Advances in Understanding the Immunological Pathways in Psoriasis
Abstract
1. Introduction
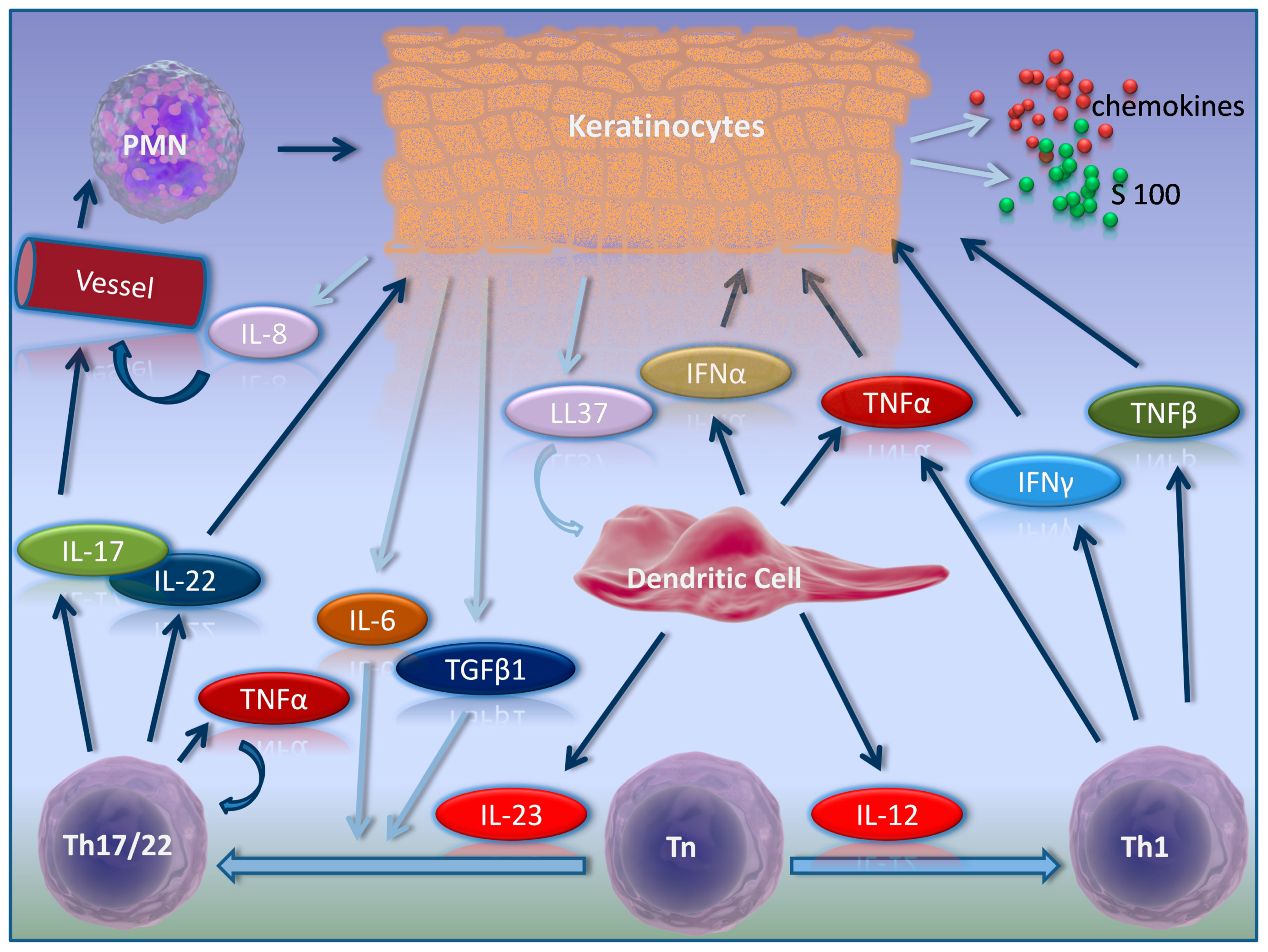
2. Psoriasis Pathogenesis in Brief
3. Activators of Inflammation in Psoriasis
3.1. Cytokines
3.1.1. IL-23
3.1.2. IL-1β
3.1.3. IL-17
3.1.4. IL-22
3.1.5. IFN-γ
3.2. T-Cells
3.2.1. Th-17 Cells
3.2.2. Th-22 Cells
3.2.3. Th-1 Cells
3.3. Other Molecules
RORC/RORγt
4. Regulatory Axis in Psoriasis
4.1. Treg Cells
4.2. TGFβ
4.3. IL-10
5. Additional Inflammatory Pathways in Psoriasis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gudjonsson, J.E.; Elder, J.T. Psoriasis. In Fitzpatrick’s Dermatology in General Medicine, 8th ed.; Goldsmith, L.A., Katz, S.I., Eds.; McGrawHill: New York, NJ, USA, 2012; pp. 169–193. [Google Scholar]
- Griffiths, C.E.M.; Barker, J.N.W.N. Psoriasis. In Rook’s Textbook of Dermatology, 8th ed.; Burns, T., Breathnach, S., Eds.; Wiley Blackwell: West Sussex, UK, 2010; pp. 20.1–20.60. [Google Scholar] [CrossRef]
- Cristophers, E.; Mrowietz, U. Psoriasis. In Braun-Falco’s Dermatology, 3rd ed.; Burgdorf, W.H.C., Plewig, G., Eds.; Springer: Berlin, Germany, 2009; pp. 506–526. [Google Scholar] [CrossRef]
- van de Kerkhof, P.C.M.; Nestle, F.O. Psoriasis. In Dermatology, 3rd ed.; Bolognia, J.L., Jorizzo, J.L., Eds.; Elsevier: Philadelphia, PA, USA, 2012; pp. 135–156. [Google Scholar]
- Mitran, M.I.; Mitran, C.I.; Sârbu, M.I.; Benea, V.; Tampa, M.; Georgescu, S.R. Therapeutic challenges in a case of psoriasis with nail onset. J. Mind Med. Sci. 2017, 4, 186–192. [Google Scholar] [CrossRef]
- Sârbu, M.I.; Georgescu, S.R.; Tampa, M.; Sârbu, A.E.; Simionescu, O. Biological therapies in psoriasis-revisited. Rom. J. Intern. Med. 2018, 56, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Sarbu, M.I.; Mitran, M.I.; Mitran, C.I.; Matei, C.; Georgescu, S.R. The Pathophysiological Mechanisms and the Quest for Biomarkers in Psoriasis, a Stress-Related Skin Disease. Dis. Markers 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, M.I.; Tampa, M.; Sarbu, A.E.; Georgescu, S.R. Sexual dysfunctions in psoriatic patients. J. Mind Med. Sci. 2014, 1, 19–27. [Google Scholar]
- Tampa, M.; Nicolae, I.L.; Ene, C.D.; Sarbu, I.; Matei, C.L.; Georgescu, S.R. Vitamin C and thiobarbituric acid reactive substances (TBARS) in psoriasis vulgaris related to psoriasis area severity index (PASI). Rev. Chim. 2017, 68, 43–47. [Google Scholar]
- Mobini, N.; Toussaint, S.; Kamino, H. Noninfectious Erythematous, Papular, and Squamous Diseases. In Lever’s Histopathology of the Skin; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 186–187. [Google Scholar]
- Sârbu, M.I.; Tampa, M.; Mitran, M.I.; Mitran, C.I.; Limbău, A.M.; Georgescu, S.R. Adverse reactions of biological therapies in patients with psoriasis. J. Mind Med Sci. 2017, 4, 4–12. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Romanelli, P.; Volpe, E.; Borsellino, G.; Romanelli, M. Scanning the Immunopathogenesis of Psoriasis. Int. J. Mol. Sci. 2018, 19, 179. [Google Scholar] [CrossRef]
- Sarbu, M.I.; Tampa, M.; Matei, C.; Mitran, C.I.; Mitran, M.I.; Pituru, S.; Pop, C.S.; Saramet, G.; Georgescu, S.R. Infliximab Biosimilar Versus Methotrexate for the Treatment of Moderate to Severe Psoriasis. Farmacia 2017, 65, 962–967. [Google Scholar]
- Caruntu, C.; Boda, D.; Dumitrascu, G.; Constantin, C.; Neagu, M. Proteomics focusing on immune markers in psoriatic arthritis. Biomark. Med. 2015, 9, 513–528. [Google Scholar] [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef]
- Krueger, J.G. An autoimmune “attack” on melanocytes triggers psoriasis and cellular hyperplasia. J. Exp. Med. 2015, 212, 2186. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Morizane, S.; Yamasaki, K.; Mühleisen, B.; Kotol, P.F.; Murakami, M.; Aoyama, Y.; Iwatsuki, K.; Hata, T.; Gallo, R.L. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J. Investig. Dermatol. 2012, 132, 135–143. [Google Scholar] [CrossRef]
- Surcel, M.; Huica, R.; Constantin, C.; Ursaciuc, C.; Neagu, M. Biomarkers Insights in Psoriasis-Regulatory Cytokines. Curr. Biomark. 2018, 7, 3–11. [Google Scholar] [CrossRef]
