Localized Delivery of Pilocarpine to Hypofunctional Salivary Glands through Electrospun Nanofiber Mats: An Ex Vivo and In Vivo Study
Abstract
1. Introduction
2. Results
2.1. Fabrication of Electrospun Nanofiber Mats
2.2. Physical and Chemical Characterization of Nanofibers
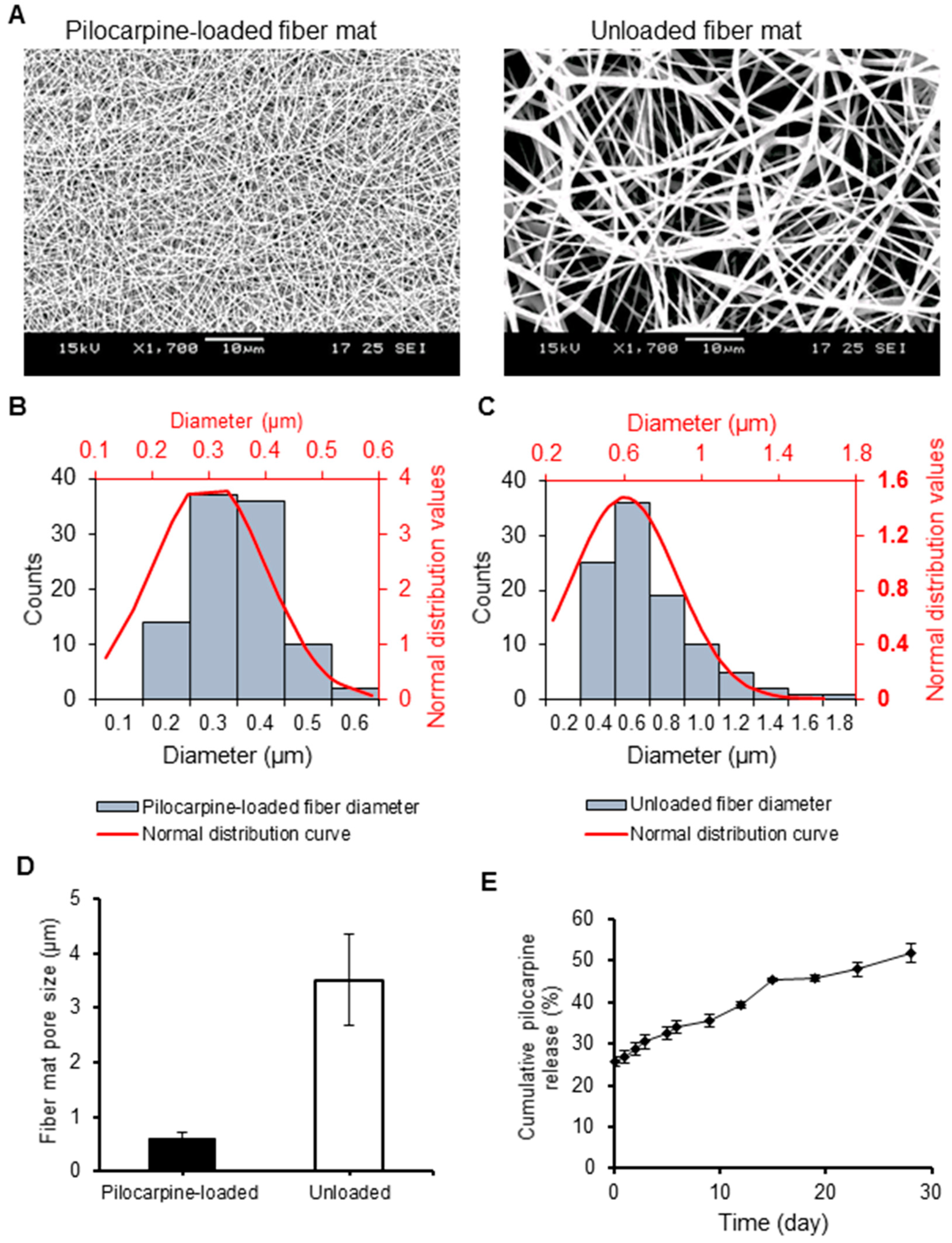
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Drug Loading Capacity, Encapsulation Efficiency, and Degradation
2.3. Short- and Long-Term In Vitro Pilocarpine Release
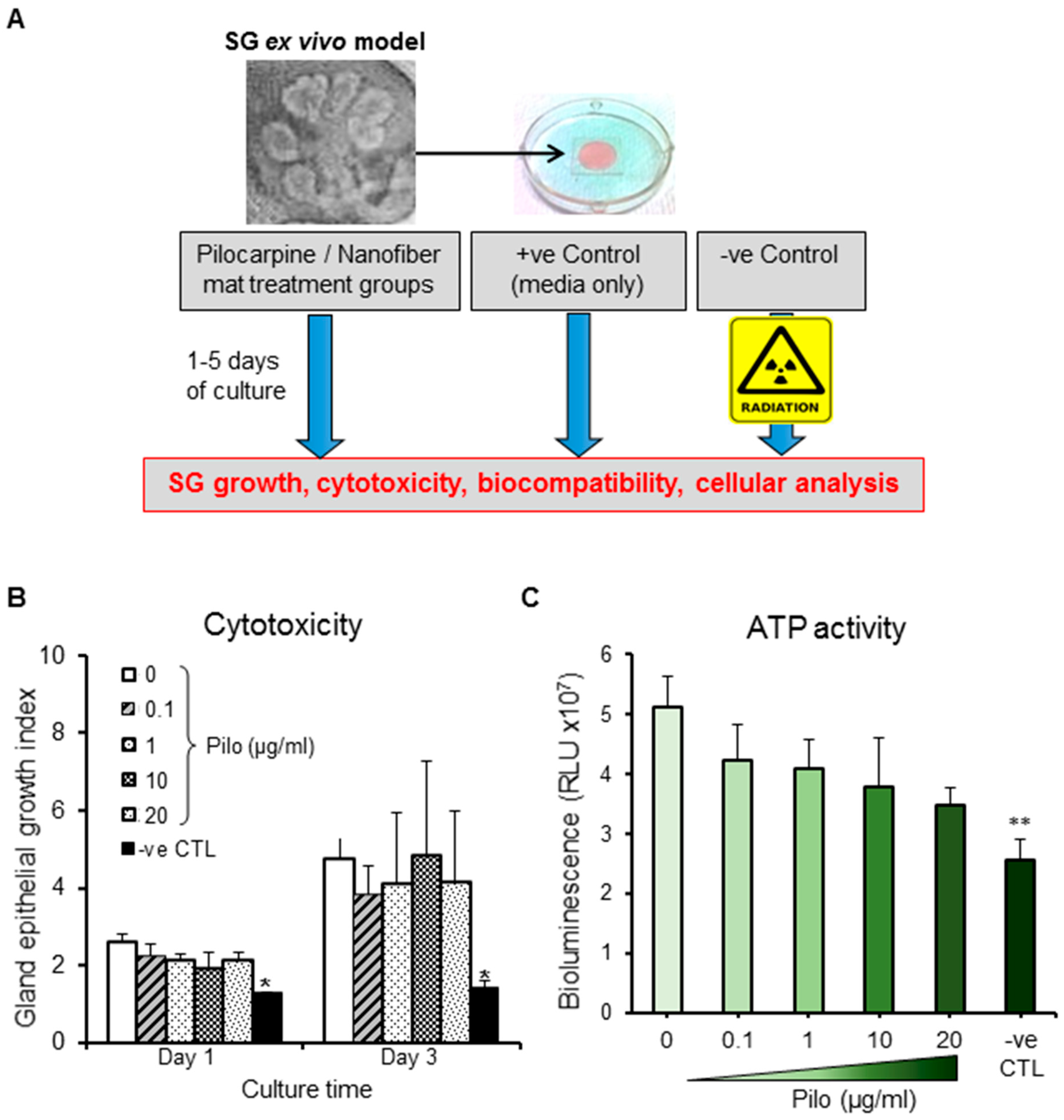
2.4. Ex Vivo Studies in Hypofunctional Salivary Glands
2.4.1. Cytotoxicity and ATP Activity of Pilocarpine
2.4.2. Biocompatibility of Nanofiber Mats (Loaded and Unloaded)
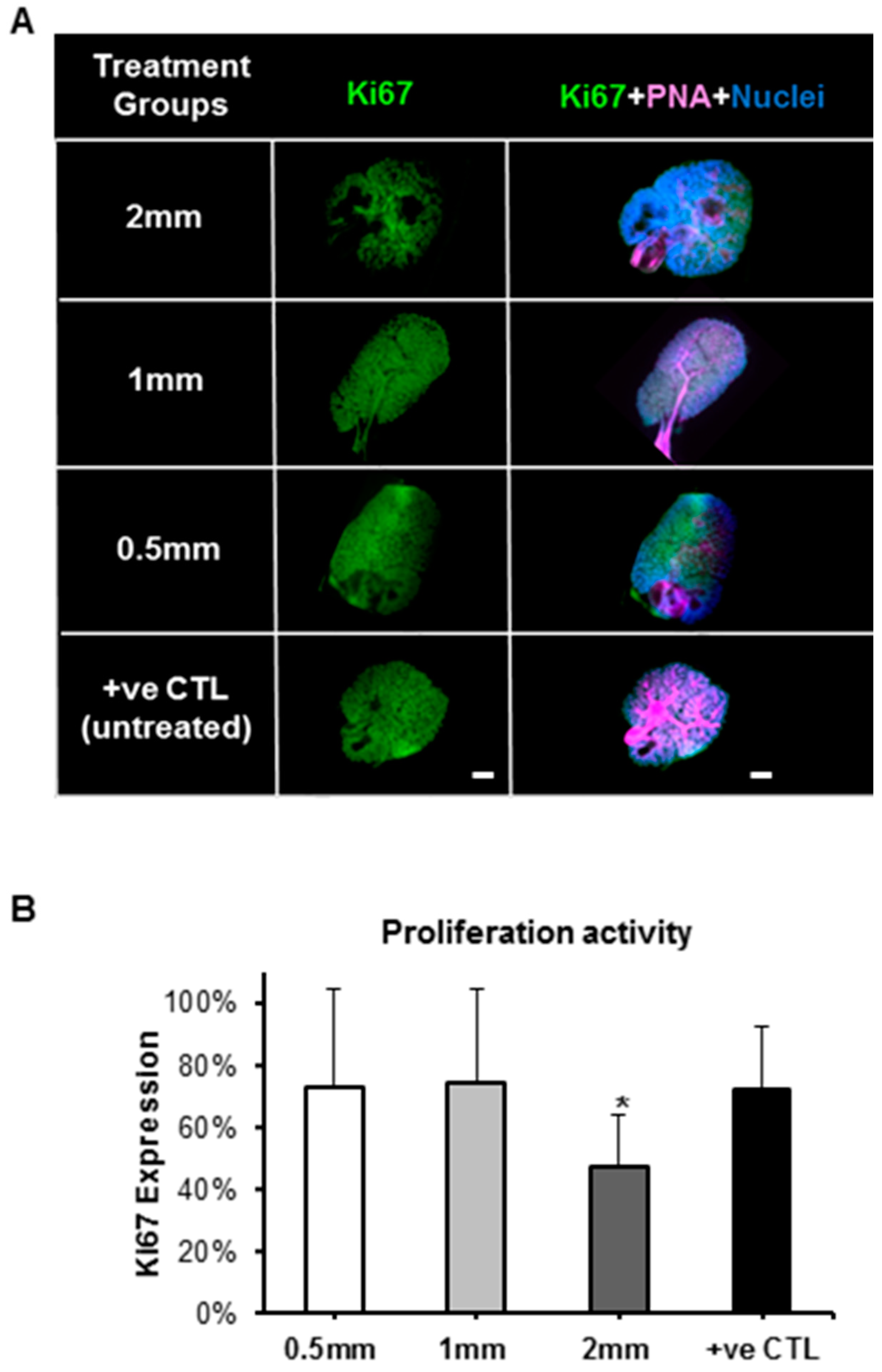
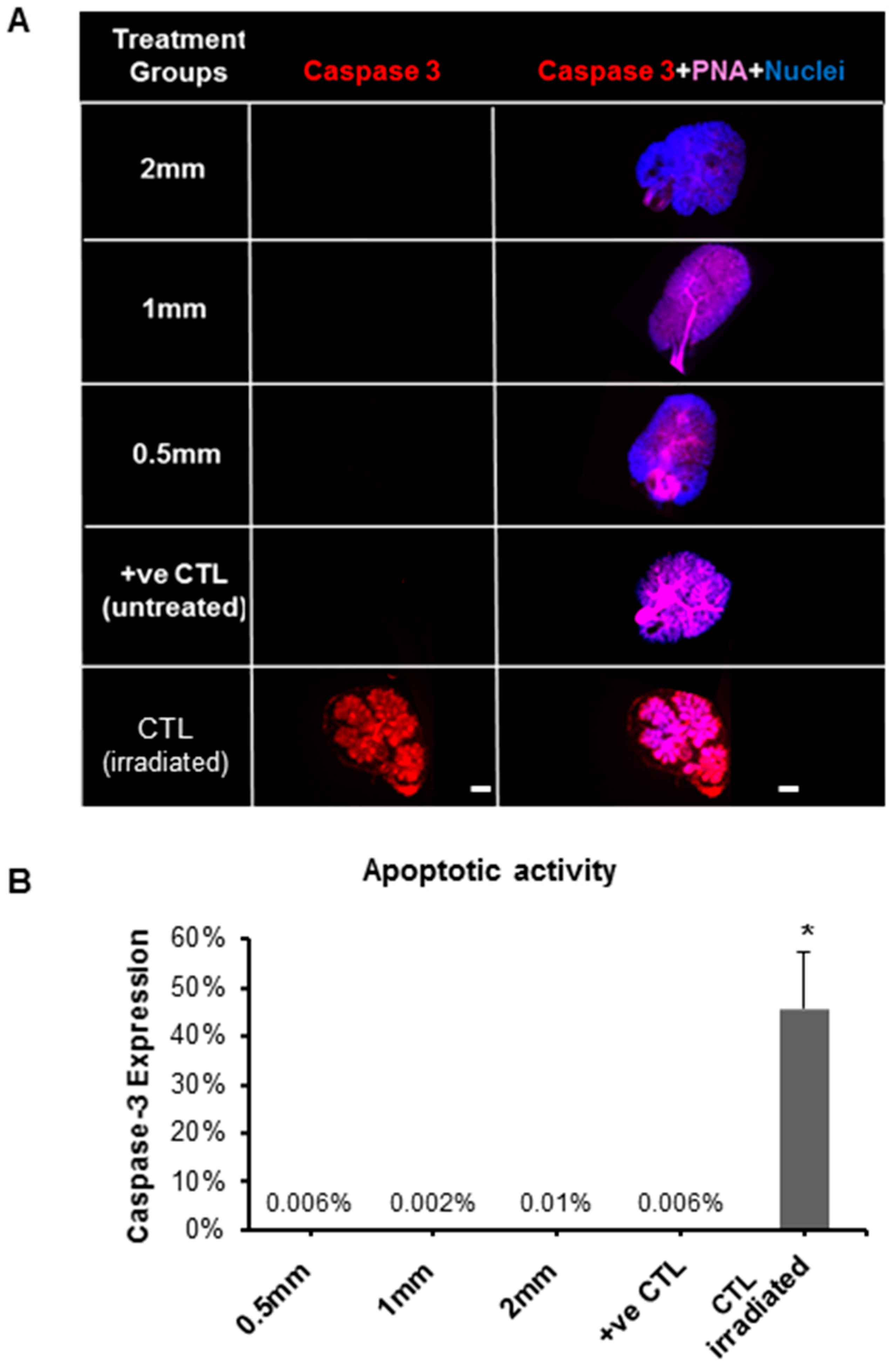
2.4.3. Biological Effects of Pilocarpine-Loaded Nanofiber Mats in SG Cellular Compartments
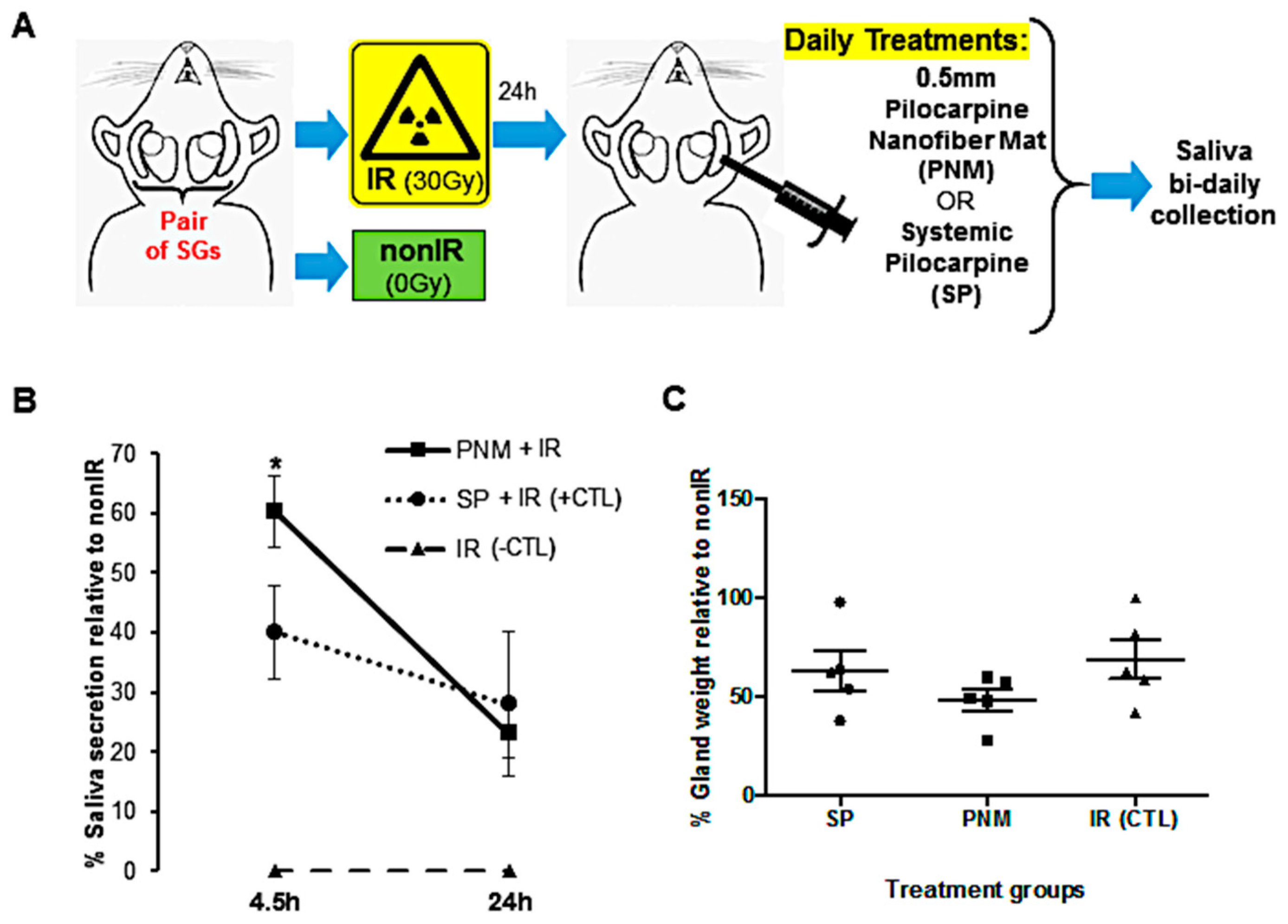
2.5. In Vivo Saliva Secretion after Intradermal Treatment with Pilocarpine-Loaded Nanofiber Mats versus Systemic Pilocarpine
3. Discussion
4. Materials and Methods
4.1. Fabrication of Electrospun Nanofiber Mats
4.2. Physical and Chemical Characterization of Nanofiber Mats
4.2.1. Scanning Electron Microscopy (SEM)
4.2.2. Drug Loading Capacity and Encapsulation Efficiency
4.2.3. Degradation Studies for the Pilocarpine Solution
4.3. Pilocarpine Drug Release Analysis from Loaded Nanofiber Mats
