New Insight on Water Status in Germinating Brassica napus Seeds in Relation to Priming-Improved Germination
Abstract
1. Introduction
2. Results
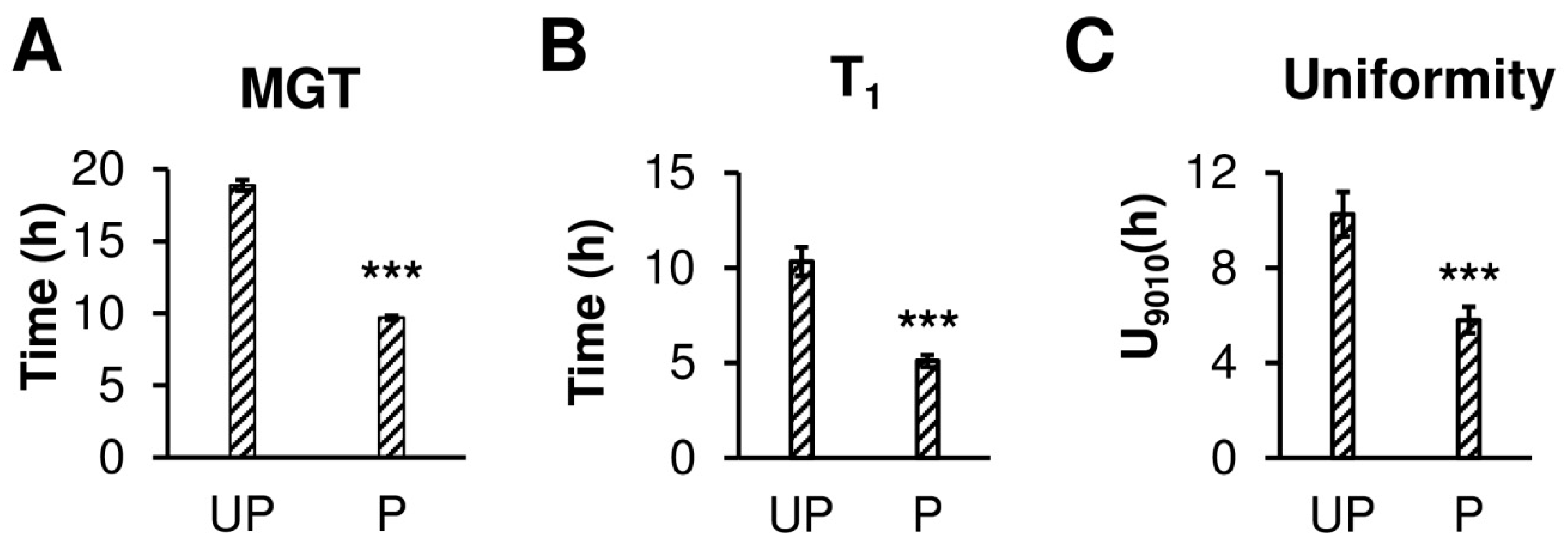
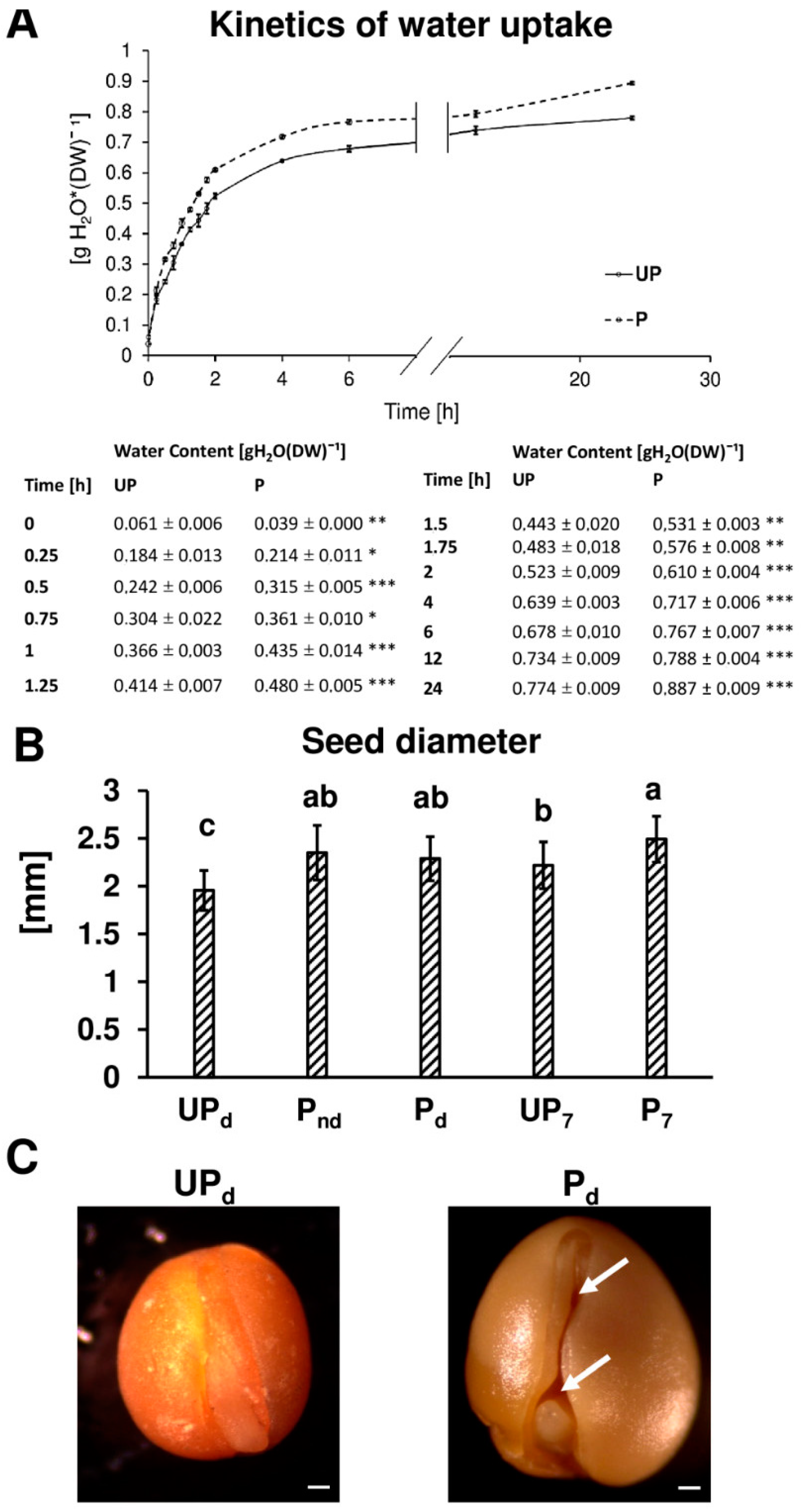
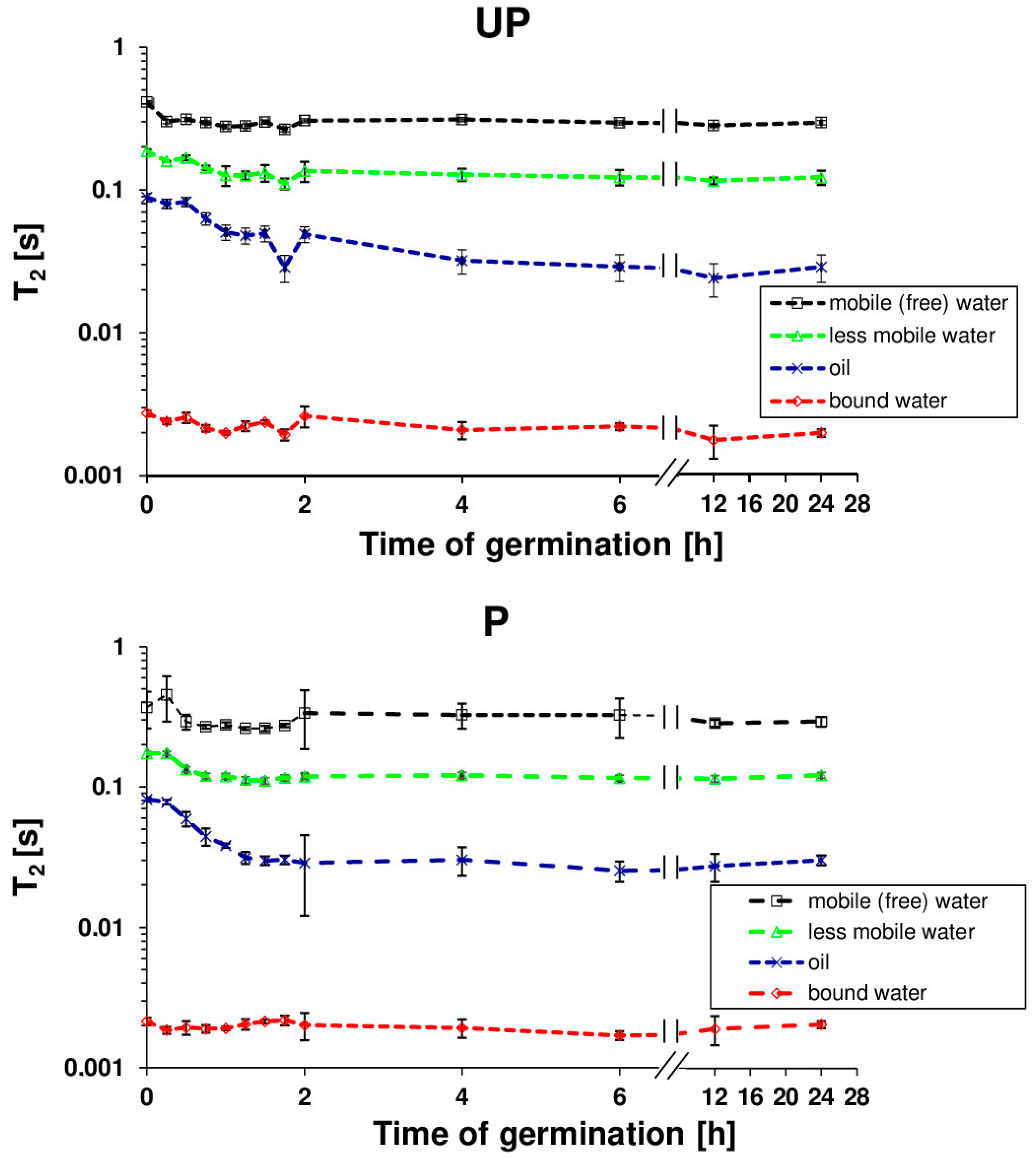
2.1. Osmopriming Increases Seed Germination Performance and Water Uptake during Germination
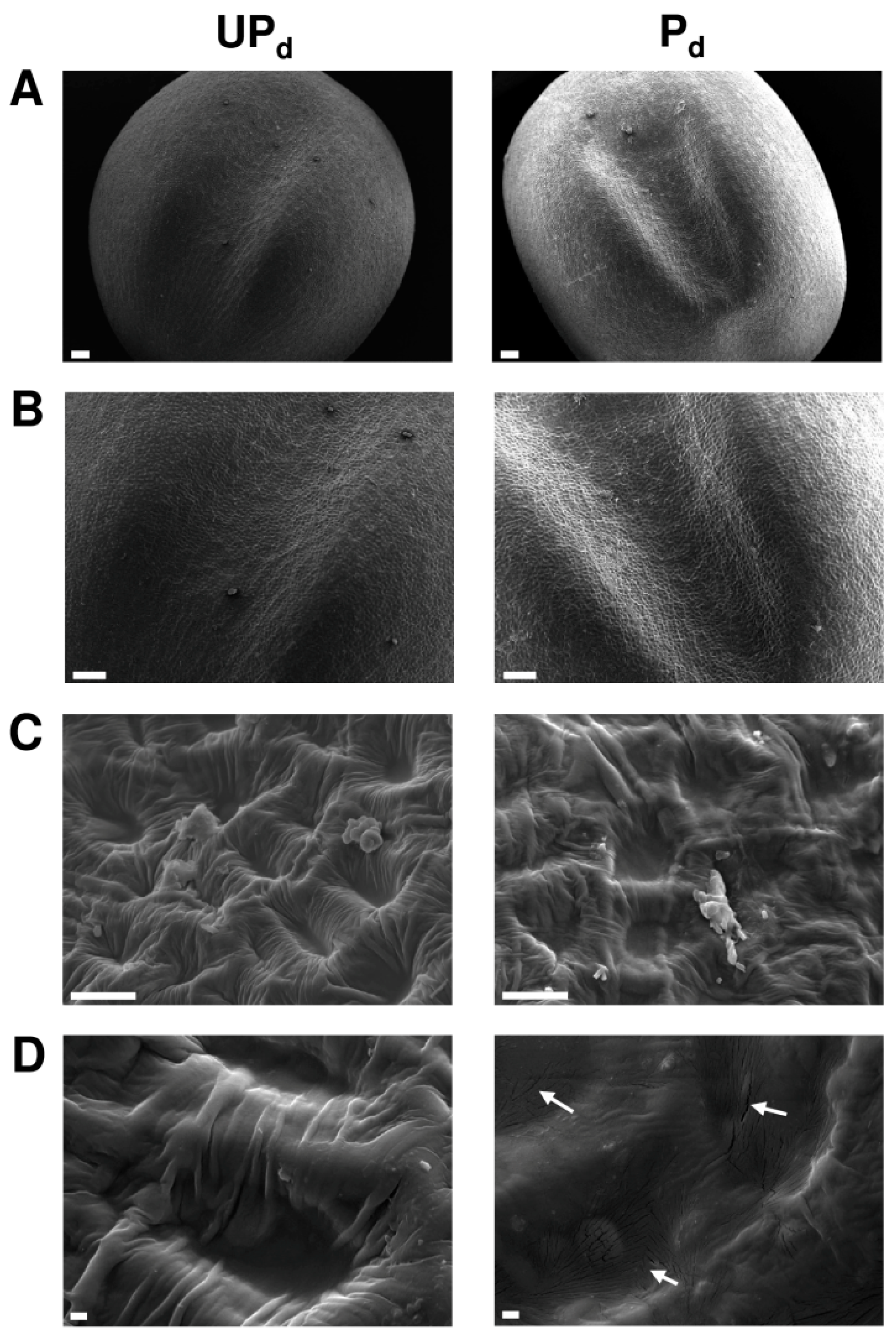
2.2. Osmopriming Changes the Microstructural Features of the Seed Coat
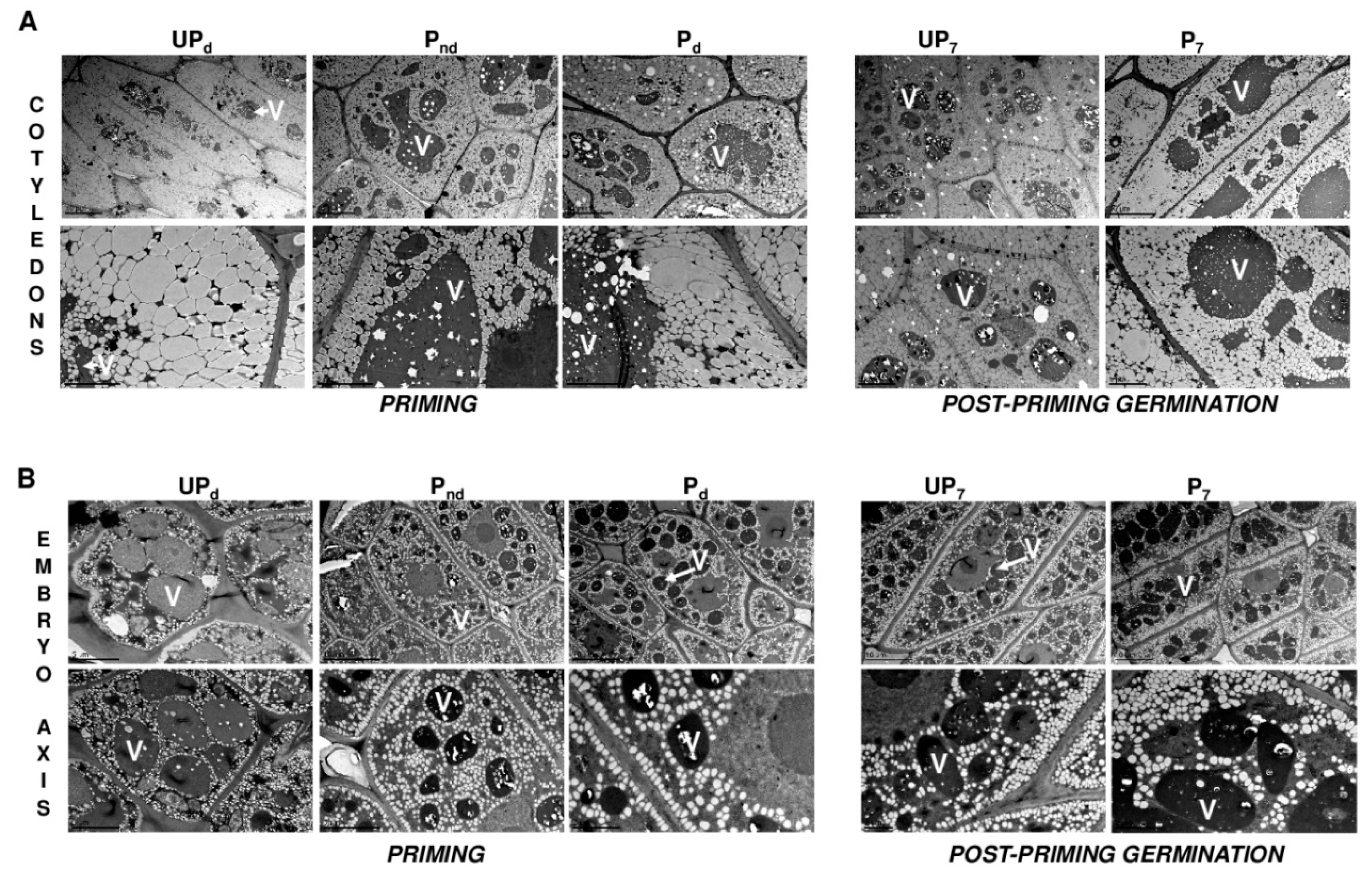
2.3. Osmopriming Caused Additional Void Spaces in Seeds
2.4. Osmopriming Increases the Vacuolization Level of Cotyledon Cells
2.5. Osmopriming Alters the Expression Pattern of Aquaporin Genes
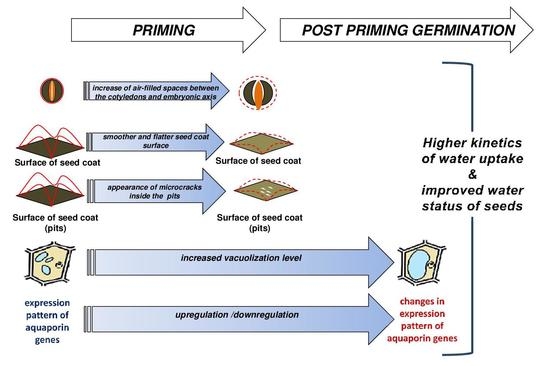
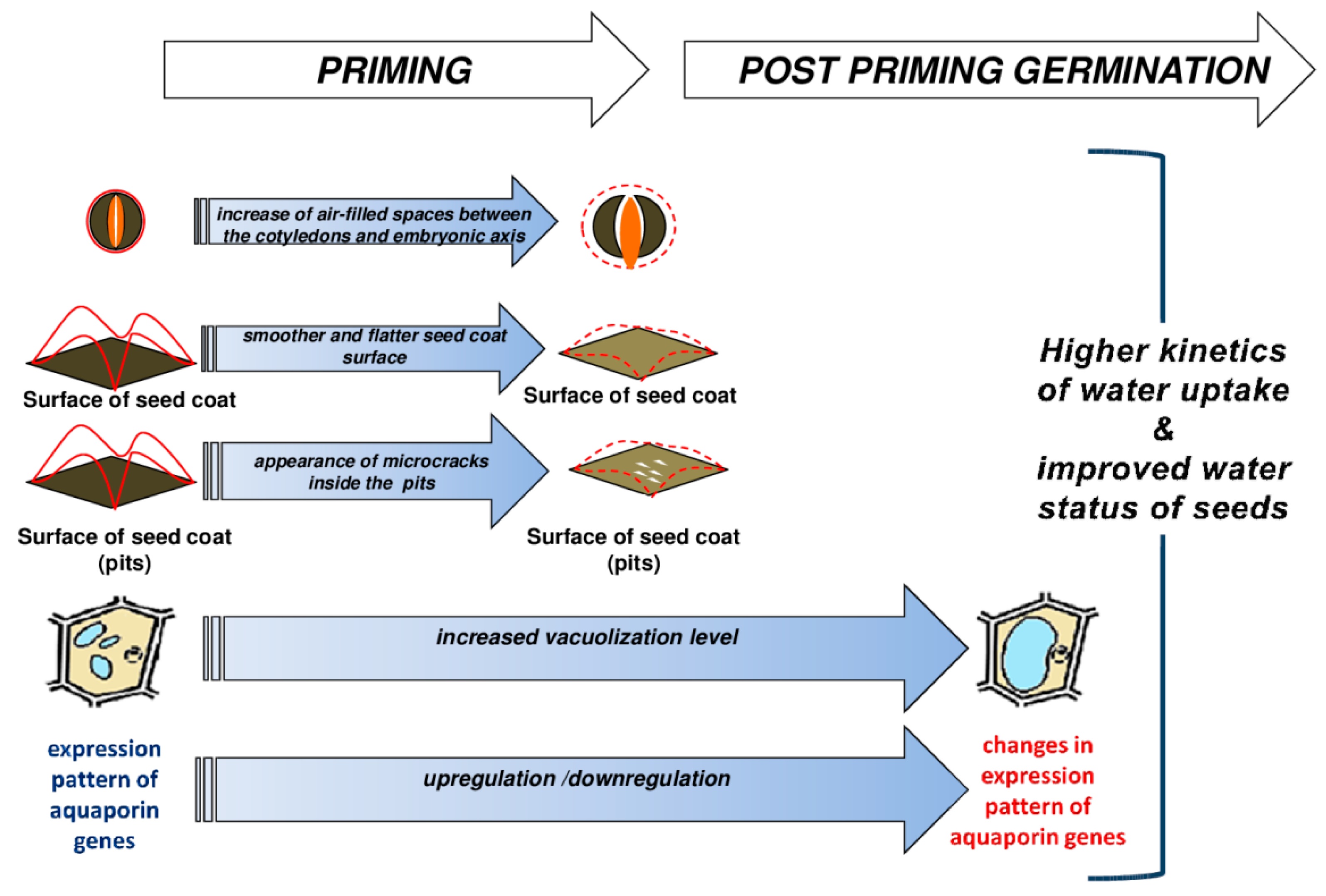
3. Discussion
4. Materials and Methods
4.1. Seed Osmopriming Treatment, Germination Conditions, and Experimental Arrangement
4.2. The Kinetics of Water Uptake by Germinating Seeds
4.3. Seed Diameter Measurement
4.4. Nuclear Magnetic Resonance Analysis
4.5. Scanning Electron Microscopy
4.6. Transmission Electron Microscope
4.7. Expression Levels of Aquaporin’s mRNAs: RNA Isolation, cDNA Synthesis and Semi-q PCR Conditions
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rakow, G. Species Origin and Economic Importance of Brassica. In Brassica; Pua, E.-C., Douglas, C.J., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2004; pp. 3–11. ISBN 978-3-662-06164-0. [Google Scholar]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer-Verlag: New York, NY, USA, 2013; ISBN 978-1-4614-4692-7. [Google Scholar]
- Copeland, L.O.; McDonald, M.F. Principles of Seed Science and Technology; Springer Science & Business Media: New York, NY, USA, 2012; ISBN 978-1-4615-1619-4. [Google Scholar]
- Souza, F.H.D.D.; Marcos-Filho, J. The seed coat as a modulator of seed-environment relationships in Fabaceae. Rev. Bras. Botânica 2001, 24, 365–375. [Google Scholar] [CrossRef]
- Moïse, J.A.; Han, S.; Gudynaitę-Savitch, L.; Johnson, D.A.; Miki, B.L.A. Seed coats: Structure, development, composition, and biotechnology. Vitro Cell. Dev. Biol. Plant 2005, 41, 620–644. [Google Scholar] [CrossRef]