- Benson, J.M.; Sachs, C.W.; Treacy, G.; Zhou, H.; Pendley, C.E.; Brodmerkel, C.M.; Shankar, G.; Mascelli, M.A. Therapeutic targeting of the IL-12/23 pathways: Generation and characterization of ustekinumab. Nat. Biotechnol. 2011, 29, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.R.; Sârbu, M.I.; Matei, C.; Ilie, M.A.; Caruntu, C.; Constantin, C.; Neagu, M.; Tampa, M. Capsaicin: Friend or foe in skin cancer and other related malignancies? Nutrients 2017, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- de Masson, A.; Bouaziz, J.D.; Battistella, M.; Bagot, M.; Bensussan, A. Immunopathologie du psoriasis-From bench to bedside. Med. Sci. (Paris) 2016, 32, 253–259. [Google Scholar] [CrossRef]
- Mahil, S.K.; Capon, F.; Barker, J.N. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin. Immunopathol. 2016, 38, 11–27. [Google Scholar] [CrossRef]
- Neuner, P.; Urbanski, A.; Trautinger, F.; Möller, A.; Kirnbauer, R.; Kapp, A.; Schöpf, E.; Schwarz, T.; Luger, T.A. Increased IL-6 production by monocytes and keratinocytes in patients with psoriasis. J. Investig. Dermatol. 1991, 97, 27–33. [Google Scholar] [CrossRef]
- Fotiadou, C.; Lazaridou, E.; Sotiriou, E.; Ioannides, D. Targeting IL-23 in psoriasis: Current perspectives. Psoriasis (Auckl) 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Eberle, F.C.; Brück, J.; Holstein, J.; Hirahara, K.; Ghoreschi, K. Recent advances in understanding psoriasis. F1000 Res. 2016, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Trepicchio, W.L.; Oestreicher, J.L.; Pittman, D.; Wang, F.; Chamian, F.; Dhodapkar, M.; Krueger, J.G. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 2004, 199, 125–130. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.; Suddason, T.; Lord, G.M. Translational Mini-Review Series on Th17 Cells: Development of mouse and human T helper 17 cells. Clin. Exp. Immunol. 2010, 159, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.C.; Hawkes, J.E.; Krueger, J.G. Interleukin 23 in the skin: Role in psoriasis pathogenesis and selective interleukin 23 blockade as treatment. Ther. Adv. Chronic Dis. 2018, 9, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Zhai, T.; Li, H.; Li, H.; Huo, R.; Shen, B.; Wang, B.; Chen, X.; Li, N.; et al. CCN1 promotes IL-1β production in keratinocytes by activating p38 MAPK signaling in psoriasis. Sci. Rep. 2017, 7, 43310. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, S.; Hattinger, E.; Bureik, D.; Batycka-Baran, A.; Schmidt, A.; Gerber, P.A.; Rothenfusser, S.; Gilliet, M.; Ruzicka, T.; Wolf, R. Th17 micro-milieu regulates NLRP1-dependent caspase-5 activity in skin autoinflammation. PLoS ONE 2017, 12, e0175153. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Gaffen, S.L. Interleukin 17 family cytokines: Signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb. Perspect. Biol. 2018, 10, a028522. [Google Scholar] [CrossRef]
- Miossec, P. Update on interleukin-17: A role in the pathogenesis of inflammatory arthritis and implication for clinical practice. RMD Open 2017, 3, e000284. [Google Scholar] [CrossRef]
- Giunta, A.; Ventura, A.; Chimenti, M.S.; Bianchi, L.; Esposito, M. Spotlight on ixekizumab for the treatment of moderate-to-severe plaque psoriasis: Design, development, and use in therapy. Drug Des. Dev. Ther. 2017, 11, 1643–1651. [Google Scholar] [CrossRef]
- Banuelos, J.; Cao, Y.; Shin, S.C.; Lu, N.Z. Immunopathology alters Th17 cell glucocorticoid sensitivity. Allergy 2017, 72, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Diani, M.; Altomare, G.; Reali, E. T helper cell subsets in clinical manifestations of psoriasis. J. Immunol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat. Inflamm. 2005, 2005, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, M.B.; Koomen, C.W.; de Waal Malefyt, R.; Wierenga, E.A.; Bos, J.D. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Investig. Dermatol. 1998, 111, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.G.; Fretzin, S.; Suárez-Fariñas, M.; Haslett, P.A.; Phipps, K.M.; Cameron, G.S.; McColm, J.; Katcherian, A.; Cueto, I.; White, T.; et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol. 2012, 130, 145–154.e9. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, M.; Talamonti, M.; De Simone, C.; D’Adamio, S.; Moretta, G.; Tambone, S.; Caldarola, G.; Fargnoli, M.C.; Peris, K.; Bianchi, L. Secukinumab in moderate-to-severe plaque psoriasis: A multi-center, retrospective, real-life study up to 52 weeks observation. Expert Opin. Biol. Ther. 2018, 18, 727–735. [Google Scholar] [CrossRef]