4.4. Ex Vivo SG Organ Culture Studies
4.4.1. SG Organ Culture Model
4.4.2. Cytotoxicity of Pilocarpine
4.4.3. Biocompatibility of Nanofiber Mats
4.4.4. Salivary Gland Cellular Analysis for Proliferation and Apoptotic Activity
4.5. In Vivo SG Hypofunctional Model
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbe, A.G.; Bock, N.; Derman, S.H.; Felsch, M.; Timmermann, L.; Noack, M.J. Self-assessment of oral health, dental health care and oral health-related quality of life among Parkinson’s disease patients. Gerodontology 2017, 34, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Barrios, R.; Sanchez-Lara, I.; Carnero-Pardo, C.; Fornieles-Rubio, F.; Montes, J.; Gonzalez-Moles, M.A.; Bravo, M. Prevalence of Drug-Induced Xerostomia in Older Adults with Cognitive Impairment or Dementia: An Observational Study. Drugs Aging 2016, 33, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Pajukoski, H.; Meurman, J.H.; Halonen, P.; Sulkava, R. Prevalence of subjective dry mouth and burning mouth in hospitalized elderly patients and outpatients in relation to saliva, medication, and systemic diseases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Silvestre, F.J.; Barrios, R.; Silvestre-Rangil, J. Treatment of xerostomia and hyposalivation in the elderly: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e355–e366. [Google Scholar] [CrossRef] [PubMed]
