Sphingosine-1-Phosphate Enhances α1-Adrenergic Vasoconstriction via S1P2–G12/13–ROCK Mediated Signaling
Abstract
1. Introduction
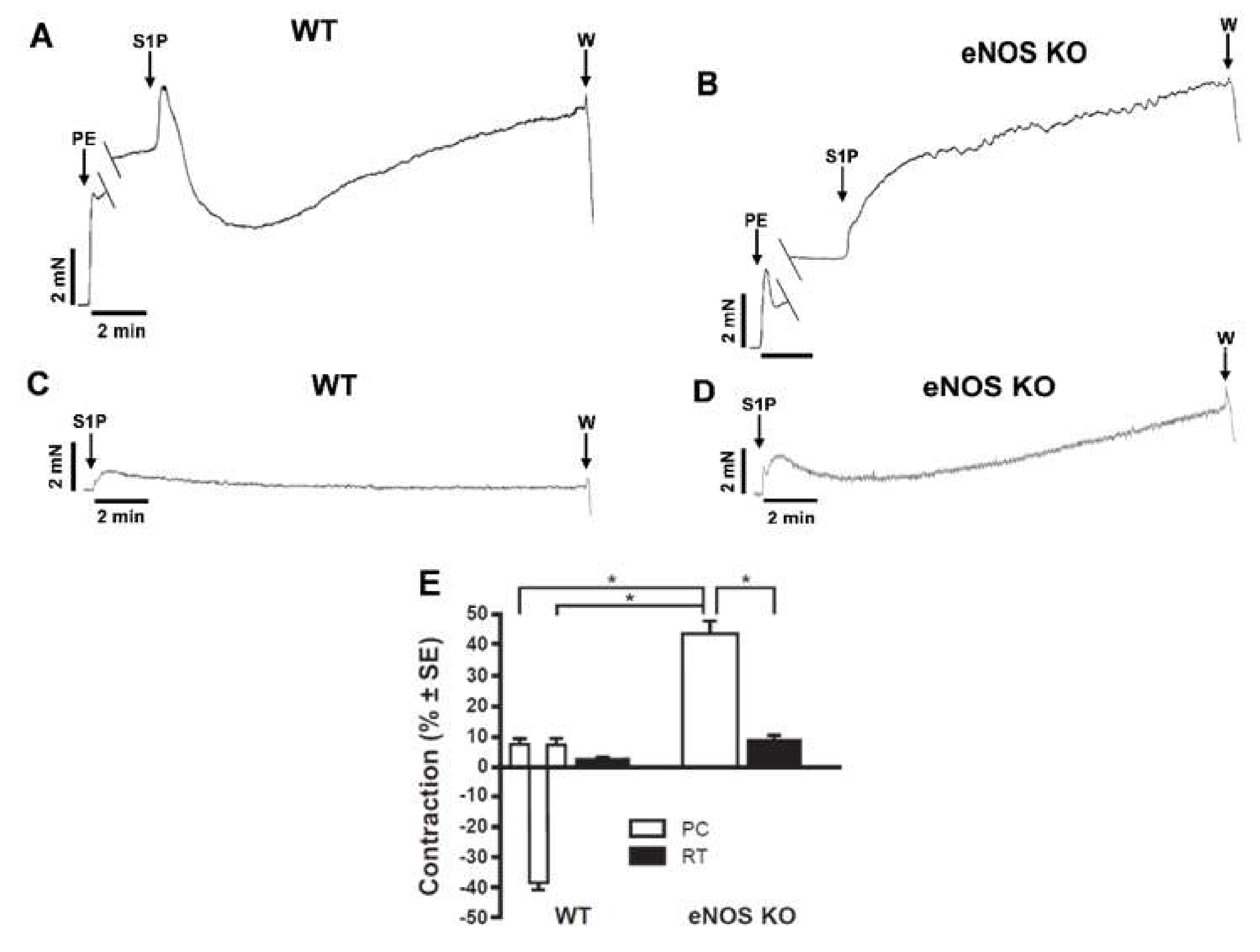