- Senra, L.; Stalder, R.; Alvarez Martinez, D.; Chizzolini, C.; Boehncke, W.H.; Brembilla, N.C. Keratinocyte-derived IL-17E contributes to inflammation in psoriasis. J. Investig. Dermatol. 2016, 136, 1970–1980. [Google Scholar] [CrossRef]
- Cochez, P.M.; Michiels, C.; Hendrickx, E.; Van Belle, A.B.; Lemaire, M.M.; Dauguet, N.; Warnier, G.; de Heusch, M.; Togbe, D.; Ryffel, B.; et al. AhR modulates the IL-22-producing cell proliferation/recruitment in imiquimod-induced psoriasis mouse model. Eur. J. Immunol. 2016, 46, 1449–1459. [Google Scholar] [CrossRef]
- Mashiko, S.; Bouguermouh, S.; Rubio, M.; Baba, N.; Bissonnette, R.; Sarfati, M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 2015, 136, 351–359.e1. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, S.G. Interleukin-22: A likely target for treatment of autoimmune diseases. Autoimmun. Rev. 2014, 13, 615–620. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, D.; Saiz, M.L.; de la Fuente, H.; Sánchez-Díaz, R.; Moreno-Gonzalo, O.; Jorge, I.; Ferrarini, A.; Vázquez, J.; Punzón, C.; Fresno, M.; et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 2016, 17, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-γ during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Pestka, S. The interferon receptors. Semin. Oncol. 1997, 24 (3 Suppl. 9), S9-18–S9-40. [Google Scholar]
- Di Meglio, P.; Duarte, J.H. CD8 T cells and IFN-γ emerge as critical players for psoriasis in a novel model of mouse psoriasiform skin inflammation. J. Investig. Dermatol. 2013, 133, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Austin, L.M.; Ozawa, M.; Kikuchi, T.; Walters, I.B.; Krueger, J.G. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-γ, interleukin-2, and tumor necrosis factor-α, defining TC1 (Cytotoxic T Lymphocyte) and TH1 effector populations: 1 a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Investig. Dermatol. 1999, 113, 752–759. [Google Scholar] [CrossRef]
- Kryczek, I.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Aphale, A.; Vatan, L.; Szeliga, W.; Wang, Y.; Liu, Y.; Welling, T.H.; et al. Induction of IL-17+ T cell trafficking and development by IFN-γ: Mechanism and pathological relevance in psoriasis. J. Immunol. 2008, 181, 4733–4741. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Abdel-Hamid, M.F.; Kotb, A.M.; Mabrouk, E.A. Serum interferon-gamma is a psoriasis severity and prognostic marker. Cutis 2009, 84, 163–168. [Google Scholar]
- Harden, J.L.; Johnson-Huang, L.M.; Chamian, M.F.; Lee, E.; Pearce, T.; Leonardi, C.L.; Haider, A.; Lowes, M.A.; Krueger, J.G. Humanized anti–IFN-γ (HuZAF) in the treatment of psoriasis. J. Allergy Clin. Immunol. 2015, 135, 553–556. [Google Scholar] [CrossRef]
- Mansouri, M.; Mansouri, P.; Raze, A.A.; Jadali, Z. The potential role of Th17 lymphocytes in patients with psoriasis. An. Bras. Dermatol. 2018, 93, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Boutet, M.A.; Nerviani, A.; Gallo Afflitto, G.; Pitzalis, C. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: The clinical importance of its divergence in skin and joints. Int. J. Mol. Sci. 2018, 19, 530. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Kuchroo, V.K. Th17 cell pathway in human immunity: Lessons from genetics and therapeutic interventions. Immunity 2015, 43, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Michalak-Stoma, A.; Bartosińska, J.; Kowal, M.; Juszkiewicz-Borowiec, M.; Gerkowicz, A.; Chodorowska, G. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis. Markers 2013, 35, 625–631. [Google Scholar] [CrossRef]
