Homeostatic Model Assessment of Insulin Resistance for Predicting the Recurrence of Hepatocellular Carcinoma after Curative Treatment
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics and Laboratory Data
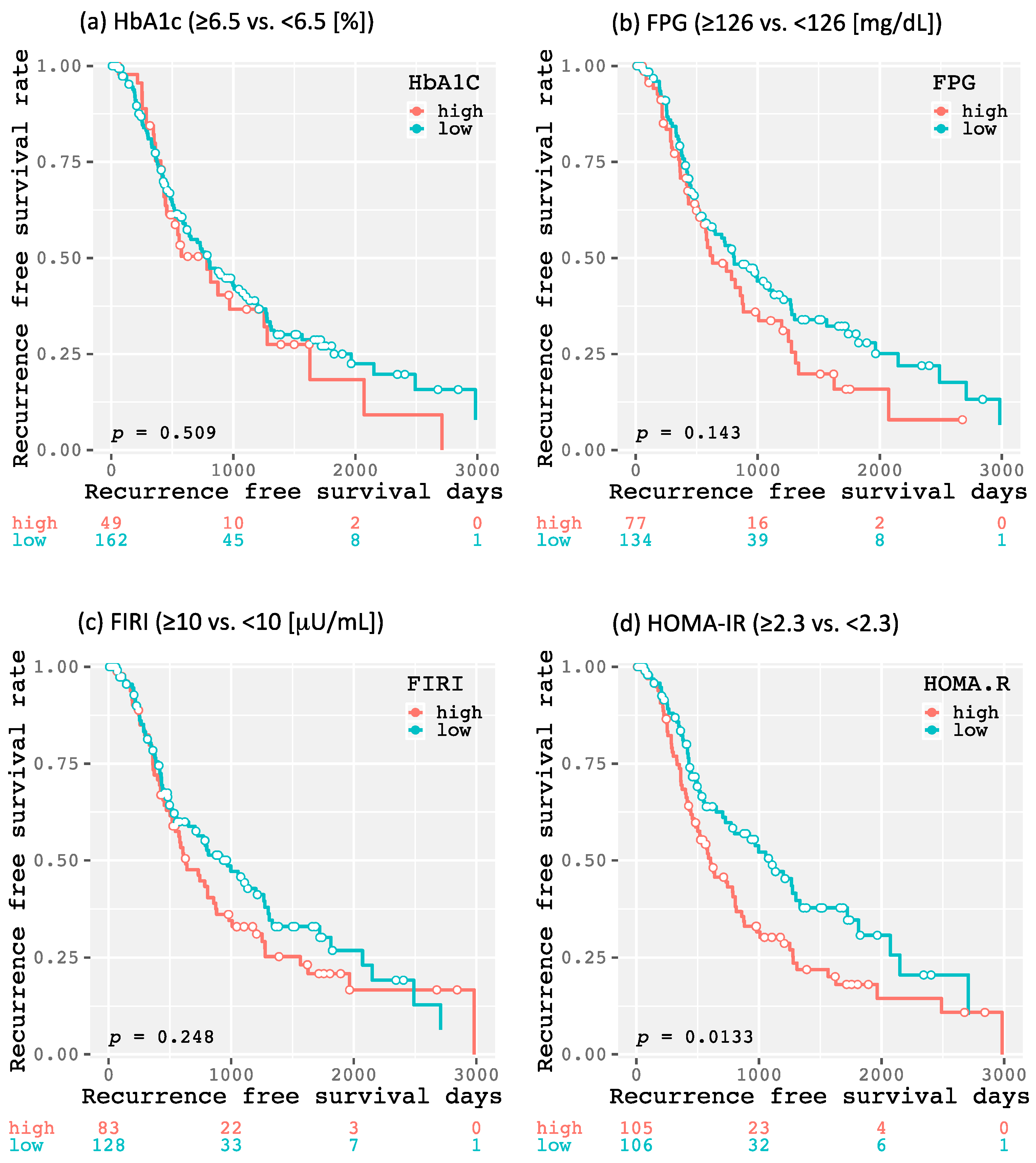
2.2. Impact of DM (Diabetes Mellitus) and Glucose Metabolism-Related Factors on Recurrence-Free Survival in Patients with HCC (Hepatocellular Carcinoma) after Curative Treatmen
3. Discussion
4. Materials and Methods
4.1. Patients, Treatment, and Determination of Recurrence
4.2. Examination of Glucose Metabolism
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Abbreviations
| HCC | hepatocellular carcinoma |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HOMA-IR | homeostasis model assessment-insulin resistance |
| DM | diabetes mellitus |
| HbA1c | hemoglobin A1c |
| FPG | fasting plasma glucose |
| FIRI | fasting immunoreactive insulin |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| AFP | alpha-fetoprotein |
| PIVKA-II | protein induced by vitamin K absence or antagonists-II |
References
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma: An epidemiologic view. J. Clin. Gastroenterol. 2002, 35, S72–S78. [Google Scholar] [CrossRef]
- Njei, B.; Rotman, Y.; Ditah, I.; Lim, J.K. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015, 61, 191–199. [Google Scholar] [CrossRef]
- Singal, A.G.; El-Serag, H.B. Hepatocellular carcinoma from epidemiology to prevention: Translating knowledge into practice. Clin. Gastroenterol. Hepatol. 2015, 13, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T. Prevention of recurrence after resection of hepatocellular carcinoma: A daunting challenge. Hepatology 2011, 54, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Hampel, H.; Javadi, F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 2006, 4, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Maeda, T.; Watanabe, S.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Increased visceral fat volume raises the risk for recurrence of hepatocellular carcinoma after curative treatment. Oncotarget 2018, 9, 14058–14067. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Imai, K.; Takai, K.; Hanai, T.; Hayashi, H.; Naiki, T.; Nishigaki, Y.; Tomita, E.; Shimizu, M.; Moriwaki, H. Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: A prospective case series study using the d-rom test. J. Cancer Res. Clin. Oncol. 2013, 139, 845–852. [Google Scholar] [CrossRef]
- Watanabe, N.; Takai, K.; Imai, K.; Shimizu, M.; Naiki, T.; Nagaki, M.; Moriwaki, H. Increased levels of serum leptin are a risk factor for the recurrence of stage I/II hepatocellular carcinoma after curative treatment. J. Clin. Biochem. Nutr. 2011, 49, 153–158. [Google Scholar] [CrossRef]
