NF-κB-Associated Pain-Related Neuropeptide Expression in Patients with Degenerative Disc Disease
Abstract
1. Introduction
2. Results
2.1. Patients’ Demographic and Clinical Data
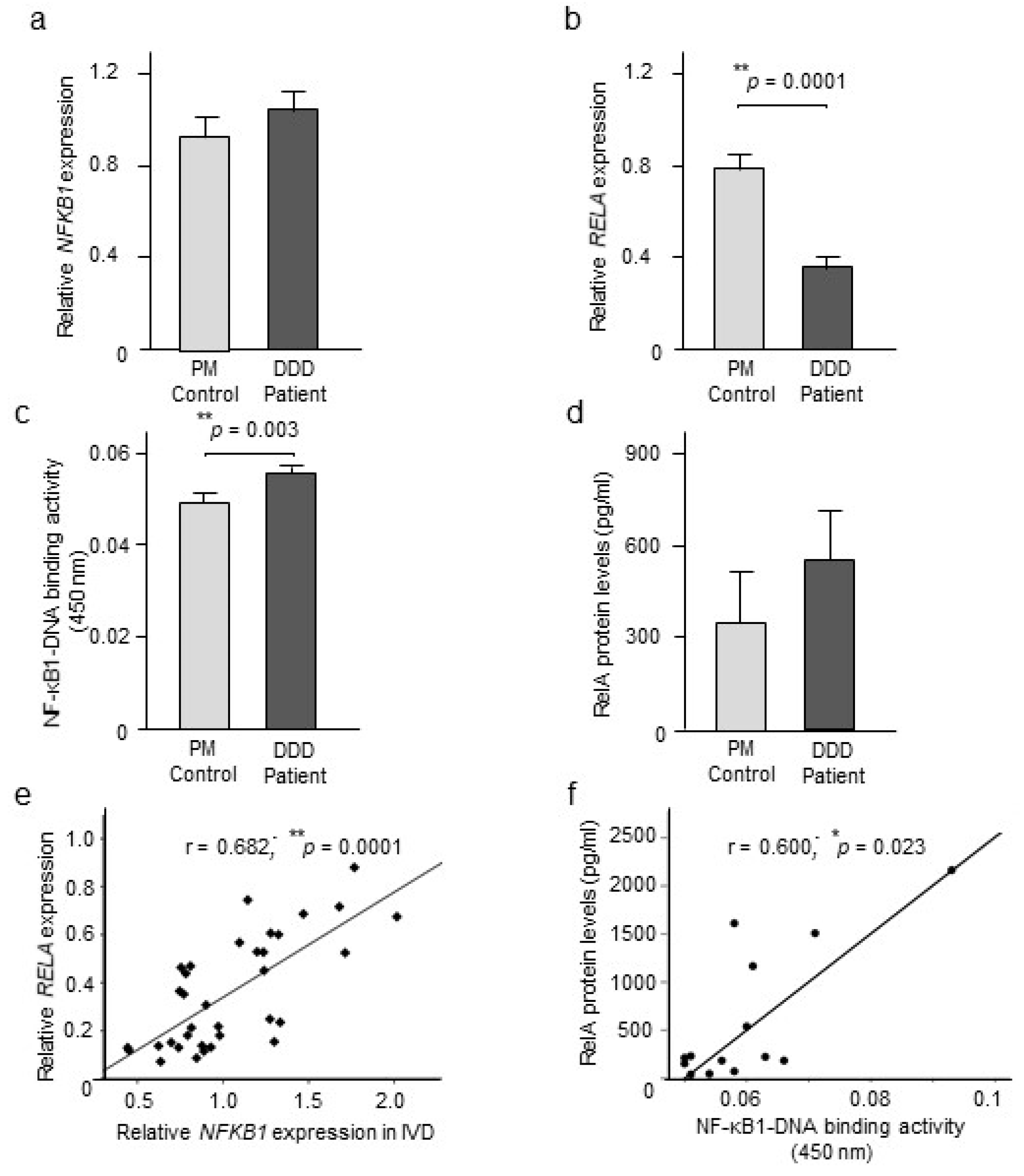
2.2. NF-κB Expression and Activity
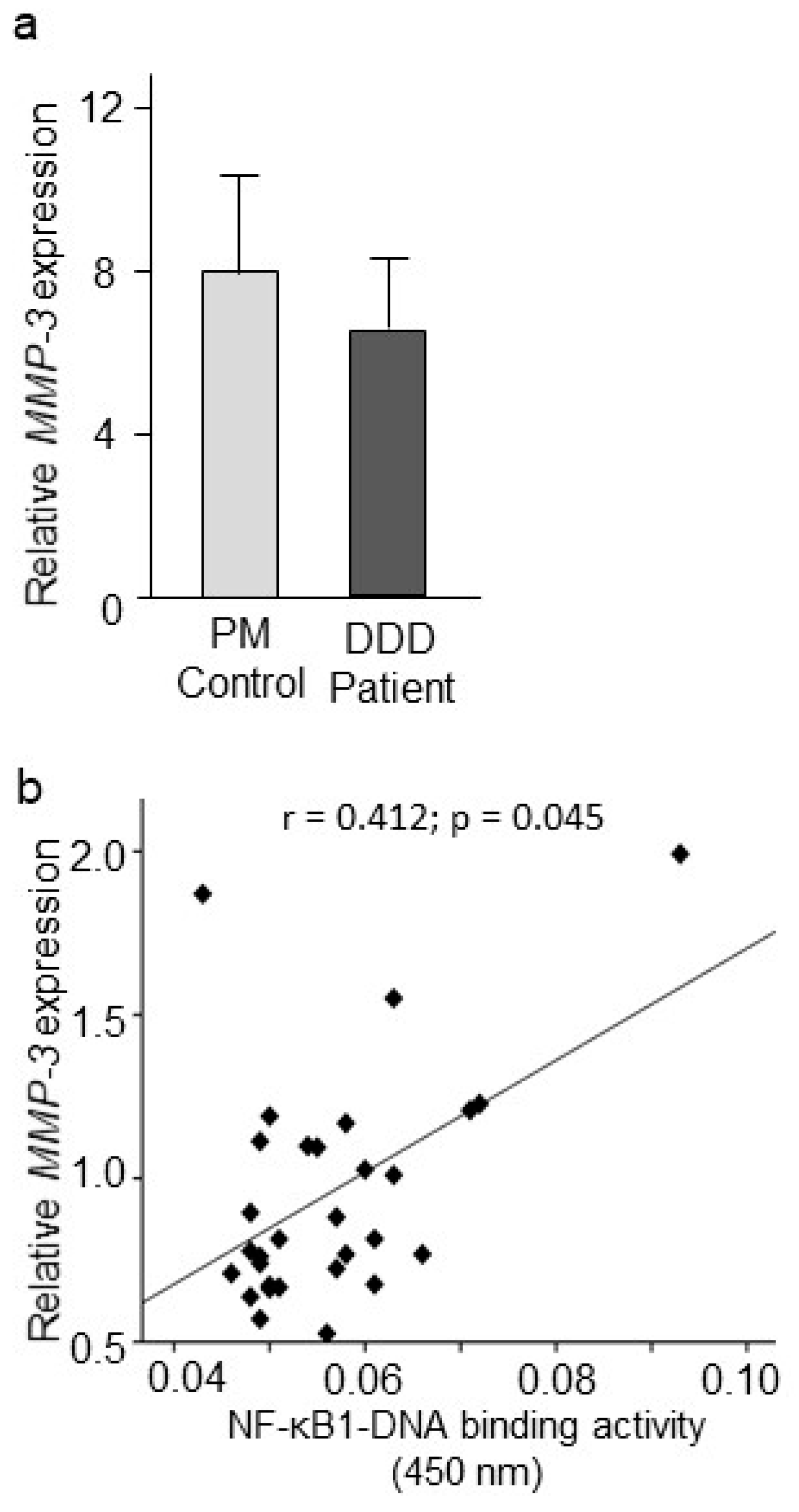
2.3. MMP-3 Gene Expression and Association with NF-κB Signaling
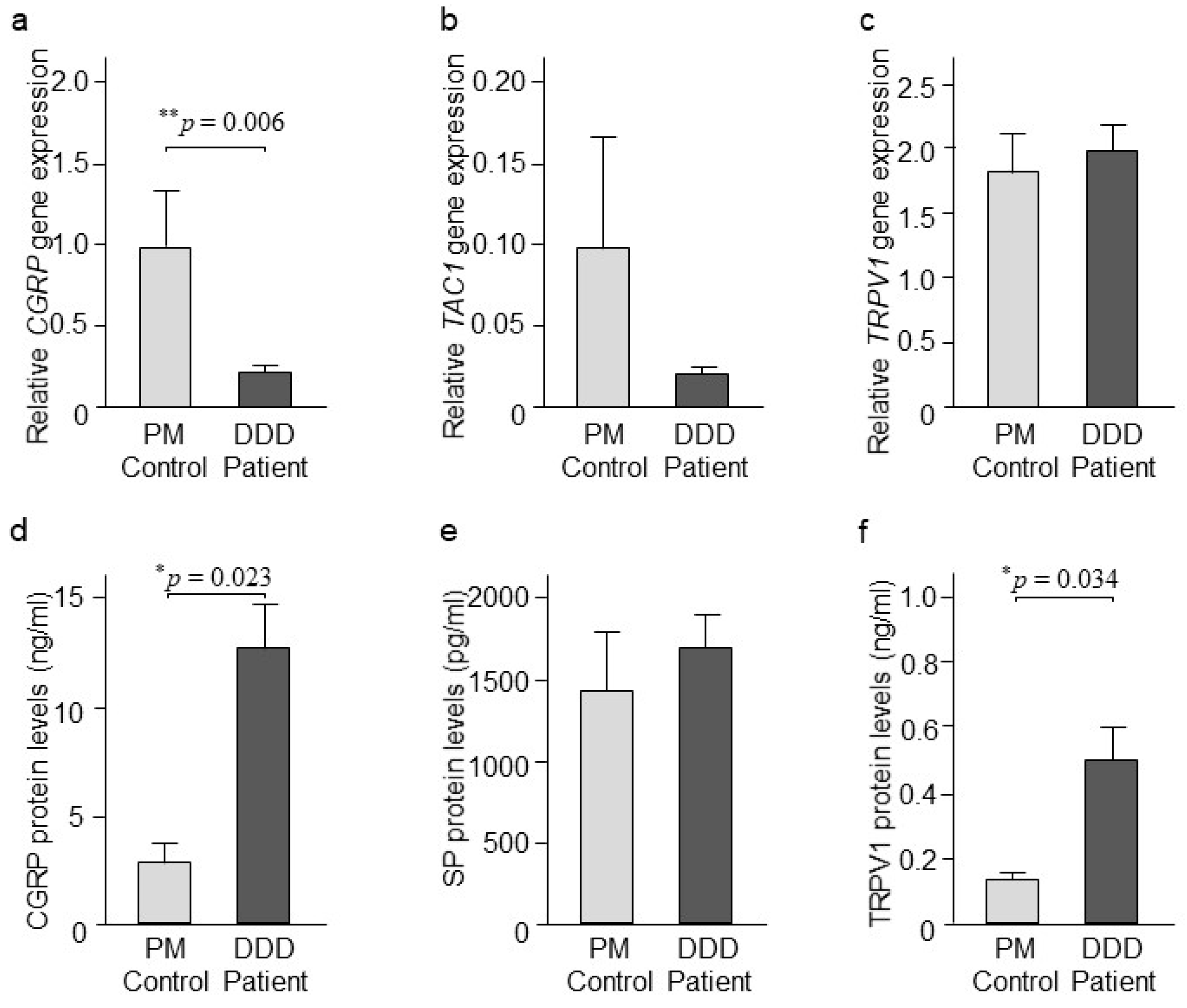
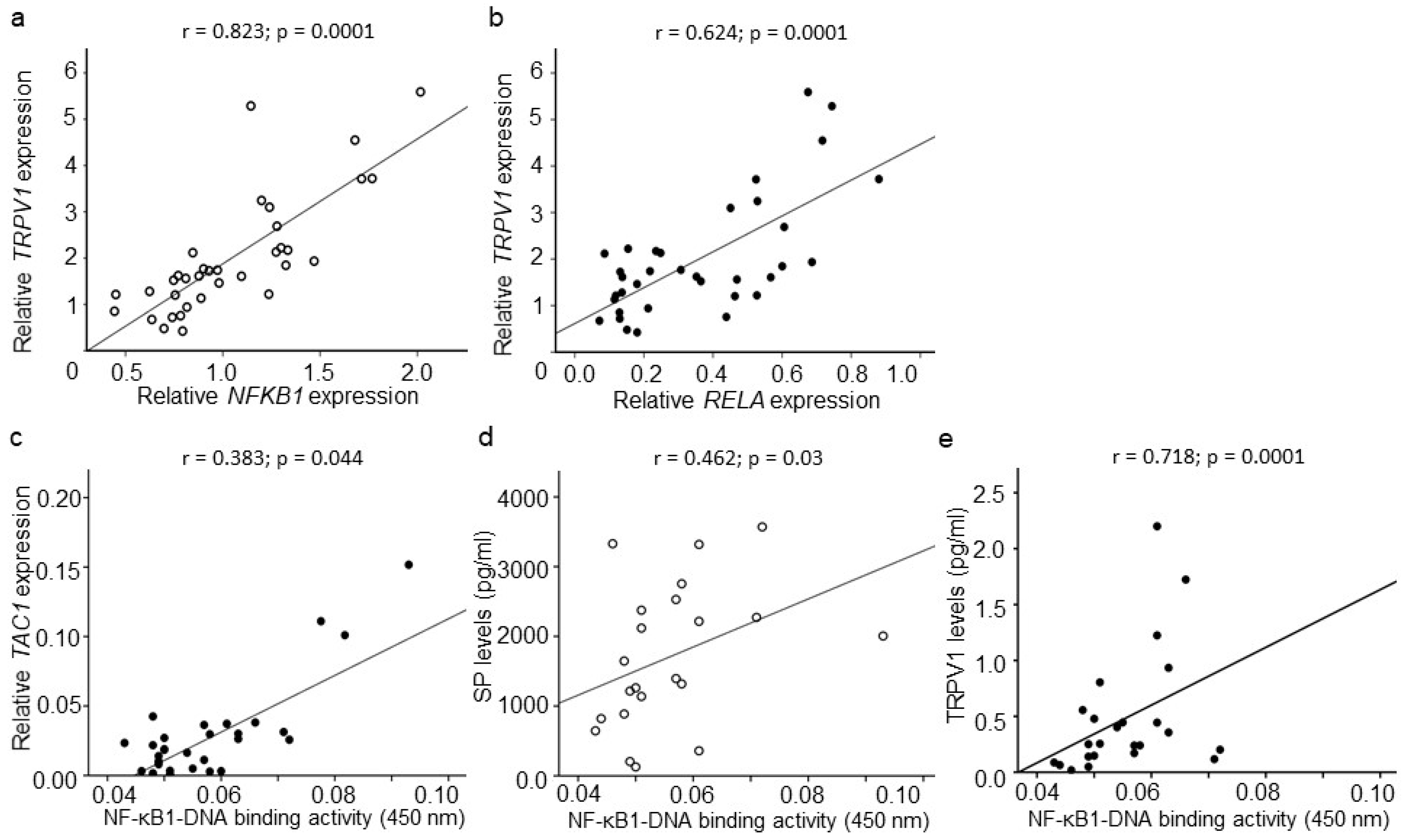
2.4. CGRP, SP, and TRPV1 Expression
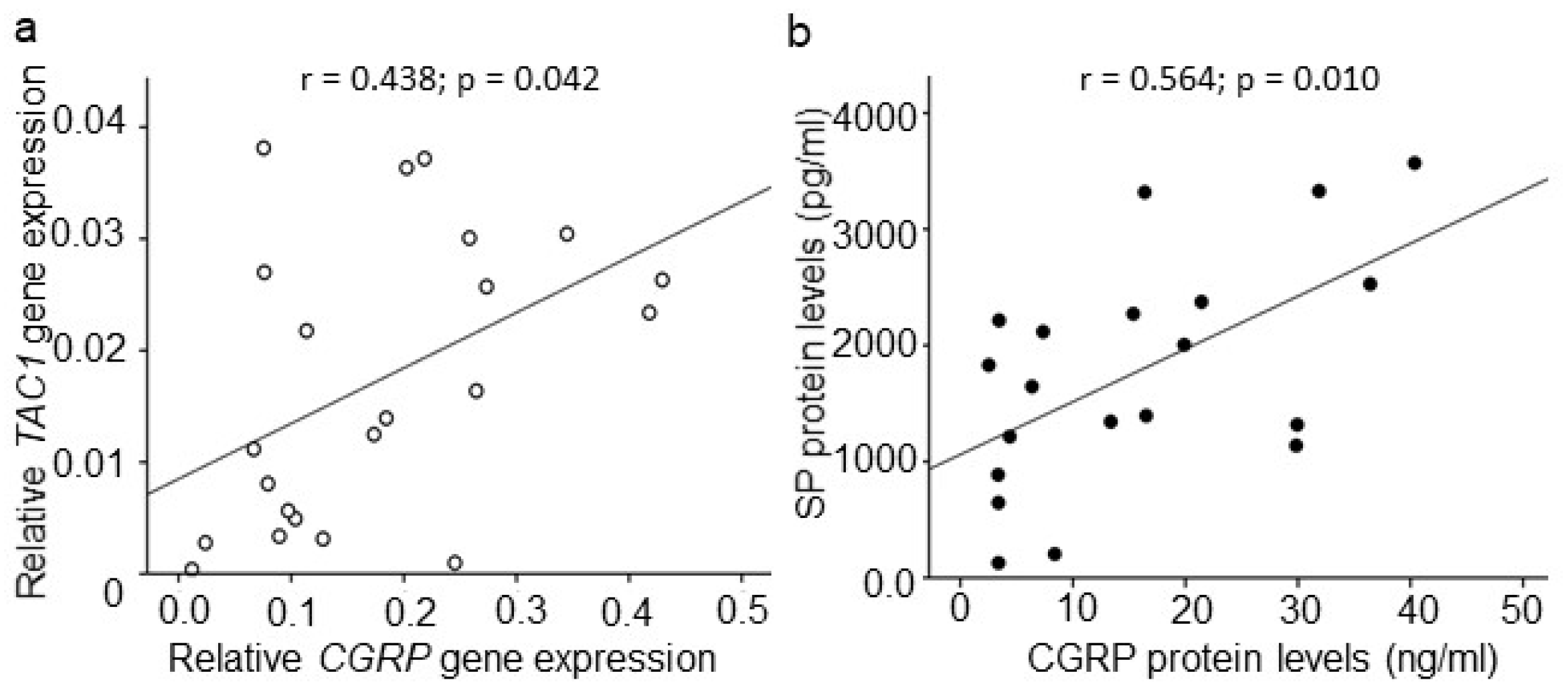
2.5. Association among CGRP, SP, and TRPV1
2.6. Association between CGRP, SP, TRPV1, and NF-κB Signaling
2.7. Association between NF-κB, CGRP, SP, TRPV1, and Clinical Symptoms
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.1.1. Patients
4.1.2. Postmortem Controls
4.2. Questionnaires
4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Cytoplasmic and Nuclear Extraction
4.5. Detection of NF-κB1–DNA Binding and RelA Expression by ELISA
4.6. CGRP, SP, and TRPV1 ELISA
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hadjipavlou, A.G.; Tzermiadianos, M.N.; Bogduk, N.; Zindrick, M.R. The pathophysiology of disc degeneration: A critical review. J. Bone Jt. Surg. Br. 2008, 90, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, P.P.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.; Welting, T.J.; van Royen, B.J.; van Dieen, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Gronblad, M.; Weinstein, J.N.; Santavirta, S. Immunohistochemical observations on spinal tissue innervation. A review of hypothetical mechanisms of back pain. Acta Orthop. Scand. 1991, 62, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J.; Peacock, T.E.; Goupille, P.; Hoyland, J.A.; O’Brien, J.; Jayson, M.I. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef]