- Radchuk, V.; Borisjuk, L. Physical, metabolic and developmental functions of the seed coat. Front. Plant Sci. 2014, 5, 510. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2017, 68, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Cholewa, E.; Mohamed, T.; Peterson, C.A.; Gijzen, M. Cracks in the palisade cuticle of soybean seed coats correlate with their permeability to water. Ann. Bot. 2004, 94, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Kennedy, J.A.; Reed, B.M. Seed-coat anatomy and proanthocyanidins contribute to the dormancy of Rubus seed. Sci. Hortic. 2011, 130, 762–768. [Google Scholar] [CrossRef]
- Woodstock, L.W. Seed imbibition: A critical period for successful germination. J. Seed Technol. 1988, 12, 1–15. [Google Scholar]
- Heil, J.R.; McCarthy, M.J.; Ozilgen, M. Magnetic resonance imaging and modeling of water up-take into dry beans. Lebensm. Wiss. Technol. Food Sci. Technol. 1992, 25, 280–285. [Google Scholar]
- Pietrzak, L.N.; Frégeau-Reid, J.; Chatson, B.; Blackwell, B. Observations on water distribution in soybean seed during hydration processes using nuclear magnetic resonance imaging. Can. J. Plant Sci. 2002, 82, 513–519. [Google Scholar] [CrossRef]
- Manz, B.; Müller, K.; Kucera, B.; Volke, F.; Leubner-Metzger, G. Water Uptake and Distribution in Germinating Tobacco Seeds Investigated in Vivo by Nuclear Magnetic Resonance Imaging. Plant Physiol. 2005, 138, 1538. [Google Scholar] [CrossRef]
- Kikuchi, K.; Koizumi, M.; Ishida, N.; Kano, H. Water Uptake by Dry Beans Observed by Micro-magnetic Resonance Imaging. Ann. Bot. 2006, 98, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, Ł.; Garnczarska, M.; Zalewski, T.; Bednarski, W.; Ratajczak, L.; Jurga, S. A comparative study of water distribution, free radical production and activation of antioxidative metabolism in germinating pea seeds. J. Plant Physiol. 2006, 163, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Garnczarska, M.; Zalewski, T.; Kempka, M. Water uptake and distribution in germinating lupine seeds studied by magnetic resonance imaging and NMR spectroscopy. Physiol. Plant. 2007, 130, 23–32. [Google Scholar] [CrossRef]
- Koizumi, M.; Kano, H. Lens: Water channel for dry broad bean seeds at germination observed by micro-magnetic resonance imaging. Am. J. Biol. Life Sci. 2014, 2, 37. [Google Scholar]