- Ali Kamkar, M.M.; Ahmad, R.; Alsmadi, O.; Behbehani, K. Insight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: Mini-review. J. Diabetes Metab. Disord. 2014, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Gao, P. Diabetes mellitus and risk of hepatocellular carcinoma. Biomed. Res. Int. 2017, 2017, 5202684. [Google Scholar] [CrossRef] [PubMed]
- Polesel, J.; Zucchetto, A.; Montella, M.; Dal Maso, L.; Crispo, A.; La Vecchia, C.; Serraino, D.; Franceschi, S.; Talamini, R. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann. Oncol. 2009, 20, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Tateishi, R.; Shiina, S.; Goto, E.; Sato, T.; Nakagawa, H.; Masuzaki, R.; Goto, T.; Hamamura, K.; Kanai, F.; et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected nash. Gut 2009, 58, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Nishigaki, Y.; Shimizu, S.; Naiki, T.; Hayashi, H.; Uematsu, T.; Sugihara, J.; Tomita, E.; Shimizu, M.; et al. Insulin resistance raises the risk for recurrence of stage i hepatocellular carcinoma after curative radiofrequency ablation in hepatitis c virus-positive patients: A prospective, case series study. Hepatol. Res. 2010, 40, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Nagata, C.; Mizoue, T.; Inoue, M.; Tsugane, S.; Sasazuki, S.; et al. Diabetes mellitus and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the japanese population. Jpn. J. Clin. Oncol. 2014, 44, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Lipworth, L.; Maso, L.D.; Talamini, R.; Montella, M.; Polesel, J.; McLaughlin, J.K.; Parpinel, M.; Franceschi, S.; Lagiou, P.; et al. Dietary glycemic load and hepatocellular carcinoma with or without chronic hepatitis infection. Ann. Oncol. 2009, 20, 1736–1740. [Google Scholar] [CrossRef]
- Hassan, M.M.; Hwang, L.Y.; Hatten, C.J.; Swaim, M.; Li, D.; Abbruzzese, J.L.; Beasley, P.; Patt, Y.Z. Risk factors for hepatocellular carcinoma: Synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002, 36, 1206–1213. [Google Scholar] [CrossRef]
- Yuan, J.M.; Govindarajan, S.; Arakawa, K.; Yu, M.C. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer 2004, 101, 1009–1017. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-kinase akt pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Matsuhisa, M.; Yamasaki, Y.; Emoto, M.; Shimabukuro, M.; Ueda, S.; Funahashi, T.; Matsuzawa, Y. A novel index of insulin resistance determined from the homeostasis model assessment index and adiponectin levels in japanese subjects. Diabetes Res. Clin. Pract. 2007, 77, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Park, Y.; McGlynn, K.A.; Hollenbeck, A.R.; Taylor, P.R.; Goldstein, A.M.; Freedman, N.D. Index-based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology 2014, 60, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Patman, G. Hepatocellular carcinoma. Working it out—Exercise reduces HCC but not steatosis in mice. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 124. [Google Scholar] [CrossRef]