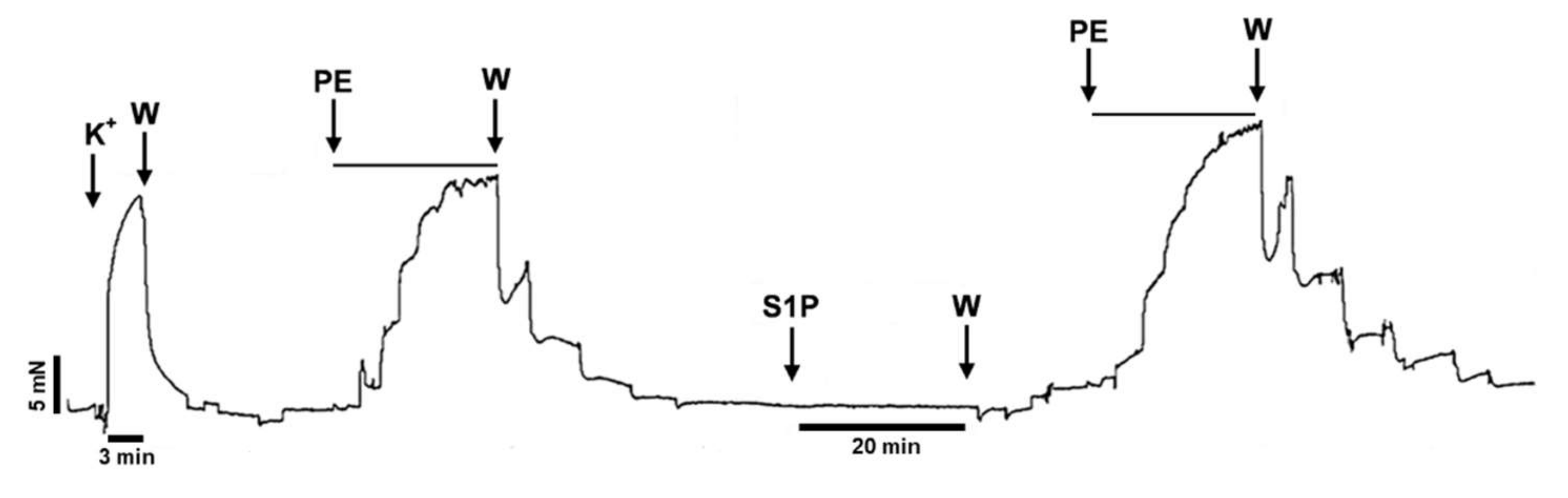