- Munz, E.; Rolletschek, H.; Oeltze-Jafra, S.; Fuchs, J.; Guendel, A.; Neuberger, T.; Ortleb, S.; Jakob, P.M.; Borisjuk, L. A functional imaging study of germinating oilseed rape seed. New Phytol. 2017, 216, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Obroucheva, N.V.; Sinkevich, I.A.; Lityagina, S.V.; Novikova, G.V. Water relations in germinating seeds. Russ. J. Plant Physiol. 2017, 64, 625–633. [Google Scholar] [CrossRef]
- De, D. Plant Cell Vacuoles: An Introduction; Csiro Publishing: Clayton North, Australia, 2000; ISBN 978-0-643-09944-9. [Google Scholar]
- Sliwinska, E.; Bassel, G.W.; Bewley, J.D. Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J. Exp. Bot. 2009, 60, 3587–3594. [Google Scholar] [CrossRef] [PubMed]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Oracz, K.; Voegele, A.; Tarkowská, D.; Jacquemoud, D.; Turečková, V.; Urbanová, T.; Strnad, M.; Sliwinska, E.; Leubner-Metzger, G. Myrigalone A Inhibits Lepidium sativum Seed Germination by Interference with Gibberellin Metabolism and Apoplastic Superoxide Production Required for Embryo Extension Growth and Endosperm Rupture. Plant Cell Physiol. 2012, 53, 81–95. [Google Scholar] [CrossRef]
- Schuurmans, J.A.M.J.; van Dongen, J.T.; Rutjens, B.P.W.; Boonman, A.; Pieterse, C.M.J.; Borstlap, A.C. Members of the aquaporin family in the developing pea seed coat include representatives of the PIP, TIP, and NIP subfamilies. Plant Mol. Biol. 2003, 53, 655–667. [Google Scholar] [CrossRef]
- Vander Willigen, C.; Postaire, O.; Tournaire-Roux, C.; Boursiac, Y.; Maurel, C. Expression and Inhibition of Aquaporins in Germinating Arabidopsis Seeds. Plant Cell Physiol. 2006, 47, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Yu, X.; Cui, D.-Y.; Sun, M.-H.; Sun, W.-N.; Tang, Z.-C.; Kwak, S.-S.; Su, W.-A. The role of water channel proteins and nitric oxide signaling in rice seed germination. Cell Res. 2007, 17, 638. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fukumoto, T.; Matsumoto, T.; Gena, P.; Frascaria, D.; Kaneko, T.; Katsuhara, M.; Zhong, S.; Sun, X.; Zhu, Y.; et al. Aquaporin OsPIP1;1 promotes rice salt resistance and seed germination. Plant Physiol. Biochem. 2013, 63, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.W.; Tao, P.; Zhang, Y.; Wang, J.B. Characterization of AQP gene expressions in Brassica napus during seed germination and in response to abiotic stresses. Biol. Plant. 2014, 58, 274–282. [Google Scholar] [CrossRef]
