.; Machalinski, B. Humoral activity of cord blood-derived stem/progenitor cells: Implications for stem cell-based adjuvant therapy of neurodegenerative disorders. PLoS ONE 2013, 8, e83833. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, B.; Girman, S.; Duan, J.; McFarland, T.; Zhang, Q.S.; Grompe, M.; Adamus, G.; Appukuttan, B.; Lund, R. Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLoS ONE 2010, 5, e9200. [Google Scholar] [CrossRef] [PubMed]
- Haddad-Mashadrizeh, A.; Bahrami, A.R.; Matin, M.M.; Edalatmanesh, M.A.; Zomorodipour, A.; Fallah, A.; Gardaneh, M.; Ahmadian Kia, N.; Sanjarmoosavi, N. Evidence for crossing the blood barrier of adult rat brain by human adipose-derived mesenchymal stromal cells during a 6-month period of post-transplantation. Cytotherapy 2013, 15, 951–960. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, S.I.; Oh, J.H.; Kim, S.M.; Jeong, C.H.; Jun, J.A.; Lee, K.S.; Oh, W.; Lee, J.K.; Jeun, S.S. Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J. Neurosci. Res. 2008, 86, 2168–2178. [Google Scholar] [CrossRef]
- Junyi, L.; Na, L.; Yan, J. Mesenchymal stem cells secrete brain-derived neurotrophic factor and promote retinal ganglion cell survival after traumatic optic neuropathy. J. Craniofac. Surg. 2015, 26, 548–552. [Google Scholar] [CrossRef]
- Zajac, C.S.; Bunger, P.C.; Moore, J.C. Neuron development in the superior colliculus of the fetal mouse following maternal alcohol exposure. Teratology 1988, 38, 37–43. [Google Scholar] [CrossRef]
- Hu, Z.L.; Li, N.; Wei, X.; Tang, L.; Wang, T.H.; Chen, X.M. Neuroprotective effects of BDNF and GDNF in intravitreally transplanted mesenchymal stem cells after optic nerve crush in mice. Int. J. Ophthalmol. 2017, 10, 35–42. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, J.H.; Sun Kim, H.; Park, C.K. Stem cell-based delivery of brain-derived neurotrophic factor gene in the rat retina. Brain Res. 2012, 1469, 10–23. [Google Scholar] [CrossRef]
- Harper, M.M.; Grozdanic, S.D.; Blits, B.; Kuehn, M.H.; Zamzow, D.; Buss, J.E.; Kardon, R.H.; Sakaguchi, D.S. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4506–4515. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Chen, W.; Cobb, M.H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.T. The eclectroretinogram: Its components and their origins. Vision Res. 1968, 8, 633–677. [Google Scholar] [CrossRef]
- Cameron, M.A.; Barnard, A.R.; Lucas, R.J. The electroretinogram as a method for studying circadian rhythms in the mammalian retina. J. Genet. 2008, 87, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.W.; Witten, C.M.; Califf, R.M. Clarifying Stem-Cell Therapy’s Benefits and Risks. N. Engl. J. Med. 2017, 376, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Langrzyk, A.; Nowak, W.N.; Stepniewski, J.; Jazwa, A.; Florczyk-Soluch, U.; Jozkowicz, A.; Dulak, J. Critical View on Mesenchymal Stromal Cells in Regenerative Medicine. Antioxid. Redox Signal. 2018, 29, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E., 2nd; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W., Jr.; Goldberg, J.L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Lois, C.; Hong, E.J.; Pease, S.; Brown, E.J.; Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002, 295, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Guo, Z.K.; Jiang, X.X.; Li, H.; Wang, X.Y.; Yao, H.Y.; Zhang, Y.; Mao, N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 2010, 5, 550–560. [Google Scholar] [CrossRef] [PubMed]