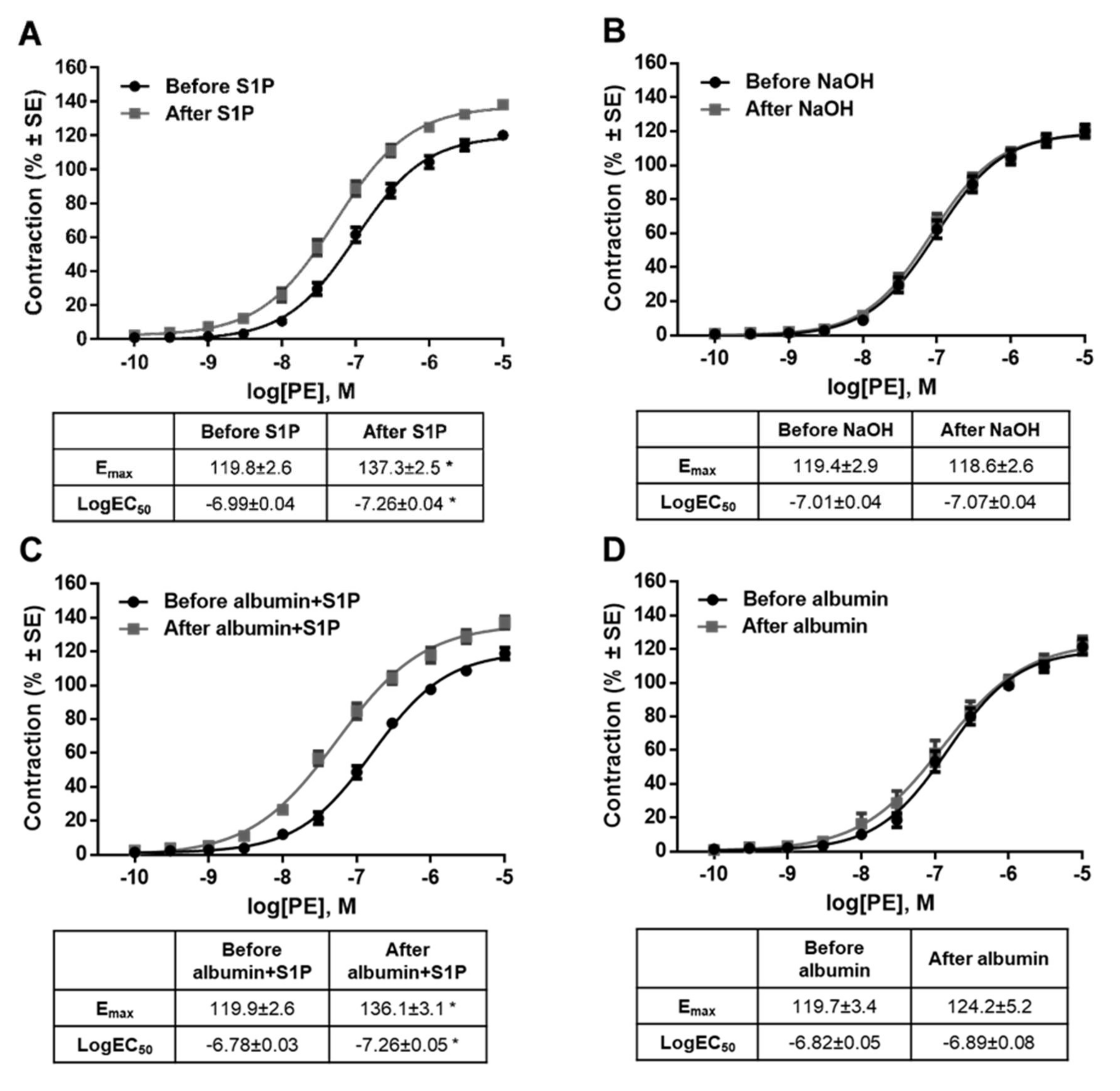
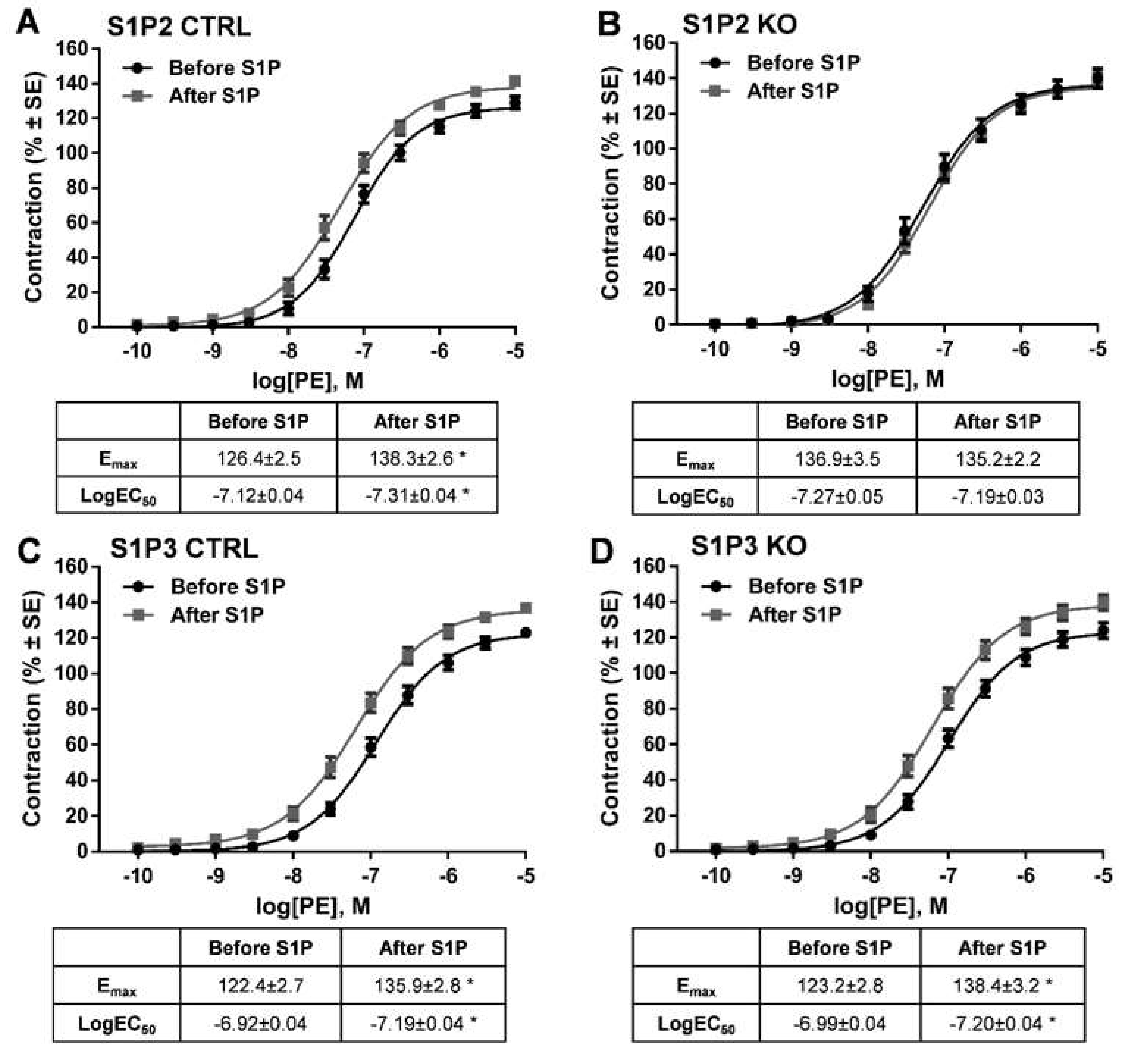