- Napenas, J.J.; Brennan, M.T.; Fox, P.C. Diagnosis and treatment of xerostomia (dry mouth). Odontology 2009, 97, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Noaiseh, G.; Baker, J.F.; Vivino, F.B. Comparison of the discontinuation rates and side-effect profiles of pilocarpine and cevimeline for xerostomia in primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2014, 32, 575–577. [Google Scholar] [PubMed]
- Hendrickson, R.G.; Morocco, A.P.; Greenberg, M.I. Pilocarpine toxicity and the treatment of xerostomia. J. Emerg. Med. 2004, 26, 429–432. [Google Scholar] [CrossRef]
- Davies, A.N.; Thompson, J. Parasympathomimetic drugs for the treatment of salivary gland dysfunction due to radiotherapy. Cochrane Database Syst. Rev. 2015, 10, CD003782. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, H.J.; Choi, J.H.; Jung, D.W.; Kwon, J.S. Effect of 0.1% pilocarpine mouthwash on xerostomia: Double-blind, randomised controlled trial. J. Oral Rehabil. 2014, 41, 226–235. [Google Scholar] [CrossRef]
- Bernardi, R.; Perin, C.; Becker, F.L.; Ramos, G.Z.; Gheno, G.Z.; Lopes, L.R.; Pires, M.; Barros, H.M. Effect of pilocarpine mouthwash on salivary flow. Braz. J. Med. Biol. Res. 2002, 35, 105–110. [Google Scholar] [CrossRef]
- Tanigawa, T.; Yamashita, J.; Sato, T.; Shinohara, A.; Shibata, R.; Ueda, H.; Sasaki, H. Efficacy and safety of pilocarpine mouthwash in elderly patients with xerostomia. Spec. Care Dentist. 