- Luan, L.; Ding, Y.; Han, S.; Zhang, Z.; Liu, X. An increased proportion of circulating Th22 and Tc22 cells in psoriasis. Cell. Immunol. 2014, 290, 196–200. [Google Scholar] [CrossRef]
- Benham, H.; Norris, P.; Goodall, J.; Wechalekar, M.D.; FitzGerald, O.; Szentpetery, A.; Smith, M.; Thomas, R.; Gaston, H. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res. Ther. 2013, 15, R136. [Google Scholar] [CrossRef]
- Cheuk, S.; Wikén, M.; Blomqvist, L.; Nylén, S.; Talme, T.; Ståhle, M.; Eidsmo, L. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 2014, 192, 3111–3120. [Google Scholar] [CrossRef]
- Romagnani, S. Th1/Th2 cells. Inflamm. Bowel Dis. 1999, 5, 285–294. [Google Scholar] [CrossRef]
- Christophers, E.; Metzler, G.; Röcken, M. Bimodal immune activation in psoriasis. Br. J. Dermatol. 2014, 170, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Soroosh, P.; De Leon-Tabaldo, A.; Luna-Roman, R.; Sablad, M.; Rozenkrants, N.; Yu, J.; Castro, G.; Banie, H.; Fung-Leung, W.P.; et al. Pharmacologic modulation of RORγt translates to efficacy in preclinical and translational models of psoriasis and inflammatory arthritis. Sci. Rep. 2016, 6, 37977. [Google Scholar] [CrossRef] [PubMed]
- Bronner, S.M.; Zbieg, J.R.; Crawford, J.J. RORγ antagonists and inverse agonists: A patent review. Expert Opin. Ther. Pat. 2017, 27, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk-Saczonek, A.; Czerwińska, J.; Placek, W. The role of regulatory T cells and anti-inflammatory cytokines in psoriasis. Acta Dermatovenerol. Alp. Panonica Adriat. 2018, 27, 17–23. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Lange, M.; Sokołowska-Wojdyło, M.; Renke, J.; Trzonkowski, P.; Sobjanek, M.; Szczerkowska-Dobosz, A.; Niedoszytko, M.; Górska, A.; Romantowski, J.; et al. The role of regulatory T cells and genes involved in their differentiation in pathogenesis of selected inflammatory and neoplastic skin diseases. Part II: The Treg role in skin diseases pathogenesis. Adv. Dermatol. Allergol./Postȩp. Dermatol. Alergol. 2017, 34, 405. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Renke, J.; Lange, M.; Trzonkowski, P.; Sobjanek, M.; Szczerkowska-Dobosz, A.; Niedoszytko, M.; Górska, A.; Romantowski, J.; et al. The role of regulatory T cells and genes involved in their differentiation in pathogenesis of selected inflammatory and neoplastic skin diseases. Part III: Polymorphisms of genes involved in Tregs’ activation and function. Postepy Dermatol. Alergol. 2017, 34, 517–525. [Google Scholar] [CrossRef]
- Yan, K.X.; Fang, X.; Han, L.; Zhang, Z.H.; Kang, K.F.; Zheng, Z.Z.; Huang, Q. Foxp3+ regulatory T cells and related cytokines differentially expressed in plaque vs. guttate psoriasis vulgaris. Br. J. Dermatol. 2010, 163, 48–56. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.Q.; Cheng, J.; Hui, R.S.; Gao, T.W. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin. Immunol. 2010, 135, 108–117. [Google Scholar] [CrossRef]
- Fujimura, T.; Okuyama, R.; Ito, Y.; Aiba, S. Profiles of Foxp3+ regulatory T cells in eczematous dermatitis, psoriasis vulgaris and mycosis fungoides. Br. J. Dermatol. 2008, 158, 1256–1263. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Karczewski, J.; Wiktorowicz, K. T regulatory CD4+ CD25high lymphocytes in peripheral blood of patients suffering from psoriasis. Postepy Dermatol. Alergol. 2010, 27, 25. [Google Scholar]
- Soler, D.C.; Sugiyama, H.; Young, A.B.; Massari, J.V.; McCormick, T.S.; Cooper, K.D. Psoriasis patients exhibit impairment of the high potency CCR5(+) T regulatory cell subset. Clin. Immunol. 2013, 149, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Bovenschen, H.J.; van de Kerkhof, P.C.; van Erp, P.E.; Woestenenk, R.; Joosten, I.; Koenen, H.J. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J. Investig. Dermatol. 2011, 131, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, D.H.; Han, L.; Deng, J.W.; Zhou, L.; He, R.; Lu, C.J.; Mi, Q.S. Roles of microRNAs in psoriasis: Immunological functions and potential biomarkers. Exp. Dermatol. 2017, 26, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zeng, J.; Yuan, J.; Deng, X.; Huang, Y.; Chen, L.; Zhang, P.; Feng, H.; Liu, Z.; Wang, Z.; et al. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J. Clin. Investig. 2018, 128, 2551–2568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, L.T.; Liang, G.P.; Zhang, P.; Deng, X.J.; Tang, Q.; Zhai, H.Y.; Chang, C.C.; Su, Y.W.; Lu, Q.J. Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clin. Immunol. 2014, 150, 22–30. [Google Scholar] [CrossRef]
