Synthesis of Fe3C@C from Pyrolysis of Fe3O4-Lignin Clusters and Its Application for Quick and Sensitive Detection of PrPSc through a Sandwich SPR Detection Assay
Abstract
1. Introduction
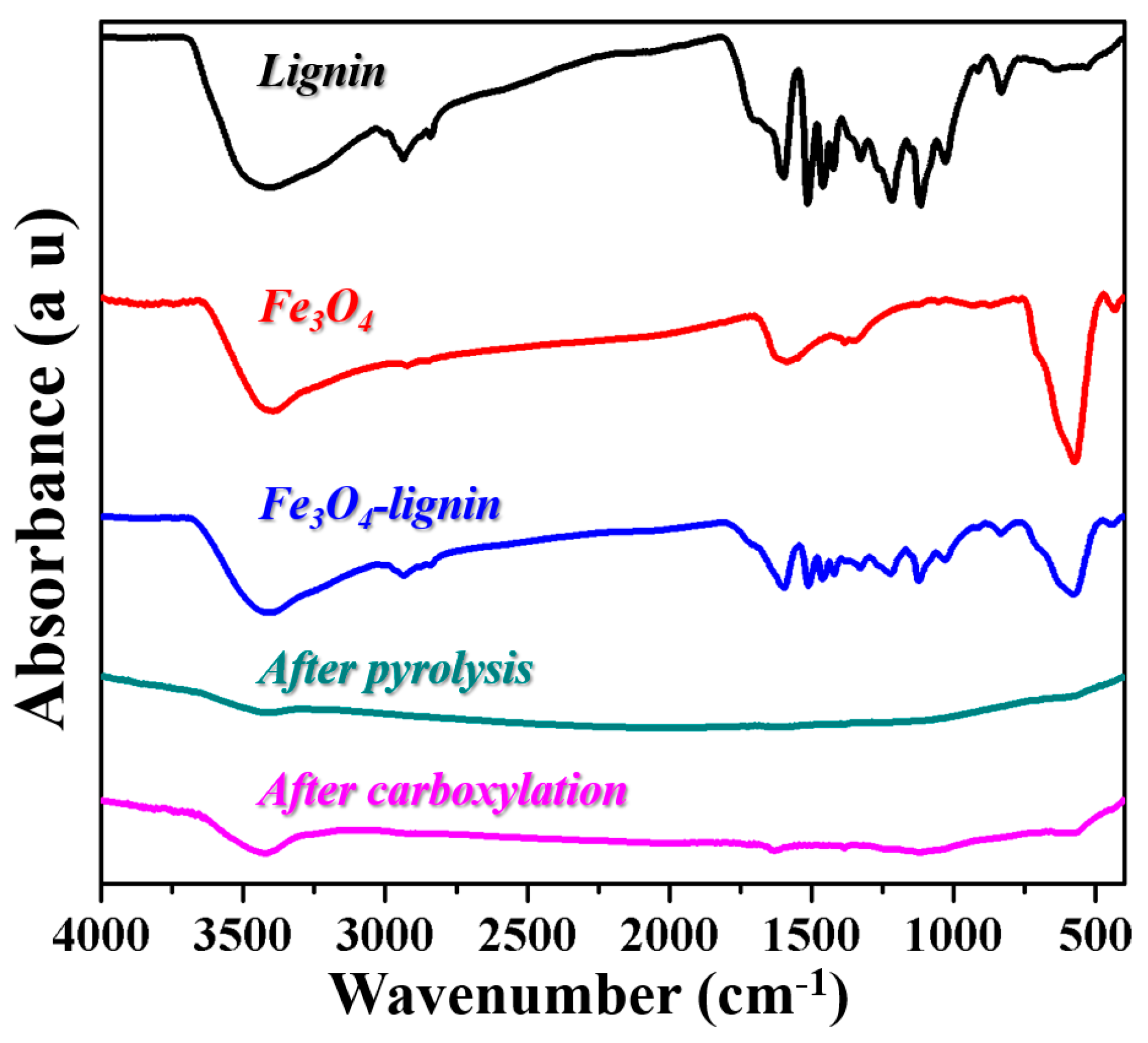
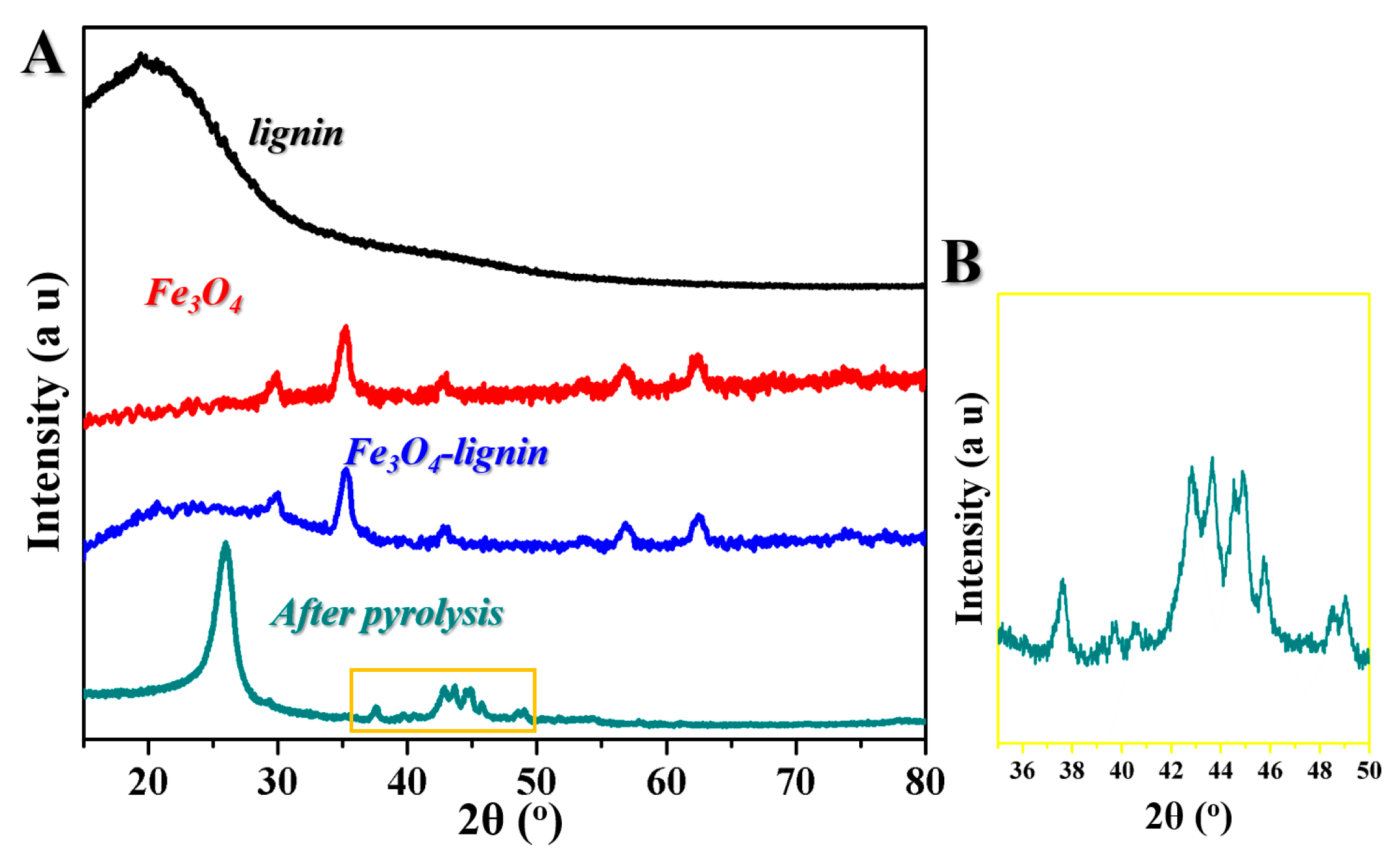
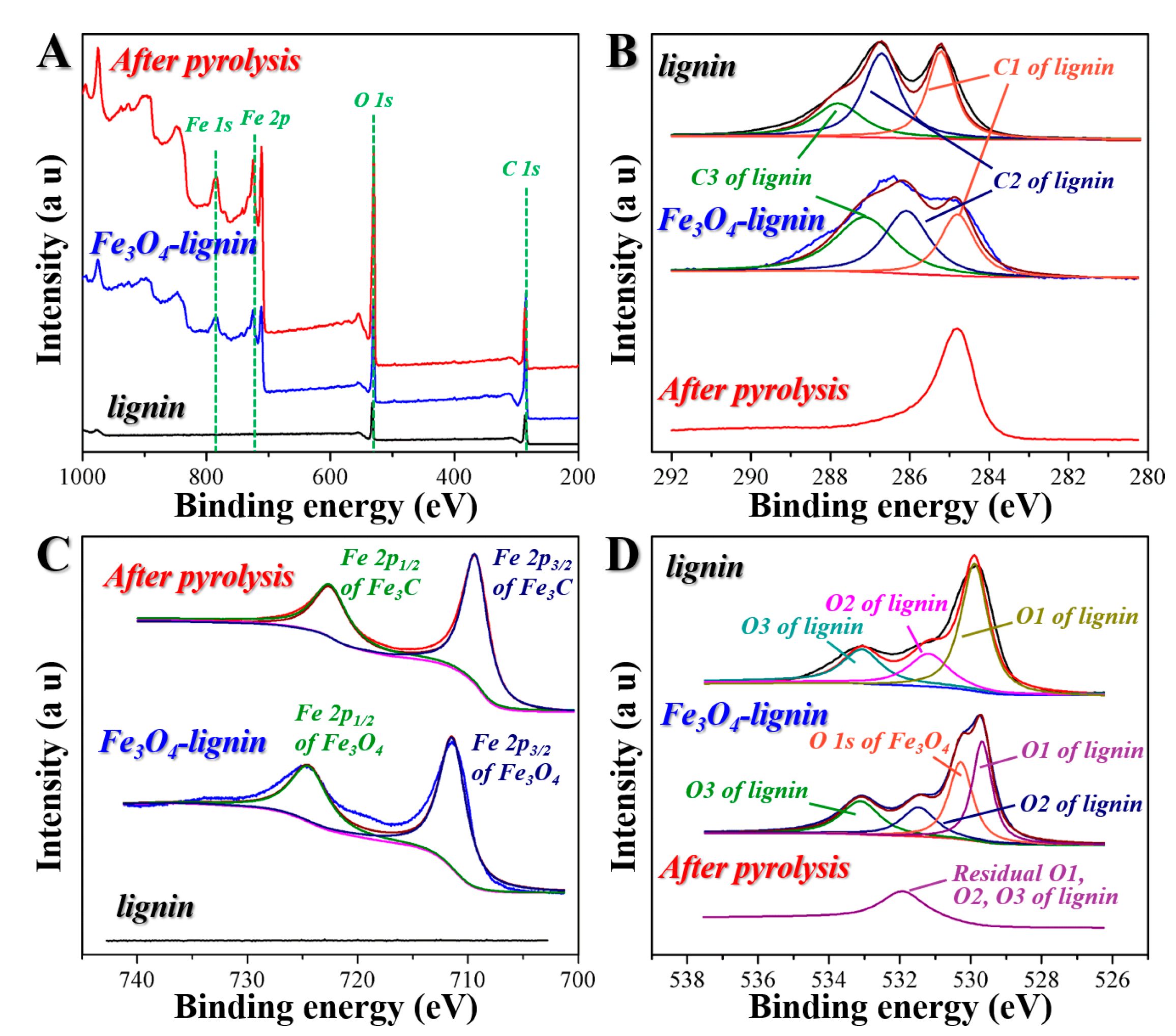
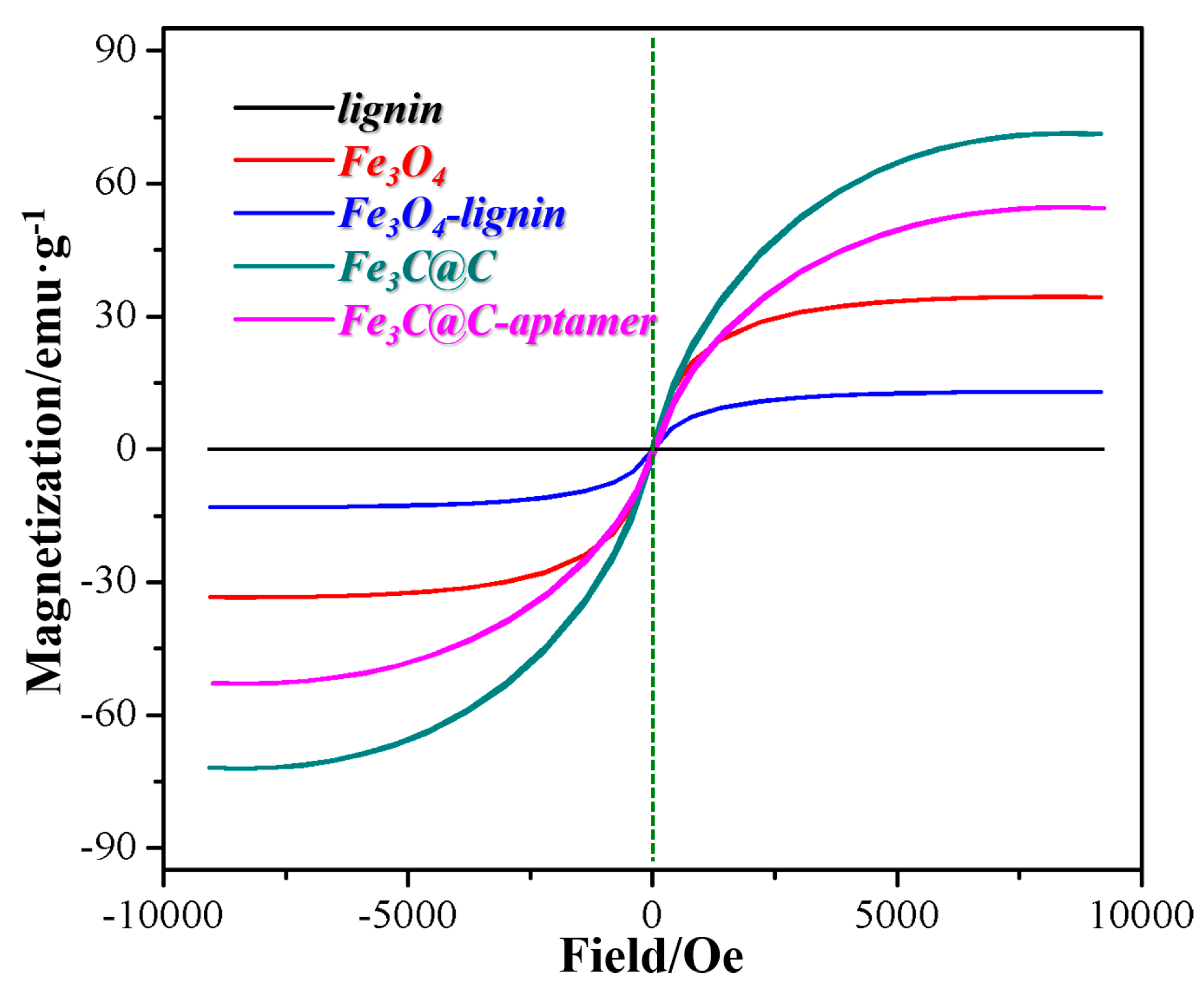
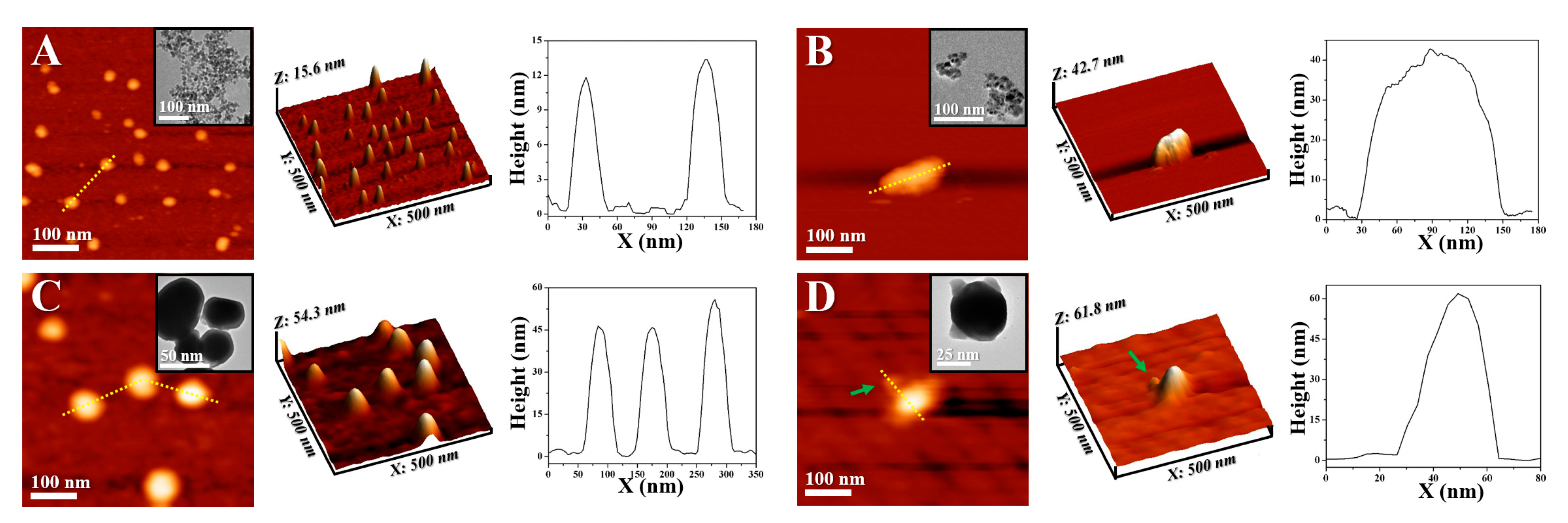
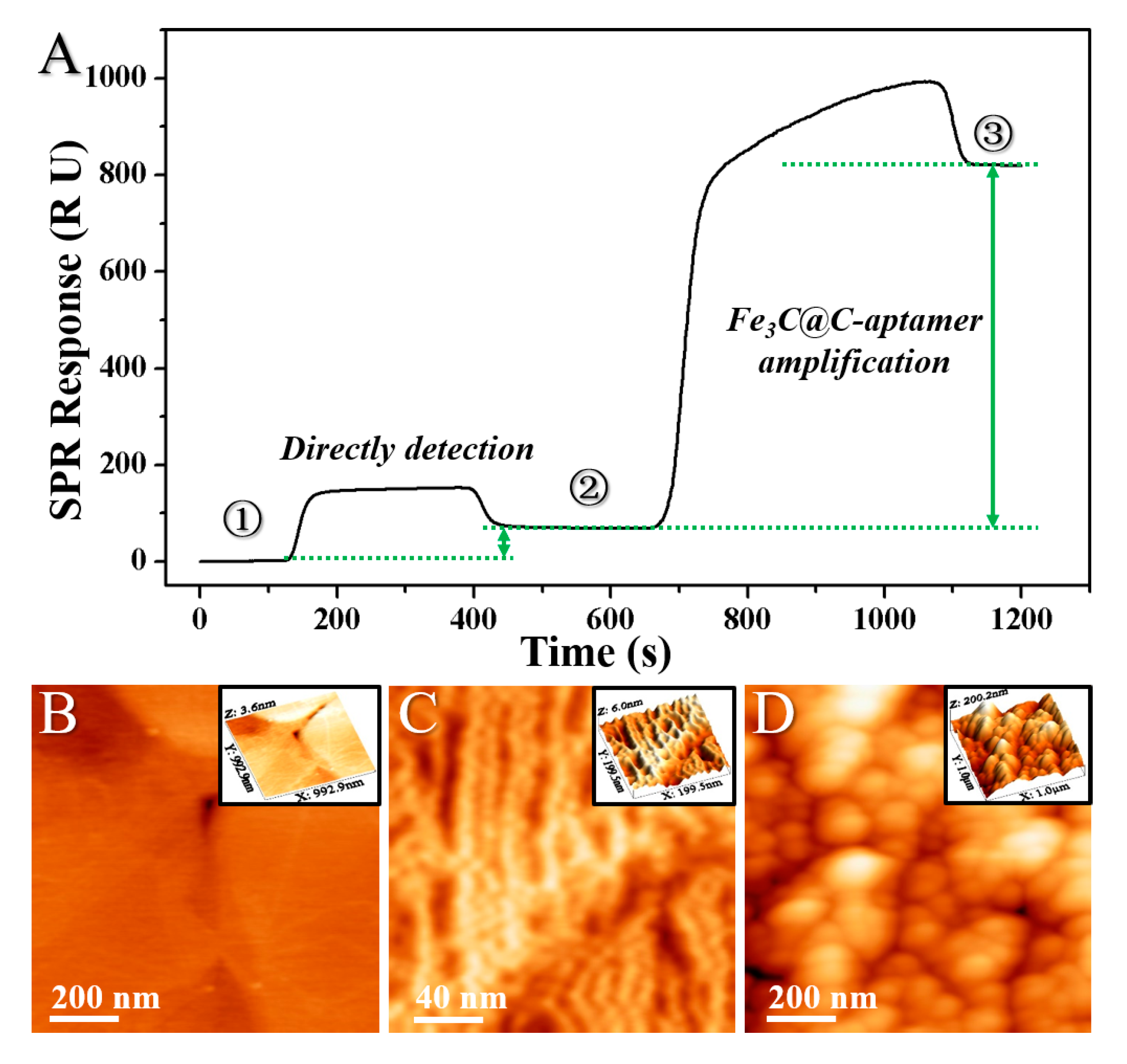
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Lignin from Natural Bio-Mass Residues
3.3. Preparation of Fe3O4 and Fe3O4-lignin
3.4. Preparation of Fe3C@C Core-Shell Nanoparticles
3.5. Preparation of Fe3C@C-Aptamer Conjugate
3.6. SPR Detection
3.7. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- MacGregor, I.R.; Drummond, O. Species differences in the blood content of the normal cellular isoform of prion protein, PrPc, measured by time-resolved fluoroimmunoassay. Vox Sang. 2001, 81, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A. Cellular Biology of Prion