- Sakai, H.; Shirakami, Y.; Shimizu, M. Chemoprevention of obesity-related liver carcinogenesis by using pharmaceutical and nutraceutical agents. World J. Gastroenterol. 2016, 22, 394–406. [Google Scholar] [CrossRef]
- Karagozian, R.; Derdak, Z.; Baffy, G. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism 2014, 63, 607–617. [Google Scholar] [CrossRef]
- American Diabetes Association. 4. Lifestyle management: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar]
- Decensi, A.; Puntoni, M.; Goodwin, P.; Cazzaniga, M.; Gennari, A.; Bonanni, B.; Gandini, S. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 2010, 3, 1451–1461. [Google Scholar] [CrossRef]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, J.W.; Wu, L.C.; Lai, M.S.; Chuang, L.M.; Chan, K.A. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology 2012, 55, 1462–1472. [Google Scholar] [CrossRef]
- Yu, M.C.; Tong, M.J.; Govindarajan, S.; Henderson, B.E. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of los Angeles county, California. J. Natl. Cancer Inst. 1991, 83, 1820–1826. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Anti-diabetic medications and the risk of hepatocellular cancer: A systematic review and meta-analysis. Am. J. Gastroenterol. 2013, 108, 881–891, quiz 892. [Google Scholar] [CrossRef]
- Hassan, M.M.; Curley, S.A.; Li, D.; Kaseb, A.; Davila, M.; Abdalla, E.K.; Javle, M.; Moghazy, D.M.; Lozano, R.D.; Abbruzzese, J.L.; et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer 2010, 116, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Franchi, M.; Nicotra, F.; Asciutto, R.; Merlino, L.; La Vecchia, C.; Corrao, G. Insulin and other antidiabetic drugs and hepatocellular carcinoma risk: A nested case-control study based on Italian healthcare utilization databases. Pharmacoepidemiol. Drug Saf. 2015, 24, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. Jsh consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar]
- Aickin, M.; Gensler, H. Adjusting for multiple testing when reporting research results: The bonferroni vs holm methods. Am. J. Public Health 1996, 86, 726–728. [Google Scholar] [CrossRef]


| All Cases (n = 211) | DM (−) (n = 140) | DM (+) (n = 71) | p-Value | |
|---|---|---|---|---|
| Sex (male/female) | 148/63 | 96/44 | 52/19 | 0.527 |
| Age (years) | 70.6 ± 9.1 | 71.0 ± 8.9 | 69.8 ± 9.4 | 0.377 |
| Etiology (HBV/HCV/others) | 28/131/52 | 21/97/22 | 7/34/30 | <0.001 |
| BMI (kg/m2) | 23.1 ± 3.1 | 22.5 ± 2.8 | 24.3 ± 3.4 | <0.001 |
| Child-Pugh score (5/6/7/8/9/10) | 137/44/18/8/2/2 | 87/29/14/7/1/2 | 50/15/4/1/10 | 0.547 |
| ALB (g/dL) | 3.8 ± 0.5 | 3.7 ± 0.5 | 3.9 ± 0.5 | 0.028 |
| ALT (IU/L) | 40.4 ± 29.9 | 39.5 ± 24.1 | 42.0 ± 39.1 | 0.577 |
| T-Bil (mg/dL) | 1.0 ± 0.6 | 1.1 ± 0.6 | 0.9 ± 0.4 | 0.022 |
| PLT (×104/μL) | 13.2 ± 6.3 | 12.8 ± 6.0 | 14.1 ± 17.1 | 0.131 |
| PT (%) | 87.1 ± 16.3 | 86.3 ± 15.5 | 88.6 ± 18.0 | 0.338 |
| FPG (mg/dL) | 112.2 ± 34.7 | 100.4 ± 15.8 | 135.5 ± 47.8 | <0.001 |