- Ajduković, J. HIF-1--a big chapter in the cancer tale. Exp. Oncol. 2016, 38, 9–12. [Google Scholar] [CrossRef]
- Han, G.; Williams, C.A.; Salter, K.; Garl, P.J.; Li, A.G.; Wang, X.J. A role for TGFbeta signaling in the pathogenesis of psoriasis. J. Investig. Dermatol. 2010, 130, 371–377. [Google Scholar] [CrossRef]
- Meki, A.R.; Al-Shobaili, H. Serum vascular endothelial growth factor, transforming growth factor β1, and nitric oxide levels in patients with psoriasis vulgaris: Their correlation to disease severity. J. Clin. Lab. Anal. 2014, 28, 496–501. [Google Scholar] [CrossRef]
- Nockowski, P.; Szepietowski, J.C.; Ziarkiewicz, M.; Baran, E. Serum concentrations of transforming growth factor beta 1 in patients with psoriasis vulgaris. Acta Dermatovenerol. Croat. 2004, 12, 2–6. [Google Scholar]
- Zhang, Y.; Meng, X.M.; Huang, X.R.; Wang, X.J.; Yang, L.; Lan, H.Y. Transforming growth factor-β1 mediates psoriasis-like lesions via a Smad3-dependent mechanism in mice. Clin. Exp. Pharmacol. Physiol. 2014, 41, 921–932. [Google Scholar] [CrossRef]
- Kitoh, A.; Nomura, T.; Kabashima, K. TGFβ1, an epidermal controller of skin dendritic cell homeostasis. J. Investig. Dermatol. 2013, 133, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Khosraviani, S.; Noel, S.; Mohan, D.; Donner, T.; Hamad, A.R. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 2015, 74, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, J.; Miller, L.; Debeljak, Ž.; Horvat, V. Pathologic patterns of interleukin 10 expressiona review. Biochem. Med. (Zagreb) 2015, 25, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Al-Balbeesi, A.O.; Halwani, M.; Alanazi, M.; Elrobh, M.; Shaik, J.P.; Khan, A.A.; Parine, N.R. Novel mutations in IL-10 promoter region -377 (C>T), -150 (C>A) and their association with psoriasis in the saudi population. Asian Pac. J. Cancer Prev. 2015, 16, 1247–1250. [Google Scholar] [CrossRef]
- Sobhan, M.R.; Farshchian, M.; Hoseinzadeh, A.; Ghasemibasir, H.R.; Solgi, G. Serum levels of IL-10 and IL-22 cytokines in patients with psoriasis. Iran. J. Immunol. 2016, 13, 317–323. [Google Scholar] [PubMed]
- Karam, R.A.; Zidan, H.E.; Khater, M.H. Polymorphisms in the TNF-α and IL-10 gene promoters and risk of psoriasis and correlation with disease severity. Cytokine 2014, 66, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulos, A.; Varna, A.; Zafiriou, E.; Liaskos, C.; Alexiou, I.; Roussaki-Schulze, A.; Vlychou, M.; Katsiari, C.; Bogdanos, D.P.; Sakkas, L.I. IL-10 producing Bregs are impaired in psoriatic arthritis and psoriasis and inversely correlate with IL-17- and IFNγ-producing T cells. Clin. Immunol. 2017, 184, 33–41. [Google Scholar] [CrossRef]
- Zanin-Zhorov, A.; Weiss, J.M.; Trzeciak, A.; Chen, W.; Zhang, J.; Nyuydzefe, M.S.; Arencibia, C.; Polimera, S.; Schueller, O.; Fuentes-Duculan, J.; et al. Cutting edge: Selective oral ROCK2 inhibitor reduces clinical scores in patients with psoriasis vulgaris and normalizes skin pathology via concurrent regulation of IL-17 and IL-10. J. Immunol. 2017, 198, 3809–3814. [Google Scholar] [CrossRef]
- Gudmundsdottir, A.B.; Omarsdottir, S.; Brynjolfsdottir, A.; Paulsen, B.S.; Olafsdottir, E.S.; Freysdottir, J. Exopolysaccharides from Cyanobacterium aponinum from the Blue Lagoon in Iceland increase IL-10 secretion by human dendritic cells and their ability to reduce the IL-17+RORγt+/IL-10+FoxP3+ ratio in CD4+ T cells. Immunol. Lett. 2015, 163, 157–162. [Google Scholar] [CrossRef]