- Krock, E.; Rosenzweig, D.H.; Chabot-Dore, A.J.; Jarzem, P.; Weber, M.H.; Ouellet, J.A.; Stone, L.S.; Haglund, L. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J. Cell. Mol. Med. 2014, 18, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Ashton, I.K.; Roberts, S.; Jaffray, D.C.; Polak, J.M.; Eisenstein, S.M. Neuropeptides in the human intervertebral disc. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1994, 12, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Doyle, P.; Minogue, B.M.; Gnanalingham, K.; Hoyland, J.A. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res. Ther. 2009, 11, R126. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Kokubo, Y.; Uchida, K.; Kobayashi, S.; Yayama, T.; Sato, R.; Nakajima, H.; Takamura, T.; Mwaka, E.; Orwotho, N.; Bangirana, A.; et al. Herniated and spondylotic intervertebral discs of the human cervical spine: Histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J. Neurosurg. Spine 2008, 9, 285–295. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, E.J.; Kim, H.M.; Lee, S.B.; Kim, H.D.; Su Kim, G.; Kim, H.H. Antioxidant alpha-lipoic acid inhibits osteoclast differentiation by reducing nuclear factor-kappaB DNA binding and prevents in vivo bone resorption induced by receptor activator of nuclear factor-kappaB ligand and tumor necrosis factor-alpha. Free Radic Biol. Med. 2006, 40, 1483–1493. [Google Scholar] [CrossRef]
- Dai, S.; Hirayama, T.; Abbas, S.; Abu-Amer, Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J. Biol. Chem. 2004, 279, 37219–37222. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Liu, N.; Luo, S.; Huang, W.; Zha, Z.; Yang, J. MicroRNA-9 regulates the development of knee osteoarthritis through the NF-kappaB1 pathway in chondrocytes. Medicine 2016, 95, e4315. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Park, S.K.; Zhou, J.L.; Taglialatela, G.; Chung, K.; Coggeshall, R.E.; Chung, J.M. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 2004, 111, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.E.; Eskew, O.; Wong, T.; Tchivileva, I.E.; Oladosu, F.A.; O’Buckley, S.C.; Nackley, A.G. Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav. Immun. 2015, 50, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Delhalle, S.; Blasius, R.; Dicato, M.; Diederich, M. A beginner’s guide to NF-kappaB signaling pathways. Ann. N. Y. Acad. Sci. 2004, 1030, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Zhongyi, S.; Sai, Z.; Chao, L.; Jiwei, T. Effects of nuclear factor kappa B signaling pathway in human intervertebral disc degeneration. Spine 2015, 40, 224–232. [Google Scholar] [CrossRef]
- Ma, T.; Guo, C.J.; Zhao, X.; Wu, L.; Sun, S.X.; Jin, Q.H. The effect of curcumin on NF-kappaB expression in rat with lumbar intervertebral disc degeneration. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1305–1314. [Google Scholar]
- Orita, S.; Miyagi, M.; Kobori, S.; Gemba, T.; Ishikawa, T.; Inoue, G.; Toyone, T.; Aoki, Y.; Eguchi, Y.; Takahashi, K.; et al. IkappaB kinase beta inhibitor downregulates pain-related neuropeptide production in the sensory neurons innervating injured lumbar intervertebral discs in the dorsal root ganglia of rats. Spine J. 2013, 13, 284–288. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Li, J.; Ahmed, M.; Hua, L.; Yakovleva, T.; Ossipov, M.H.; Bakalkin, G.; Stark, A. Attenuation of pain and inflammation in adjuvant-induced arthritis by the proteasome inhibitor MG132. Arthritis Rheum. 2010, 62, 2160–2169. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Li, J.; Erlandsson-Harris, H.; Stark, A.; Bakalkin, G.; Ahmed, M. Suppression of pain and joint destruction by inhibition of the proteasome system in experimental osteoarthritis. Pain 2012, 153, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.A.; Connor, M. TRPV1 antagonists as a potential treatment for hyperalgesia. Recent Pat. Cns Drug Discov. 