2015, 35, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Spivakovsky, S.; Spivakovsky, Y. Parasympathomimetic drugs for dry mouth due to radiotherapy. Evid. Based Dent. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.C.; Baum, B.J. Salivary enhancement: Current status and future therapies. J. Dent. Educ. 2001, 65, 1096–1101. [Google Scholar]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Liu, L.; Lu, Y.; Sun, T.; Shen, C.; Chen, X.; Chen, Q.; An, S.; He, X.; Ruan, C.; et al. T7 Peptide-Functionalized PEG-PLGA Micelles Loaded with Carmustine for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces 2016, 8, 27465–27473. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Fu, Y.; Arifin, D.Y.; Kee, I.; Zheng, L.; Lee, H.S.; Chow, P.K.; Wang, C.H. The use of submicron/nanoscale PLGA implants to deliver paclitaxel with enhanced pharmacokinetics and therapeutic efficacy in intracranial glioblastoma in mice. Biomaterials 2010, 31, 5199–5207. [Google Scholar] [CrossRef]
- Davoodi, P.; Ng, W.C.; Yan, W.C.; Srinivasan, M.P.; Wang, C.H. Double-Walled Microparticles-Embedded Self-Cross-Linked, Injectable, and Antibacterial Hydrogel for Controlled and Sustained Release of Chemotherapeutic Agents. ACS Appl. Mater. Interfaces 2016, 8, 22785–22800. [Google Scholar] [CrossRef]
- Feng, F.; Neoh, K.Y.; Davoodi, P.; Wang, C.H. Coaxial double-walled microspheres for combined release of cytochrome c and doxorubicin. J. Control Release 2017, 259, e30–e31. [Google Scholar] [CrossRef]
- Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Effective Co-delivery of Nutlin-3a and p53 genes via Core-shell Microparticles for Disruption of MDM2-p53 Interaction and Reactivation of p53 in Hepatocellular Carcinoma. J. Mater. Chem. B 2017, 5, 5816–5834. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, P.; Feng, F.; Xu, Q.; Yan, W.C.; Tong, Y.W.; Srinivasan, M.P.; Sharma, V.K.; Wang, C.H. Coaxial electrohydrodynamic atomization: Microparticles for drug delivery applications. J. Control. Release 2015, 205, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tan, R.S.; Wang, C.H. Biodegradable microparticles and fiber fabrics for sustained delivery of cisplatin to treat C6 glioma in vitro. J. Biomed. Mater. Res. A 2008, 85, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.L.; Vidyanand, S.; James, J.; Kumar, G.S.V. Pilocarpine-loaded poly (DL-lactic-co-glycolic acid) nanoparticles as potential candidates for controlled drug delivery with enhanced ocular pharmacological response. J. Appl. Polym. Sci. 2012, 124, 2030–2036. [Google Scholar] [CrossRef]
- Kao, H.J.; Lin, H.R.; Lo, Y.L.; Yu, S.P. Characterization of pilocarpine-loaded chitosan/Carbopol nanoparticles. J. Pharm. Pharmacol. 2006, 58, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.C.; Williams, R.A. Precision emulsification for droplet and capsule production. Adv. Powder Technol. 2014, 25, 122–135. [Google Scholar] [CrossRef]