- Mabuchi, T.; Hwang, S.T. ACKR2: Nature’s Decoy Receptor Lures Unsuspecting Chemokines in Psoriasis. J. Investig. Dermatol. 2017, 137, 7–11. [Google Scholar] [CrossRef]
- Singh, M.D.; King, V.; Baldwin, H.; Burden, D.; Thorrat, A.; Holmes, S.; McInnes, I.B.; Nicoll, R.; Shams, K.; Pallas, K.; et al. Elevated expression of the chemokine-scavenging receptor D6 is associated with impaired lesion development in psoriasis. Am. J. Pathol. 2012, 181, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Shams, K.; Kurowska-Stolarska, M.; Schütte, F.; Burden, A.D.; McKimmie, C.S.; Graham, G.J. MicroRNA-146 and cell trauma down-regulate expression of the psoriasis-associated atypical chemokine receptor ACKR2. J. Biol. Chem. 2018, 293, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Shams, K.; Wilson, G.J.; Singh, M.; van den Bogaard, E.H.; Le Brocq, M.L.; Holmes, S.; Schalkwijk, J.; Burden, A.D.; McKimmie, C.S.; Graham, G.J. Spread of psoriasiform inflammation to remote tissues is restricted by the atypical chemokine receptor ACKR2. J. Investig. Dermatol. 2017, 137, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ding, Y.; Yi, X.; Zheng, J. CD19+ B cell subsets in the peripheral blood and skin lesions of psoriasis patients and their correlations with disease severity. Braz. J. Med. Biol. Res. 2016, 49, e5374. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Kamata, M.; Ishiura, N.; Shibata, S.; Asano, Y.; Tada, Y.; Sugaya, M.; Kadono, T.; Tedder, T.F.; Sato, S. Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation. J. Leukoc. Biol. 2013, 94, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Darabi, K.; Jaiswal, R.; Hostetler, S.G.; Bechtel, M.A.; Zirwas, M.J.; Witman, P. A new kid on the block: IL-10+ regulatory B cells and a possible role in psoriasis. J. Pediatr. Pharmacol. Ther. 2009, 14, 148–153. [Google Scholar] [PubMed]
- Thomas, J.; Küpper, M.; Batra, R.; Jargosch, M.; Atenhan, A.; Baghin, V.; Krause, L.; Lauffer, F.; Biedermann, T.; Theis, F.J.; et al. Is the humoral immunity dispensable for the pathogenesis of psoriasis? J. Eur. Acad. Dermatol. Venereol. 2019, 33, 115–122. [Google Scholar] [CrossRef]
- Harden, J.L.; Lewis, S.M.; Lish, S.R.; Suárez-Fariñas, M.; Gareau, D.; Lentini, T.; Johnson-Huang, L.M.; Krueger, J.G.; Lowes, M.A. The tryptophan metabolism enzyme L-kynureninase is a novel inflammatory factor in psoriasis and other inflammatory diseases. J. Allergy Clin. Immunol. 2016, 137, 1830–1840. [Google Scholar] [CrossRef]
- Korenfeld, D.; Gorvel, L.; Munk, A.; Man, J.; Schaffer, A.; Tung, T.; Mann, C.; Klechevsky, E. A type of human skin dendritic cell marked by CD5 is associated with the development of inflammatory skin disease. JCI Insight 2017, 2, 96101. [Google Scholar] [CrossRef]
- Buerger, C.; Shirsath, N.; Lang, V.; Berard, A.; Diehl, S.; Kaufmann, R.; Boehncke, W.H.; Wolf, P. Inflammation dependent mTORC1 signaling interferes with the switch from keratinocyte proliferation to differentiation. PLoS ONE 2017, 12, e0180853. [Google Scholar] [CrossRef]
- Luan, C.; Chen, X.; Hu, Y.; Hao, Z.; Osland, J.M.; Chen, X.; Gerber, S.D.; Chen, M.; Gu, H.; Yuan, R. Overexpression and potential roles of NRIP1 in psoriasis. Oncotarget 2016, 7, 74236–74246. [Google Scholar] [CrossRef] [PubMed]
- Andrianne, M.; Assabban, A.; La, C.; Mogilenko, D.; Salle, D.S.; Fleury, S.; Doumont, G.; Van Simaeys, G.; Nedospasov, S.A.; Blackshear, P.J.; et al. Tristetraprolin expression by keratinocytes controls local and systemic inflammation. JCI Insight 2017, 2, 92979. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, W.; Yuan, Y.; Ayithan, N.; Imai, Y.; Wu, X.; Miller, H.; Olson, M.; Feng, Y.; Huang, Y.H.; et al. Immune-checkpoint protein VISTA critically regulates the IL-23/IL-17 inflammatory axis. Sci. Rep. 2017, 7, 1485. [Google Scholar] [CrossRef] [PubMed]