- Novikova, G.V.; Tournaire-Roux, C.; Sinkevich, I.A.; Lityagina, S.V.; Maurel, C.; Obroucheva, N. Vacuolar biogenesis and aquaporin expression at early germination of broad bean seeds. Plant Physiol. Biochem. 2014, 82, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; Araujo, S., Balestrazzi, A., Eds.; InTech: Daimlerstraße, Germany, 2016; ISBN 978-953-51-2658-4. [Google Scholar]
- Chaumont, F.; Barrieu, F.; Wojcik, E.; Chrispeels, M.J.; Jung, R. Aquaporins Constitute a Large and Highly Divergent Protein Family in Maize. Plant Physiol. 2001, 125, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Yaneff, A.; Vitali, V.; Amodeo, G. PIP1 aquaporins: Intrinsic water channels or PIP2 aquaporin modulators? FEBS Lett. 2015, 589, 3508–3515. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, Ł.; Lechowska, K.; Kubala, Sz.; Garnczarska, M. Molecular processes induced in primed seedsߞrising the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 2016, 203, 116–126. [Google Scholar] [CrossRef]
- Kubala, S.; Garnczarska, M.; Wojtyla, Ł.; Clippe, A.; Kosmala, A.; Żmieńko, A.; Lutts, S.; Quinet, M. Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Sci. 2015, 231, 94–113. [Google Scholar] [CrossRef]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. J. Plant Physiol. 2015, 183, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yağmur, M.; Kaydan, D. Alleviation of osmotic stress of water and salt in germination and seedling growth of triticale with seed priming treatments. Afr. J. Biotechnol. 2008, 7, 2156–2162. [Google Scholar]
- Galhaut, L.; de Lespinay, A.; Walker, D.J.; Bernal, M.P.; Correal, E.; Lutts, S. Seed Priming of Trifolium repens L. Improved Germination and Early Seedling Growth on Heavy Metal-Contaminated Soil. Water. Air. Soil Pollut. 2014, 225, 1905. [Google Scholar] [CrossRef]
- Gao, Y.-P.; Young, L.; Bonham-Smith, P.; Gusta, L.V. Characterization and expression of plasma and tonoplast membrane aquaporins in primed seed of Brassica napus during germination under stress conditions. Plant Mol. Biol. 1999, 40, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Fessehaie, A.; Arora, R. Aquaporin expression during seed osmopriming and post-priming germination in spinach. Biol. Plant. 2013, 57, 193–198. [Google Scholar] [CrossRef]