- Chua, K.N.; Chai, C.; Lee, P.C.; Tang, Y.N.; Ramakrishna, S.; Leong, K.W.; Mao, H.Q. Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials 2006, 27, 6043–6051. [Google Scholar] [CrossRef]
- Blakney, A.K.; Little, A.B.; Jiang, Y.; Woodrow, K.A. In vitro-ex vivo correlations between a cell-laden hydrogel and mucosal tissue for screening composite delivery systems. Drug. Deliv. 2016, 24, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable electrospun fibers for drug delivery. J. Control Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.W.; Jeong, L.; Bae, S.H.; Min, B.C.; Youk, J.H.; Park, W.H. Effect of organosoluble salts on the nanofibrous structure of electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Int. J. Biol. Macromol. 2004, 34, 249–256. [Google Scholar] [CrossRef]
- Han, F.; Zhang, H.; Zhao, J.; Zhao, Y.; Yuan, X. Diverse release behaviors of water-soluble bioactive substances from fibrous membranes prepared by emulsion and suspension electrospinning. J. Biomater. Sci. Polym. Ed. 2013, 24, 1244–1259. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.J.; Schmale, I.L.; Mickelsen, D.; Hansen, M.E.; Newlands, S.D.; Benoit, D.S.W.; Korshuno, V.A.; Ovitt, C.E. Localized Delivery of Amifostine Enhances Salivary Gland Radioprotection. J. Dent. Res. 2018, 97, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Agban, Y.; Lian, J.; Prabakar, S.; Seyfoddin, A.; Rupenthal, I.D. Nanoparticle cross-linked collagen shields for sustained delivery of pilocarpine hydrochloride. Int. J. Pharm. 