- Surcel, M.; Huică, R.I.; Munteanu, A.N.; Isvoranu, G.; Pîrvu, I.R.; Ciotaru, D.; Constantin, C.; Bratu, O.; Căruntu, C.; Neagu, M.; et al. Phenotypic changes of lymphocyte populations in psoriasiform dermatitis animal model. Exp. Ther. Med. 2019, 17, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Sidler, D.; Wu, P.; Herro, R.; Claus, M.; Wolf, D.; Kawakami, Y.; Kawakami, T.; Burkly, L.; Croft, M. TWEAK mediates inflammation in experimental atopic dermatitis and psoriasis. Nat. Commun. 2017, 8, 15395. [Google Scholar] [CrossRef] [PubMed]
- Carceller, E.; Ballegeer, M.; Deckers, J.; Riccardi, C.; Bruscoli, S.; Hochepied, T.; Libert, C.; Pérez, P. Overexpression of Glucocorticoid-induced Leucine Zipper (GILZ) increases susceptibility to Imiquimod-induced psoriasis and involves cutaneous activation of TGF-β1. Sci. Rep. 2016, 6, 38825. [Google Scholar] [CrossRef]
- He, X.; Shen, C.; Lu, Q.; Li, J.; Wei, Y.; He, L.; Bai, R.; Zheng, J.; Luan, N.; Zhang, Z.; et al. Prokineticin 2 plays a pivotal role in psoriasis. EBioMedicine 2016, 13, 248–261. [Google Scholar] [CrossRef]
- Tanigawa, H.; Miyata, K.; Tian, Z.; Aoi, J.; Kadomatsu, T.; Fukushima, S.; Ogata, A.; Takeda, N.; Zhao, J.; Zhu, S.; et al. Upregulation of ANGPTL6 in mouse keratinocytes enhances susceptibility to psoriasis. Sci. Rep. 2016, 6, 34690. [Google Scholar] [CrossRef]
- Sweeney, C.M.; Russell, S.E.; Malara, A.; Kelly, G.; Hughes, R.; Tobin, A.M.; Adamzik, K.; Walsh, P.T.; Kirby, B.; Sweeney, C.; et al. Human ß-Defensin 3 and Its Mouse Ortholog Murine ß-Defensin 14 Activate Langerhans Cells and Exacerbate Psoriasis-Like Skin Inflammation in Mice. J. Investig. Dermatol. 2016, 136, 723–727. [Google Scholar] [CrossRef]
- Dou, R.; Liu, Z.; Yuan, X.; Xiangfei, D.; Bai, R.; Bi, Z.; Yang, P.; Yang, Y.; Dong, Y.; Su, W.; et al. PAMs ameliorates the imiquimod-induced psoriasis-like skin disease in mice by inhibition of translocation of NF-κB and production of inflammatory cytokines. PLoS ONE 2017, 12, e0176823. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, S.; Su, Z.; Song, S.; Xu, H.; Chen, G.; Cao, W.; Yin, S.; Gao, Q.; Wang, H. Heme oxygenase-1 induction attenuates imiquimod-induced psoriasiform inflammation by negative regulation of Stat3 signaling. Sci. Rep. 2016, 6, 21132. [Google Scholar] [CrossRef] [PubMed]
- Di, T.T.; Ruan, Z.T.; Zhao, J.X.; Wang, Y.; Liu, X.; Wang, Y.; Li, P. Astilbin inhibits Th17 cell differentiation and ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via Jak3/Stat3 signaling pathway. Int. Immunopharmacol. 2016, 32, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Di, T.; Wang, Y.; Wang, Y.; Liu, X.; Liang, D.; Li, P. Paeoniflorin inhibits imiquimod-induced psoriasis in mice by regulating Th17 cell response and cytokine secretion. Eur. J. Pharmacol. 2016, 772, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, M.; Xie, X.; Di, T.; Zhao, J.; Lin, Y.; Xu, X.; Li, N.; Zhai, Y.; Wang, Y.; et al. Paeonol ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice by inhibiting the maturation and activation of dendritic cells. Int. J. Mol. Med. 2017, 39, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Park, K.H.; Yun, C.O.; Kang, K.S.; Kim, T.Y. Effects of human mesenchymal stem cells transduced with superoxide dismutase on imiquimod-induced psoriasis-like skin inflammation in mice. Antioxid. Redox Signal. 2016, 24, 233–248. [Google Scholar] [CrossRef] [PubMed]
| Biological Effect | Cell/Molecule/Pathway | Model Used to Demonstrate the Effect | Reference |
|---|---|---|---|
| Cell-Type Involvement/Effects | |||
| Inflammation | CD5+ dendritic cell by inducing cytotoxic T cells and Th22 cells | Skin samples from psoriatic patients and healthy controls | [104] |
| Inflammation | Significantly increased peripheral T-CD8a+ lymphocyte and NK1.1+ cell percentages, decreased peripheral T-CD4+ and B lymphocyte percentages. | Samples from imiquimod experimental psoriasis mouse model | [109] |
| Cytokines/Chemokines-Type Effects | |||
| Inflammation | TWEAK (TNF superfamily molecule) | TWEAK-deficient mice bred on the C57BL/6 background; Fn14-deficient mice bred on BALB/c background | [110] |
| Anti-inflammatory | MCPIP1/Regnase-1 via restriction of IL-17A and IL-17C signaling | Skin biopsies from psoriatic patients; Zc3h12a−/− mice; Il17ra−/− mice; Il17a−/− mice. | [33] |