- Murley, M.R. Seeds of the Cruciferae of Northeastern North America. Am. Midl. Nat. 1951, 46, 1–81. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, J.; Liu, A.; Wu, X. Seed Coat Microsculpturing Changes during Seed Development in Diploid and Amphidiploid Brassica Species. Ann. Bot. 2004, 93, 555–566. [Google Scholar] [CrossRef]
- Bove, J.; Jullien, M.; Grappin, P. Functional genomics in the study of seed germination. Genome Biol. 2001, 3, reviews1002.1. [Google Scholar] [CrossRef]
- Obroucheva, N.V. Hydration up to Threshold Levels as the Triggering Agent of the Processes Preparing Germination in Quiescent Seeds. In Basic and Applied Aspects of Seed Biology: Proceedings of the Fifth International Workshop on Seeds, Reading, 1995; Ellis, R.H., Black, M., Murdoch, A.J., Hong, T.D., Eds.; Current Plant Science and Biotechnology in Agriculture; Springer: Dordrecht, The Netherlands, 1997; pp. 555–562. ISBN 978-94-011-5716-2. [Google Scholar]
- Soeda, Y.; Konings, M.C.J.M.; Vorst, O.; van Houwelingen, A.M.M.L.; Stoopen, G.M.; Maliepaard, C.A.; Kodde, J.; Bino, R.J.; Groot, S.P.C.; van der Geest, A.H.M. Gene Expression Programs during Brassica oleracea Seed Maturation, Osmopriming, and Germination Are Indicators of Progression of the Germination Process and the Stress Tolerance Level. Plant Physiol. 2005, 137, 354. [Google Scholar] [CrossRef]
- Yacoubi, R.; Job, C.; Belghazi, M.; Chaibi, W.; Job, D. Toward Characterizing Seed Vigor in Alfalfa Through Proteomic Analysis of Germination and Priming. J. Proteome Res. 2011, 10, 3891–3903. [Google Scholar] [CrossRef]
- Nagarajan, S.; Pandita, V.K.; Joshi, D.K.; Sinha, J.P.; Modi, B.S. Characterization of water status in primed seeds of tomato (Lycopersicon esculentum Mill.) by sorption properties and NMR relaxation times. Seed Sci. Res. 2005, 15, 99–111. [Google Scholar] [CrossRef]
- Parera, C.A.; Cantliffe, D.J. Improved Germination and Modified Imbibition of shrunken-2 Sweet Corn by Seed Disinfection and Solid Matrix Priming. J. Am. Soc. Hortic. Sci. 1991, 116, 942–945. [Google Scholar]
- Osborne, D.J.; Boubriak, I.; Leprince, O. Rehydration of dried systems: Membranes and the nuclear genome. In Desiccation and Survival in Plants: Drying without Dying; Black, M., Pritchard, H.W., Eds.; CABI: Wallingford, UK, 2002; pp. 343–364. ISBN 978-0-85199-534-2. [Google Scholar]