2006, 1, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Fernihough, J.; Gentry, C.; Bevan, S.; Winter, J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci. Lett. 2005, 388, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.L.; Chandran, P.; Joshi, S.K.; Jarvis, M.F.; Kym, P.R.; McGaraughty, S. TRPV1-related modulation of spinal neuronal activity and behavior in a rat model of osteoarthritic pain. Brain Res. 2011, 1369, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Sappington, R.M.; Calkins, D. Contribution of TRPV1 to microglia-derived IL-6 and NFkappaB translocation with elevated hydrostatic pressure. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3004–3017. [Google Scholar] [CrossRef] [PubMed]
- Moneta, G.B.; Videman, T.; Kaivanto, K.; Aprill, C.; Spivey, M.; Vanharanta, H.; Sachs, B.L.; Guyer, R.D.; Hochschuler, S.H.; Raschbaum, R.F.; et al. Reported pain during lumbar discography as a function of anular ruptures and disc degeneration. A re-analysis of 833 discograms. Spine 1994, 19, 1968–1974. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Nerlich, A.; Mittermaier, N.; Weiler, C.; Lumenta, C.; Wuertz, K.; Boos, N. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur. Spine J. 2009, 18, 1573–1586. [Google Scholar] [CrossRef]
- Berenbaum, F. Signaling transduction: Target in osteoarthritis. Curr. Opin. Rheumatol. 2004, 16, 616–622. [Google Scholar] [CrossRef]
- Acharyya, S.; Villalta, S.A.; Bakkar, N.; Bupha-Intr, T.; Janssen, P.M.; Carathers, M.; Li, Z.W.; Beg, A.A.; Ghosh, S.; Sahenk, Z.; et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Investig. 2007, 117, 889–901. [Google Scholar] [CrossRef]
- Nerlich, A.G.; Bachmeier, B.E.; Schleicher, E.; Rohrbach, H.; Paesold, G.; Boos, N. Immunomorphological analysis of RAGE receptor expression and NF-kappaB activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann. N. Y. Acad. Sci. 2007, 239–248. [Google Scholar] [CrossRef]
- Akeda, K.; An, H.; Gemba, T.; Okuma, M.; Miyamoto, K.; Chujo, T.; Kitahara, S.; Masuda, K. A new gene therapy approach: In vivo transfection of “naked” NF-kB decoy oligonucleotide restored disc degeneration in the rabbit annular needle puncture model. Trans. Orthop. Res. Soc. 2005, 30, 45. [Google Scholar]