Diseases. Clin. Microbiol. Rev. 1999, 12, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Shyng, S.L.; Huber, M.T.; Harris, D.A. A prion protein cycles between the cell surface and an endocytic compartment in cultured neuroblastoma cells. J. Biol. Chem. 1993, 268, 15922–15928. [Google Scholar] [PubMed]
- Biasini, E.; Turnbaugh, J.A.; Unterberger, U.; Harris, D.A. Prion protein at the crossroads of physiology and disease. Trends Neurosci. 2012, 35, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.M.; Baldwin, M.; Nguyen, J.; Gasset, M.; Serban, A.; Groth, D.; Mehlhorn, I.; Huang, Z.; Fletterick, R.J.; Cohen, F.E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA 1993, 90, 10962–10966. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.S.; Hosszu, L.L.; Power, A.; Hill, A.F.; Kenney, J.; Saibil, H.; Craven, C.J.; Waltho, J.P.; Clarke, A.R.; Collinge, J. Reversible conversion of monomeric human prion protein between native and fibrilogenic conformations. Science 1999, 283, 1935–1937. [Google Scholar] [CrossRef]
- Chen, B.; Morales, R.; Barria, M.A.; Soto, C. Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat. Methods 2010, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Englund, H.; Sehlin, D.; Johansson, A.-S.; Nilsson, L.N.G.; Gellerfors, P.; Paulie, S.; Lannfelt, L.; Pettersson, F.E. Sensitive ELISA detection of amyloid-beta protofibrils in biological samples. J. Neurochem. 2007, 103, 334–345. [Google Scholar] [PubMed]
- Varshney, M.; Waggoner, P.S.; Tan, C.P.; Aubin, K.; Montagna, R.A.; Craighead, H.G. Prion protein detection using nanomechanical resonator arrays and secondary mass labeling. Anal. Chem. 2008, 80, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Fujii, F.; Horiuchi, M.; Ueno, M.; Sakata, H.; Nagao, I.; Tamura, M.; Kinjo, M. Detection of prion protein immune complex for bovine spongiform encephalopathy diagnosis using fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy. Anal. Biochem. 2007, 370, 131–141. [Google Scholar] [CrossRef]