- Powell, A.A.; Oliveira, M.D.A.; Matthews, S. The Role of Imbibition Damage in Determining the Vigour of White and Coloured Seed Lots of Dwarf French Beans (Phaseolus vulgaris). J. Exp. Bot. 1986, 37, 716–722. [Google Scholar] [CrossRef]
- Chachalis, D.; Smith, M.L. Imbibition behavior of soybean (Glycine max (L.) Merrill) accessions with different testa characteristics. Seed Sci. Technol. 2000, 28, 321–331. [Google Scholar]
- Nakayama, N.; Hashimoto, S.; Shimada, S.; Takahashi, M.; Kim, Y.-H.; Oya, T.; Arihara, J. The Effect of Flooding Stress at the Germination Stage on the Growth of Soybean in Relation to Initial Seed Moisture Content. Jpn. J. Crop Sci. 2004, 73, 323–329. [Google Scholar] [CrossRef]
- Isobe, S.; Ishida, N.; Koizumi, M.; Kano, H.; Hazlewood, C.F. Effect of electric field on physical states of cell-associated water in germinating morning glory seeds observed by 1H-NMR. Biochim. Biophys. Acta 1999, 1426, 17–31. [Google Scholar] [CrossRef]
- Krishnan, P.; Joshi, D.K.; Nagarajan, S.; Moharir, A.V. Characterization of germinating and non-viable soybean seeds by nuclear magnetic resonance (NMR) spectroscopy. Seed Sci. Res. 2004, 14, 355–362. [Google Scholar] [CrossRef]
- Vu, D.T.; Velusamy, V.; Park, E. Structure and chemical composition of wild soybean seed coat related to its permeability. Pak. J. Bot. 2014, 46, 1847–1857. [Google Scholar]
- Wolf, W.J.; Baker, F.L.; Bernard, R.L. Soybean seed-coat structural features: Pits, deposits and cracks. Scan. Electron Microsc. 1981, 3, 531–544. [Google Scholar]
- Liu, Y.; Bino, R.J.; van der Burg, W.J.; Groot, S.P.C.; Hilhorst, H.W.M. Effects of osmotic priming on dormancy and storability of tomato (Lycopersicon esculentum Mill.) seeds. Seed Sci. Res. 1996, 6, 49–55. [Google Scholar] [CrossRef]
- Downie, B.; Gurusinghe, S.; Bradford, K.J. Internal anatomy of individual tomato seeds: Relationship to abscisic acid and germination physiology. Seed Sci. Res. 1999, 9, 117–128. [Google Scholar]
- Pandita, V.K.; Nagara Jan, S.; Sinha, J.P.; Modi, B.S. Physiological and biochemical changes induced by priming in tomato seed and its relation to germination and field emergence characteristics. Indian J. Plant Physiol. 2003, 8, 249–254. [Google Scholar]