- Ahmed, A.S.; Ahmed, M.; Li, J.; Gu, H.F.; Bakalkin, G.; Stark, A.; Harris, H.E. Proteasome inhibitor MG132 modulates inflammatory pain by central mechanisms in adjuvant arthritis. Int. J. Rheum Dis. 2017, 20, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, G.; Shimaoka, M.; Fukuoka, T.; Hiroi, T.; Inoue, T.; Hashimoto, N.; Sakaguchi, T.; Sawa, Y.; Morishita, R.; Kiyono, H.; et al. NF-kappa B decoy suppresses cytokine expression and thermal hyperalgesia in a rat neuropathic pain model. Neuroreport 2001, 12, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.D.; Guo, Q.L.; Wang, E.; Ye, Z.; He, Z.H.; Zou, W.Y.; Cheng, Z.G.; Wang, Y.J. Intrathecal infusion of pyrrolidine dithiocarbamate for the prevention and reversal of neuropathic pain in rats using a sciatic chronic constriction injury model. Reg. Anesth Pain Med. 2010, 35, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Millet, I.; Phillips, R.J.; Sherwin, R.S.; Ghosh, S.; Voll, R.E.; Flavell, R.A.; Vignery, A.; Rincon, M. Inhibition of NF-kappaB activity and enhancement of apoptosis by the neuropeptide calcitonin gene-related peptide. J. Biol. Chem. 2000, 275, 15114–15121. [Google Scholar] [CrossRef] [PubMed]
- Azzolina, A.; Guarneri, P.; Lampiasi, N. Involvement of p38 and JNK MAPKs pathways in Substance P-induced production of TNF-alpha by peritoneal mast cells. Cytokine 2002, 18, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Fong, G.; Backman, L.J.; Hart, D.A.; Danielson, P.; McCormack, B.; Scott, A. Substance P enhances collagen remodeling and MMP-3 expression by human tenocytes. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2013, 31, 91–98. [Google Scholar] [CrossRef]
- Roberts, S.; Caterson, B.; Menage, J.; Evans, E.H.; Jaffray, D.C.; Eisenstein, S.M. Matrix metalloproteinases and aggrecanase: Their role in disorders of the human intervertebral disc. Spine 2000, 25, 3005–3013. [Google Scholar] [CrossRef]