- Coleman, B.M.; Nisbet, R.M.; Han, S.; Cappai, R.; Hatters, D.M.; Hill, A.F. Conformational detection of prion protein with biarsenical labeling and FlAsH fluorescence. Biochem. Biophys. Res. Commun. 2009, 380, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Reuter, T.; Gilroyed, B.H.; Alexander, T.W.; Mitchell, G.; Balachandran, A.; Czub, S.; McAllister, T.A. Prion protein detection via direct immuno-quantitative real-time PCR. J. Microbiol. Methods 2009, 78, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Situ, C.; Buijs, J.; Mooney, M.H.; Elliott, C.T. Advances in surface plasmon resonance biosensor technology towards high-throughput, food-safety analysis. Trac-Trends Anal. Chem. 2010, 29, 1305–1315. [Google Scholar] [CrossRef]
- Hoa, X.D.; Kirk, A.G.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007, 23, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Soelberg, S.D.; Stevens, R.C.; Limaye, A.P.; Furlong, C.E. Surface Plasmon Resonance Detection Using Antibody-Linked Magnetic Nanoparticles for Analyte Capture, Purification, Concentration, and Signal Amplification. Anal. Chem. 2009, 81, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Reekmans, G.; Saerens, D.; Friedt, J.M.; Frederix, F.; Francis, L.; Muyldermans, S.; Campitelli, A.; Van Hoof, C. Prostate-specific antigen immunosensing based on mixed self-assembled monolayers, camel antibodies and colloidal gold enhanced sandwich assays. Biosens. Bioelectron. 2005, 21, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Z.; Munir, A.; Zhou, H.S. Fe3O4 nanoparticles-enhanced SPR sensing for ultrasensitive sandwich bio-assay. Talanta 2011, 84, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.-P.; Yao, G.-H.; Fan, L.-X.; Qiu, J.-D. Magnetic Fe3O4@Au composite-enhanced surface plasmon resonance for ultrasensitive detection of magnetic nanoparticle-enriched alpha-fetoprotein. Anal. Chim. Acta 2012, 737, 22–28. [Google Scholar] [CrossRef]
- Lou, Z.; Han, H.; Zhou, M.; Wan, J.; Sun, Q.; Zhou, X.; Gu, N. Fabrication of Magnetic Conjugation Clusters via Intermolecular Assembling for Ultrasensitive Surface Plasmon Resonance (SPR) Detection in a Wide Range of Concentrations. Anal. Chem. 2017, 89, 13472–13479. [Google Scholar] [CrossRef]