| FIRI (μU/mL) | 12.1 ± 16.1 | 11. 2 ± 9.7 | 14.0 ± 24.2 | 0.243 |
| HbA1c (%) | 6.1 ± 1.2 | 5.6 ± 0.7 | 7.0 ± 1.4 | <0.001 |
| HOMA-IR | 3.5 ± 6.5 | 2.9 ± 3.0 | 4.8 ± 10.3 | 0.047 |
| TG (mg/dL) | 102.6 ± 58.0 | 93.6 ± 40.4 | 120.2 ± 79.6 | 0.003 |
| AFP (ng/dL) | 552 ± 2440 | 626 ± 2376 | 409 ± 2573 | 0.542 |
| PIVKA-II (mAU/mL) | 6440 ± 46 580 | 2482 ± 17 335 | 14 244 ± 76 284 | 0.085 |
| Stage (I/II/III/IV) | 76/93/38/4 | 52/59/26/3 | 24/34/12/1 | 0.911 |
| Initial treatment (resection/RFA) | 99/112 | 63/77 | 36/35 | 0.523 |
| Co-existing diseases (yes/no) | ||||
| Renal disease | 11/200 | 6/134 | 5/66 | 0.513 |
| Heart disease | 27/184 | 14/126 | 13/58 | 0.125 |
| Neurologic disease | 12/199 | 6/134 | 6/65 | 0.224 |
| Hypertension | 86/125 | 42/98 | 44/27 | <0.001 |
| Hyperlipidemia | 17/194 | 6/134 | 11/60 | 0.007 |
| NAFLD/NASH | 16/195 | 3/137 | 13/58 | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Sex (male vs. female) | 1.221 (0.829–1.798) | 0.311 | ||
| Age (years) | 0.993 (0.973–1.014) | 0.525 | ||
| BMI (kg/m2) | 1.061 (1.000–1.125) | 0.049 | 1.047 (0.986–1.111) | 0.134 |
| Child (A vs. B/C) | 1.032 (0.609–1.746) | 0.908 | ||
| Albumin (g/dL) | 0.939 (0.647–1.361) | 0.738 | ||
| PLT (×104/mL) | 0.988 (0.959–1.018) | 0.434 | ||
| HOMA-IR (≥2.3 vs. <2.3) | 1.565 (1.095–2.239) | 0.014 | 1.485 (1.030–2.139) | 0.034 |
| AFP (ng/dL) | 1.000 (0.999–1.000) | 0.503 | ||
| PIVKA-II (mAU/mL) | 1.000 (1.000–1.000) | 0.168 | ||
| Stage (I vs. II/III/IV) | 0.913 (0.632–1.320) | 0.628 | ||
| Initial treatment (RFA vs. resection) | 1.103 (0.771-1.577) | 0.592 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, K.; Takai, K.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Homeostatic Model Assessment of Insulin Resistance for Predicting the Recurrence of Hepatocellular Carcinoma after Curative Treatment. Int. J. Mol. Sci. 2019, 20, 605. https://doi.org/10.3390/ijms20030605
Imai K, Takai K, Hanai T, Suetsugu A, Shiraki M, Shimizu M. Homeostatic Model Assessment of Insulin Resistance for Predicting the Recurrence of Hepatocellular Carcinoma after Curative Treatment. International Journal of Molecular Sciences. 2019; 20(3):605. https://doi.org/10.3390/ijms20030605
Chicago/Turabian StyleImai, Kenji, Koji Takai, Tatsunori Hanai, Atsushi Suetsugu, Makoto Shiraki, and Masahito Shimizu. 2019. "Homeostatic Model Assessment of Insulin Resistance for Predicting the Recurrence of Hepatocellular Carcinoma after Curative Treatment" International Journal of Molecular Sciences 20, no. 3: 605. https://doi.org/10.3390/ijms20030605
APA StyleImai, K., Takai, K., Hanai, T., Suetsugu, A., Shiraki, M., & Shimizu, M. (2019). Homeostatic Model Assessment of Insulin Resistance for Predicting the Recurrence of Hepatocellular Carcinoma after Curative Treatment. International Journal of Molecular Sciences, 20(3), 605. https://doi.org/10.3390/ijms20030605