| Bioactive Molecules-Type Effects | |||
| Inflammation | Upregulated L-kynureninase (KYNU) | Skin and blood samples from psoriatic patients and healthy controls | [103] |
| Inflammation | Nuclear receptor interacting protein 1 (NRIP1) via the regulation of RelA/p65 | Skin and blood samples from psoriatic and healthy patients; HaCaT cells; C57BL/6J (B6) and Nrip1−/− mice | [106] |
| Inflammation | aberrant mTORC1 signaling | Spontaneously immortalized human keratinocyte cell line (HaCaT); NHK (normal human keratinocytes). | [105] |
| Inflammation | Tristetraprolin (TTP) deficiency | Zfp36-deficient mice (Zfp36−/−); LoxP-flanked Zfp36 mice (Zfp36fl/fl); LysM-Cre mice; CD11c-Cre mice; K14-Cre mice; Zfp36ΔEPTnfΔEP mice | [107] |
| Inflammation | VISTA (V-domain Immunoglobulin Suppressor of T cell Activation) deficiency via hyperactivation of Erk1/2 and Jnk1/2. | C57BL/6 mice; Vsir−/− mice | [108] |
| Inflammation | Overexpression of GILZ (Glucocorticoid-induced Leucine Zipper) via activation of TGF-β1 | GILZ-Tg (transgenic)mice; GILZ-Wt | [111] |
| Inflammation | High expression of PK2 (prokineticin 2) induces production of IL-1 in macrophages | K14-VEGF transgenic mice; Kunming mice; C57BL/6 mice | [112] |
| Inflammation | Upregulation of epidermal ANGPTL6 promotes hyperproliferation of keratinocytes | K14-Angptl6 Tg mice; skin biopsies from psoriasis patients. | [113] |
| Inflammation | Human β-Defensin 3 and Murine β-Defensin 14 via Langerhans cell activation | Skin biopsies from psoriatic patients; C57BL/6 mice. | [114] |
| Anti-inflammatory | PAM (plant antimicrobial solution) via inhibition of inflammatory NF-κB signaling pathway | HaCaT cells; Female BALB/c mice | [115] |
| Anti-inflammatory | Luteolin-7-glucoside via inhibition of IL-22/STAT3 pathway | HEKn cells (Human Epidermal Keratinocytes, neonatal); C57BL/6 mice | [46] |
| Anti-inflammatory | Astilbin inhibits Th17 cell differentiation via Jak3/Stat3 signaling pathway | BALB/c mice | [117] |
| Anti-inflammatory | Heme oxygenase-1 (HO-1) by negative regulation of STAT3 signaling | HaCaT cells; biopsies from psoriatic patients; BALB/c mice | [116] |
| Anti-inflammatory | Paeoniflorin by regulating Th17 cell response via phosphorylation of STAT3 | BALB/c mice; C57BL/6 mice | [118] |
| Anti-inflammatory | Paeonol by inhibiting the maturation and activation of DC via the TLR7/8 signaling pathway | BALB/c mice | [119] |
| Anti-inflammatory | Superoxide dismutase (SOD3)-transduced MSCs (Mesenchymal Stem Cells) via inhibition of signaling pathways toll-like receptor-7, nuclear factor-kappa B, p38 mitogen-activated kinase, and Janus kinase–signal transducer and activator of transcription | C57BL/6 mice | [120] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgescu, S.-R.; Tampa, M.; Caruntu, C.; Sarbu, M.-I.; Mitran, C.-I.; Mitran, M.-I.; Matei, C.; Constantin, C.; Neagu, M. Advances in Understanding the Immunological Pathways in Psoriasis. Int. J. Mol. Sci. 2019, 20, 739. https://doi.org/10.3390/ijms20030739
Georgescu S-R, Tampa M, Caruntu C, Sarbu M-I, Mitran C-I, Mitran M-I, Matei C, Constantin C, Neagu M. Advances in Understanding the Immunological Pathways in Psoriasis. International Journal of Molecular Sciences. 2019; 20(3):739. https://doi.org/10.3390/ijms20030739
Chicago/Turabian StyleGeorgescu, Simona-Roxana, Mircea Tampa, Constantin Caruntu, Maria-Isabela Sarbu, Cristina-Iulia Mitran, Madalina-Irina Mitran, Clara Matei, Carolina Constantin, and Monica Neagu. 2019. "Advances in Understanding the Immunological Pathways in Psoriasis" International Journal of Molecular Sciences 20, no. 3: 739. https://doi.org/10.3390/ijms20030739
APA StyleGeorgescu, S.-R., Tampa, M., Caruntu, C., Sarbu, M.-I., Mitran, C.-I., Mitran, M.-I., Matei, C., Constantin, C., & Neagu, M. (2019). Advances in Understanding the Immunological Pathways in Psoriasis. International Journal of Molecular Sciences, 20(3), 739. https://doi.org/10.3390/ijms20030739