- Xiang, J.; Li, J.; Zhang, X.; Ye, Q.; Xu, J.; Shen, X. Magnetic carbon nanofibers containing uniformly dispersed Fe/Co./Ni nanoparticles as stable and high-performance electromagnetic wave absorbers. J. Mater. Chem. A 2014, 2, 16905–16914. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.-J.; Wang, J.-Q.; Hu, J.-S.; Wei, Z.; Wan, L.-J. Understanding the High Activity of Fe-N-C Electrocatalysts in Oxygen Reduction: Fe/Fe3C Nanoparticles Boost the Activity of Fe-N-x. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yao, H.; Daniels, R.A.; Song, W.; Zheng, H.; Jin, L.; Suib, S.L.; He, J. A facile synthesis of Fe3C@mesoporous carbon nitride nanospheres with superior electrocatalytic activity. Nanoscale 2016, 8, 5441–5445. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, B.; Guo, C.; Wang, K.; Zhang, H.; Xu, B. Molecular-level insights of early-stage prion protein aggregation on mica and gold surface determined by AFM imaging and molecular simulation. Colloid Surf. B-Biointerfaces 2015, 135, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Rhie, A.; Kirby, L.; Sayer, N.; Wellesley, R.; Disterer, P.; Sylvester, I.; Gill, A.; Hope, J.; James, W.; Tahiri-Alaoui, A. Characterization of 2′-fluoro-RNA aptamers that bind preferentially to disease-associated conformations of prion protein and inhibit conversion. J. Biol. Chem. 2003, 278, 39697–39705. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Han, H.; Mao, D.; Jiang, Y.; Song, J. Qualitative and Quantitative Detection of PrPSc Based on the Controlled Release Property of Magnetic Microspheres Using Surface Plasmon Resonance (SPR). Nanomaterials 2018, 8, 107. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Lou, Z.; Wang, W.; Yang, L.; Li, Y. Synthesis of Fe3C@C from Pyrolysis of Fe3O4-Lignin Clusters and Its Application for Quick and Sensitive Detection of PrPSc through a Sandwich SPR Detection Assay. Int. J. Mol. Sci. 2019, 20, 741. https://doi.org/10.3390/ijms20030741
Yuan C, Lou Z, Wang W, Yang L, Li Y. Synthesis of Fe3C@C from Pyrolysis of Fe3O4-Lignin Clusters and Its Application for Quick and Sensitive Detection of PrPSc through a Sandwich SPR Detection Assay. International Journal of Molecular Sciences. 2019; 20(3):741. https://doi.org/10.3390/ijms20030741
Chicago/Turabian StyleYuan, Chenglong, Zhichao Lou, Weikai Wang, Lintian Yang, and Yanjun Li. 2019. "Synthesis of Fe3C@C from Pyrolysis of Fe3O4-Lignin Clusters and Its Application for Quick and Sensitive Detection of PrPSc through a Sandwich SPR Detection Assay" International Journal of Molecular Sciences 20, no. 3: 741. https://doi.org/10.3390/ijms20030741
APA StyleYuan, C., Lou, Z., Wang, W., Yang, L., & Li, Y. (2019). Synthesis of Fe3C@C from Pyrolysis of Fe3O4-Lignin Clusters and Its Application for Quick and Sensitive Detection of PrPSc through a Sandwich SPR Detection Assay. International Journal of Molecular Sciences, 20(3), 741. https://doi.org/10.3390/ijms20030741