- Purmessur, D.; Freemont, A.J.; Hoyland, J.A. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res. Ther. 2008, 10, R99. [Google Scholar] [CrossRef]
- Song, X.X.; Shi, S.; Guo, Z.; Li, X.F.; Yu, B.W. Estrogen receptors involvement in intervertebral discogenic pain of the elderly women: Colocalization and correlation with the expression of Substance P in nucleus pulposus. Oncotarget 2017, 8, 38136–38144. [Google Scholar] [CrossRef]
- Zacest, A.C.; Vink, R.; Manavis, J.; Sarvestani, G.T.; Blumbergs, P.C. Substance P immunoreactivity increases following human traumatic brain injury. Acta Neurochir. Suppl. 2010, 106, 211–216. [Google Scholar] [PubMed]
- Lorente, L. New Prognostic Biomarkers in Patients with Traumatic Brain Injury. Arch. Trauma Res. 2015, 4, e30165. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Geppetti, P. Substance p. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Aoki, Y.; Ohtori, S.; Ino, H.; Douya, H.; Ozawa, T.; Saito, T.; Moriya, H.; Takahashi, K. Disc inflammation potentially promotes axonal regeneration of dorsal root ganglion neurons innervating lumbar intervertebral disc in rats. Spine 2004, 29, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Gavenis, K.; Schumacher, C.; Schneider, U.; Eisfeld, J.; Mollenhauer, J.; Schmidt-Rohlfing, B. Expression of ion channels of the TRP family in articular chondrocytes from osteoarthritic patients: Changes between native and in vitro propagated chondrocytes. Mol. Cell. Biochem. 2009, 321, 135–143. [Google Scholar] [CrossRef]
- Idris, A.I.; Landao-Bassonga, E.; Ralston, S.H. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone 2010, 46, 1089–1099. [Google Scholar] [CrossRef]
- Engler, A.; Aeschlimann, A.; Simmen, B.R.; Michel, B.A.; Gay, R.E.; Gay, S.; Sprott, H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2007, 359, 884–888. [Google Scholar] [CrossRef]