- Shijneva, I.A.; Novikova, G.V.; Obroucheva, N.V. Aquaporins of tonoplast and plasmalemma in axial organs of germinating broad bean seeds. Dokl. Biochem. Biophys. 2007, 413, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, J.; Neubohn, B.; Müntz, K. Different functions of vicilin and legumin are reflected in the histopattern of globulin mobilization during germination of vetch (Vicia sativa L.). Planta 2000, 211, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kuraś, M. Activation of rape (Brassica napus L.) embryo during seed germination. V. The first zones of ultrastructural changes and their expansion. Acta Soc. Bot. Pol. 1987, 56, 77–91. [Google Scholar] [CrossRef]
- Bassel, G.W.; Stamm, P.; Mosca, G.; Barbier de Reuille, P.; Gibbs, D.J.; Winter, R.; Janka, A.; Holdsworth, M.J.; Smith, R.S. Mechanical constraints imposed by 3D cellular geometry and arrangement modulate growth patterns in the Arabidopsis embryo. Proc. Natl. Acad. Sci. USA 2014, 111, 8685–8690. [Google Scholar] [CrossRef] [PubMed]
- Bots, M.; Feron, R.; Uehlein, N.; Weterings, K.; Kaldenhoff, R.; Mariani, T. PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. J. Exp. Bot. 2005, 56, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Joosen, R.V.L.; Kodde, J.; Willems, L.A.J.; Ligterink, W.; van der Plas, L.H.W.; Hilhorst, H.W.M. Germinator: A software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J. 2010, 62, 148–159. [Google Scholar] [CrossRef] [PubMed]
- El-Kassaby, Y.A.; Moss, I.; Kolotelo, D.; Stoehr, M. Seed Germination: Mathematical Representation and Parameters Extraction. For. Sci. 2008, 54, 220–227. [Google Scholar]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Provencher, S.W. An eigenfunction expansion method for the analysis of exponential decay curves. J. Chem. Phys. 1976, 64, 2772–2777. [Google Scholar] [CrossRef]
- Provencher, S.W.; Vogel, R.H. Information loss with transform methods in system identification: A new set of transforms with high information content. Math. Biosci. 1980, 50, 251–262. [Google Scholar] [CrossRef]
- Borek, S.; Ratajczak, W.; Ratajczak, L. Ultrastructural and enzymatic research on the role of sucrose in mobilization of storage lipids in germinating yellow lupine seeds. Plant Sci. 2006, 170, 441–452. [Google Scholar] [CrossRef]