2016, 501, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Al-Handawi, M.B.; Al-Kaabi, L.; Ali, L.; Salman Ashraf, S.; Thiemann, T.; Al-Hindawi, B.; Meetani, M. Intermolecular interactions between cucurbit [7]uril and pilocarpine. Int. J. Pharm. 2014, 460, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Adine, C.; Ng, K.K.; Rungarunlert, S.; Souza, G.R.; Ferreira, J.N. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials 2018, 180, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.N.; Zheng, C.; Lombaert, I.M.A.; Goldsmith, C.M.; Cotrim, A.P.; Symonds, J.M.; Patel, V.N.; Hoffman, M.P. Neurturin Gene Therapy Protects Parasympathetic Function to Prevent Irradiation-Induced Murine Salivary Gland Hypofunction. Mol. Ther. Methods Clin. Dev. 2018, 23, 172–180. [Google Scholar] [CrossRef]
- Lin, A.L.; Johnson, D.A.; Wu, Y.; Wong, G.; Ebersole, J.L.; Yeh, C.K. Measuring short-term gamma-irradiation effects on mouse salivary gland function using a new saliva collection device. Arch. Oral Biol. 2001, 46, 1085–1089. [Google Scholar] [CrossRef]
- Baum, B.J.; Afione, S.; Chiorini, J.A.; Cotrim, A.P.; Goldsmith, C.M.; Zheng, C. Gene Therapy of Salivary Diseases. Methods Mol. Biol. 2017, 1537, 107–123. [Google Scholar] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthumariappan, S.; Ng, W.C.; Adine, C.; Ng, K.K.; Davoodi, P.; Wang, C.-H.; Ferreira, J.N. Localized Delivery of Pilocarpine to Hypofunctional Salivary Glands through Electrospun Nanofiber Mats: An Ex Vivo and In Vivo Study. Int. J. Mol. Sci. 2019, 20, 541. https://doi.org/10.3390/ijms20030541
Muthumariappan S, Ng WC, Adine C, Ng KK, Davoodi P, Wang C-H, Ferreira JN. Localized Delivery of Pilocarpine to Hypofunctional Salivary Glands through Electrospun Nanofiber Mats: An Ex Vivo and In Vivo Study. International Journal of Molecular Sciences. 2019; 20(3):541. https://doi.org/10.3390/ijms20030541
Chicago/Turabian StyleMuthumariappan, Sujatha, Wei Cheng Ng, Christabella Adine, Kiaw Kiaw Ng, Pooya Davoodi, Chi-Hwa Wang, and Joao N. Ferreira. 2019. "Localized Delivery of Pilocarpine to Hypofunctional Salivary Glands through Electrospun Nanofiber Mats: An Ex Vivo and In Vivo Study" International Journal of Molecular Sciences 20, no. 3: 541. https://doi.org/10.3390/ijms20030541
APA StyleMuthumariappan, S., Ng, W. C., Adine, C., Ng, K. K., Davoodi, P., Wang, C.-H., & Ferreira, J. N. (2019). Localized Delivery of Pilocarpine to Hypofunctional Salivary Glands through Electrospun Nanofiber Mats: An Ex Vivo and In Vivo Study. International Journal of Molecular Sciences, 20(3), 541. https://doi.org/10.3390/ijms20030541