- Yoshino, K.; Suzuki, M.; Kawarai, Y.; Sakuma, Y.; Inoue, G.; Orita, S.; Yamauchi, K.; Aoki, Y.; Ishikawa, T.; Miyagi, M.; et al. Increase of TRPV1-immunoreactivity in dorsal root ganglia neurons innervating the femur in a rat model of osteoporosis. Yonsei Med. J. 2014, 55, 1600–1605. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharm. Rev. 1999, 51, 159–212. [Google Scholar]
- Chu, C.; Zavala, K.; Fahimi, A.; Lee, J.; Xue, Q.; Eilers, H.; Schumacher, M.A. Transcription factors Sp1 and Sp4 regulate TRPV1 gene expression in rat sensory neurons. Mol. Pain 2011, 7, 44. [Google Scholar] [CrossRef]
- Cho, H.K.; Ahn, S.H.; Kim, S.Y.; Choi, M.J.; Hwang, S.J.; Cho, Y.W. Changes in the Expressions of Iba1 and Calcitonin Gene-Related Peptide in Adjacent Lumbar Spinal Segments after Lumbar Disc Herniation in a Rat Model. J. Korean Med. Sci. 2015, 30, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Wang, X.; Wan, Z.; Liu, Y.; Leng, Y. Dexmedetomidine Relieves Acute Inflammatory Visceral Pain in Rats through the ERK Pathway, Toll-Like Receptor Signaling, and TRPV1 Channel. J. Mol. Neurosci. 2018, 66, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, S.Y.; Lim, S.Y.; Kieff, E.; Song, Y.J. Role of Ca2+/calmodulin-dependent kinase II-IRAK1 interaction in LMP1-induced NF-kappaB activation. Mol. Cell Biol. 2014, 34, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.G.; Namer, B.; Reeh, P.W.; Fischer, M.J.M. TRPA1 and TRPV1 Antagonists Do Not Inhibit Human Acidosis-Induced Pain. J. Pain Off. J. Am. Pain Soc. 2017, 18, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Kuliwaba, J.S.; Fazzalari, N.L.; Findlay, D.M. Stability of RNA isolated from human trabecular bone at post-mortem and surgery. Biochim. Et Biophys. Acta 2005, 1740, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.C.; Couper, J.; Davies, J.B.; O’Brien, J.P. The Oswestry low back pain disability questionnaire. Physiotherapy 1980, 66, 271–273. [Google Scholar] [PubMed]
| Subject Characteristics | DDD Patients | PM Controls | Differences | |