| Coexpression | Method | First Set | Second Set | ||
|---|---|---|---|---|---|
| Antibody | Dilution | Antibody | Dilution | ||
| I Ab * | rabbit anti-BDNF 1 | 1:100 | rabbit anti-GFP 1 | 1:100 | |
| BDNF and GFP | II Ab ** | goat anti-rabbit HRP 2 | 1:100 | goat anti-rabbit HRP 2 | 1:100 |
| Detection | Tyramide Alexa-Fluor 594 2 | 1:100 | Tyramide Alexa-Fluor 488 3 | 1:100 | |
| I Ab | goat anti-PCNA 4 | 1:100 | rabbit anti-GFP 1 | 1:100 | |
| PCNA and GFP | II Ab | donkey anti-goat HRP 5 | 1:100 | chicken anti-rabbit HRP 6 | 1:100 |
| Detection | Tyramide Alexa-Fluor 594 2 | 1:100 | Tyramide Alexa-Fluor 488 3 | 1:100 | |
| I Ab | rabbit anti-opsin blue 5 | 1:100 | mouse anti-rhodopsin 1 | 1:1000 | |
| opsin blue and rhodopsin | II Ab | goat anti-rabbit HRP 2 | 1:100 | goat anti-mouse HRP 3 | 1:100 |
| Detection | Tyramide Alexa-Fluor 594 2 | 1:100 | Tyramide Alexa-Fluor 488 3 | 1:100 | |
| I Ab | mouse anti-rhodopsin 1 | 1:1000 | rabbit anti-opsin red/green 5 | 1:250 | |
| rhodopsin and opsin red/green | II Ab | goat anti-mouse HRP 3 | 1:100 | goat anti-rabbit HRP 2 | 1:100 |
| Detection | Tyramide Alexa-Fluor 488 3 | 1:100 | Tyramide Alexa-Fluor 594 2 | 1:100 | |
| Gene | Primer | Sequence |
|---|---|---|
| mGAPDH | Forward | 5′-AGGTCGGTGTGAACGGATTT-3′ |
| Reverse | 5′-TGTAGACCATGTAGTTGAGGT-3′ | |
| mBDNF | Forward | 5′-GCACTGGAACTCGCAATGC-3′ |
| Reverse | 5′-GTAAGGGCCCGAACATACGA-3′ | |
| mTRKB | Forward | 5′-TTGACCCGGAGAACATCACG-3′ |
| Reverse | 5′-CCACAAACTTTAAGCCGGAATCC-3′ | |
| mPCNA | Forward | 5′-CTTGGTACAGCTTACTCTGCG-3′ |
| Reverse | 5′-AGTTGCTCCACATCTAAGTCCAT-3′ | |
| mBCL-XL | Forward | 5′-GACAAGGAGATGCAGGTATTGG-3′ |
| Reverse | 5′-TCCCGTAGAGATCCACAAAAGT-3′ | |
| mBAX | Forward | 5′-TGAAGACAGGGGCCTTTTTG-3′ |
| Reverse | 5′-AATTCGCCGGAGACACTCG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lejkowska, R.; Kawa, M.P.; Pius-Sadowska, E.; Rogińska, D.; Łuczkowska, K.; Machaliński, B.; Machalińska, A. Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice. Int. J. Mol. Sci. 2019, 20, 777. https://doi.org/10.3390/ijms20030777
Lejkowska R, Kawa MP, Pius-Sadowska E, Rogińska D, Łuczkowska K, Machaliński B, Machalińska A. Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice. International Journal of Molecular Sciences. 2019; 20(3):777. https://doi.org/10.3390/ijms20030777
Chicago/Turabian StyleLejkowska, Renata, Miłosz Piotr Kawa, Ewa Pius-Sadowska, Dorota Rogińska, Karolina Łuczkowska, Bogusław Machaliński, and Anna Machalińska. 2019. "Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice" International Journal of Molecular Sciences 20, no. 3: 777. https://doi.org/10.3390/ijms20030777
APA StyleLejkowska, R., Kawa, M. P., Pius-Sadowska, E., Rogińska, D., Łuczkowska, K., Machaliński, B., & Machalińska, A. (2019). Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice. International Journal of Molecular Sciences, 20(3), 777. https://doi.org/10.3390/ijms20030777