- Karnovsky, M. A Formaldehyde-Glutaraldehyde Fixative of High Osmolality for Use in Electron Microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Asif, M.H.; Dhawan, P.; Nath, P. A simple procedure for the isolation of high quality RNA from ripening banana fruit. Plant Mol. Biol. Report. 2000, 18, 109–115. [Google Scholar] [CrossRef]


| Gene | GenBank Accession Number | Forward Primer 5′–3′ | Revers Primer 5′–3′ | Annealing Temperature (°C) |
|---|---|---|---|---|
| PIP1.1 | KF277205.1 | CACTGTTTTGACCGTCATGG | TCCAAGACCACTTCCTTTGG | 55 |
| PIP1.2 | KF277206.1 | CTTGCTTCCTGGTCCTTCTG | GGCTCCACCTCCTAGAGCTT | 55 |
| PIP1.3 | KF277207.1 | CTTTCGGTGGCATGATCTTT | AGCGGAGAAGACGGTGTAGA | 60 |
| PIP1.4 | KF277208.1 | ACATCAGCTCAGTCCGACAA | CCTAGCCAAGAACAGACCGA | 60 |
| PIP2 | AF118383.1 | CGAGTTCGTAGCCACTCTCC | AACCGCTCTAACCAGCGATA | 55 |
| PIP2.2 | KF277209.1 | GTGACGTTCGGCTTGTTCTT | AGTGGCCAAGTGTACCATGA | 55 |
| PIP2.5 | KF277210.1 | CCCTTTACCCTGACCAGTGT | CGGAGAAGACGGTGTAGAC | 60 |
| PIP2.7 | KF277211.1 | CCCTTTACCCTGACCAGTGT | CGGAGAAGACGGTGTAGACT | 55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechowska, K.; Kubala, S.; Wojtyla, Ł.; Nowaczyk, G.; Quinet, M.; Lutts, S.; Garnczarska, M. New Insight on Water Status in Germinating Brassica napus Seeds in Relation to Priming-Improved Germination. Int. J. Mol. Sci. 2019, 20, 540. https://doi.org/10.3390/ijms20030540
Lechowska K, Kubala S, Wojtyla Ł, Nowaczyk G, Quinet M, Lutts S, Garnczarska M. New Insight on Water Status in Germinating Brassica napus Seeds in Relation to Priming-Improved Germination. International Journal of Molecular Sciences. 2019; 20(3):540. https://doi.org/10.3390/ijms20030540
Chicago/Turabian StyleLechowska, Katarzyna, Szymon Kubala, Łukasz Wojtyla, Grzegorz Nowaczyk, Muriel Quinet, Stanley Lutts, and Małgorzata Garnczarska. 2019. "New Insight on Water Status in Germinating Brassica napus Seeds in Relation to Priming-Improved Germination" International Journal of Molecular Sciences 20, no. 3: 540. https://doi.org/10.3390/ijms20030540
APA StyleLechowska, K., Kubala, S., Wojtyla, Ł., Nowaczyk, G., Quinet, M., Lutts, S., & Garnczarska, M. (2019). New Insight on Water Status in Germinating Brassica napus Seeds in Relation to Priming-Improved Germination. International Journal of Molecular Sciences, 20(3), 540. https://doi.org/10.3390/ijms20030540