|---|---|---|---|---|
| Study subjects (N) | 40 | 18 | ||
| Average Age (years ± SD) | 44.5 (± 10.0) | 42.7 (± 13.0) | n.s | |
| Gender (F/M) | 22/18 | 6/12 | ||
| BMI (kg/m2) | 25.4 (± 5.4) | 28.37 (± 5.1) | p = 0.0001 | |
| Post-mortem interval (h) | - | 49.6 (± 15.5) | ||
| Anti-nociceptive medication | 20/40 | |||
| VAS Back (mm) | 32.01 (± 19.98) | - | ||
| VAS Leg (mm) | 4.71 (± 9.37) | - | ||
| Oswestry Disability Index (ODI) | 31.59 (± 12.25) | - | ||
| Subjects included in biochemical analysis | ||||
| qRT-PCR | N | 35/40 | 17/18 | |
| Gender (F/M) | 18/17 | 6/11 | ||
| Age years (±SD) | 45.17 (±9.7) | 41.7 (±12.8) | n.s | |
| ELISA | N | 36/40 | 18/18 | |
| Gender (F/M) | 19/17 | 6/12 | ||
| Age years (±SD) | 44.30 (±9.2) | 42.7 (±13.0) | n.s | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.S.; Berg, S.; Alkass, K.; Druid, H.; Hart, D.A.; Svensson, C.I.; Kosek, E. NF-κB-Associated Pain-Related Neuropeptide Expression in Patients with Degenerative Disc Disease. Int. J. Mol. Sci. 2019, 20, 658. https://doi.org/10.3390/ijms20030658
Ahmed AS, Berg S, Alkass K, Druid H, Hart DA, Svensson CI, Kosek E. NF-κB-Associated Pain-Related Neuropeptide Expression in Patients with Degenerative Disc Disease. International Journal of Molecular Sciences. 2019; 20(3):658. https://doi.org/10.3390/ijms20030658
Chicago/Turabian StyleAhmed, Aisha S., Svante Berg, Kanar Alkass, Henrik Druid, David A. Hart, Camilla I. Svensson, and Eva Kosek. 2019. "NF-κB-Associated Pain-Related Neuropeptide Expression in Patients with Degenerative Disc Disease" International Journal of Molecular Sciences 20, no. 3: 658. https://doi.org/10.3390/ijms20030658
APA StyleAhmed, A. S., Berg, S., Alkass, K., Druid, H., Hart, D. A., Svensson, C. I., & Kosek, E. (2019). NF-κB-Associated Pain-Related Neuropeptide Expression in Patients with Degenerative Disc Disease. International Journal of Molecular Sciences, 20(3), 658. https://doi.org/10.3390/ijms20030658