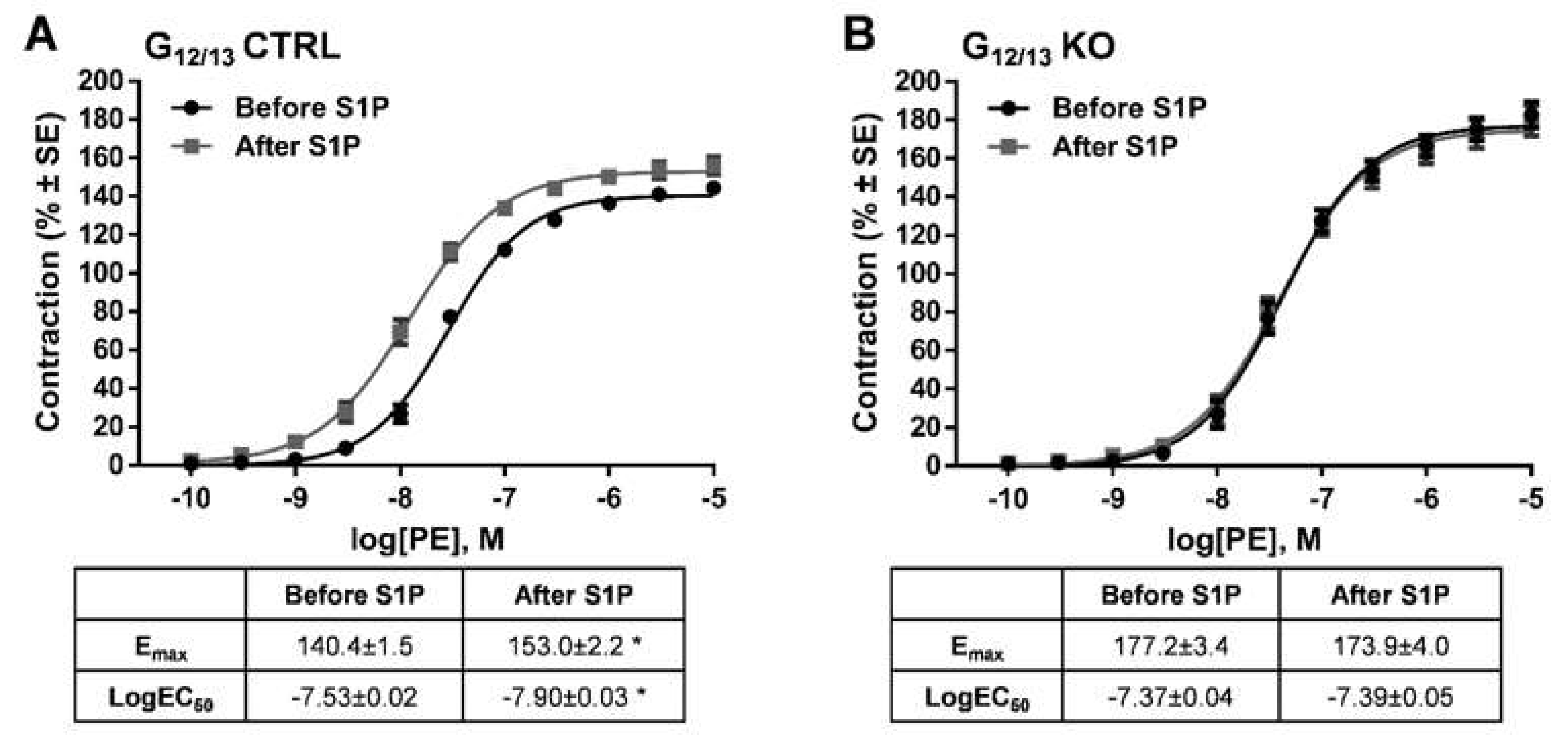
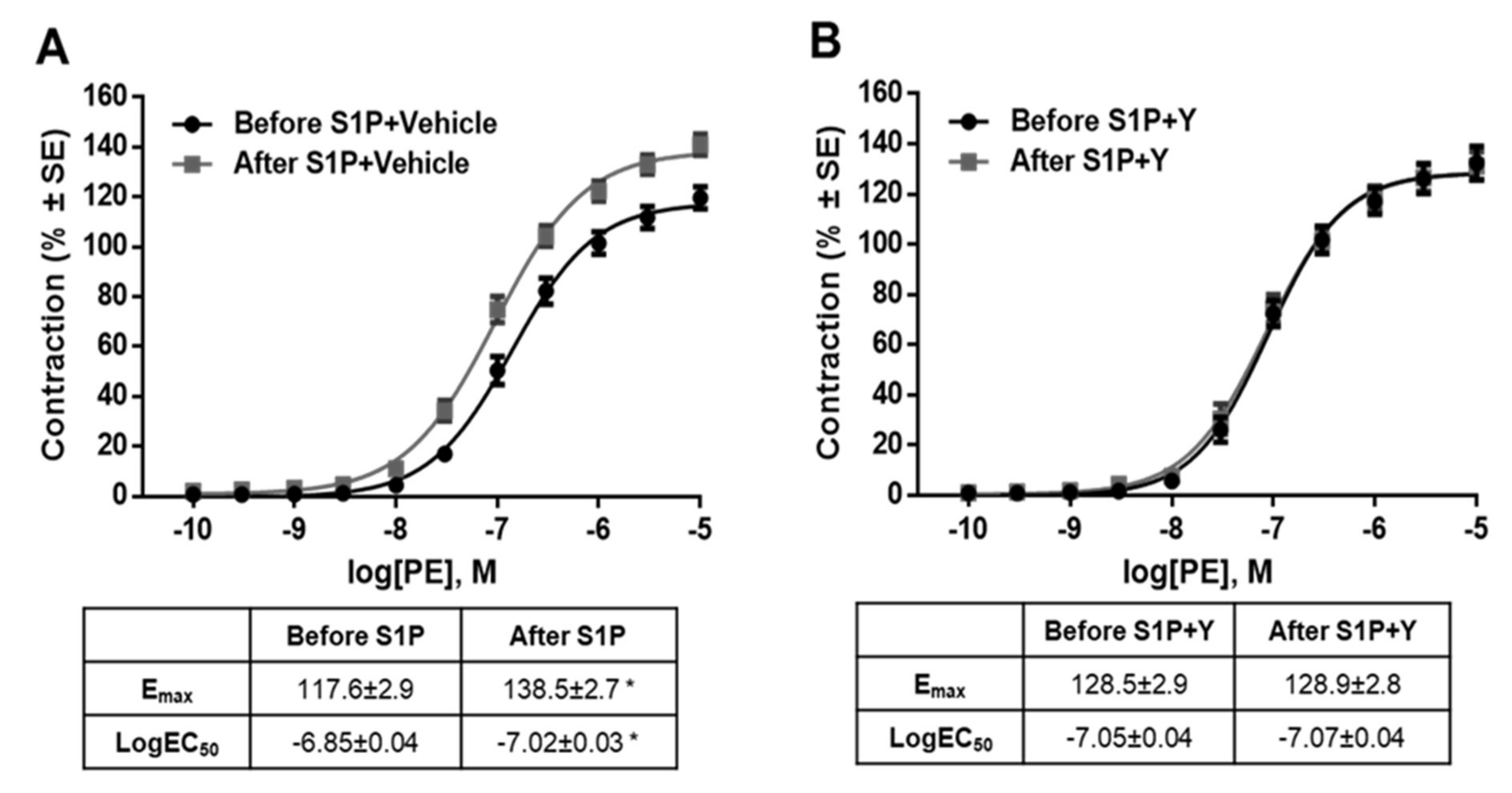
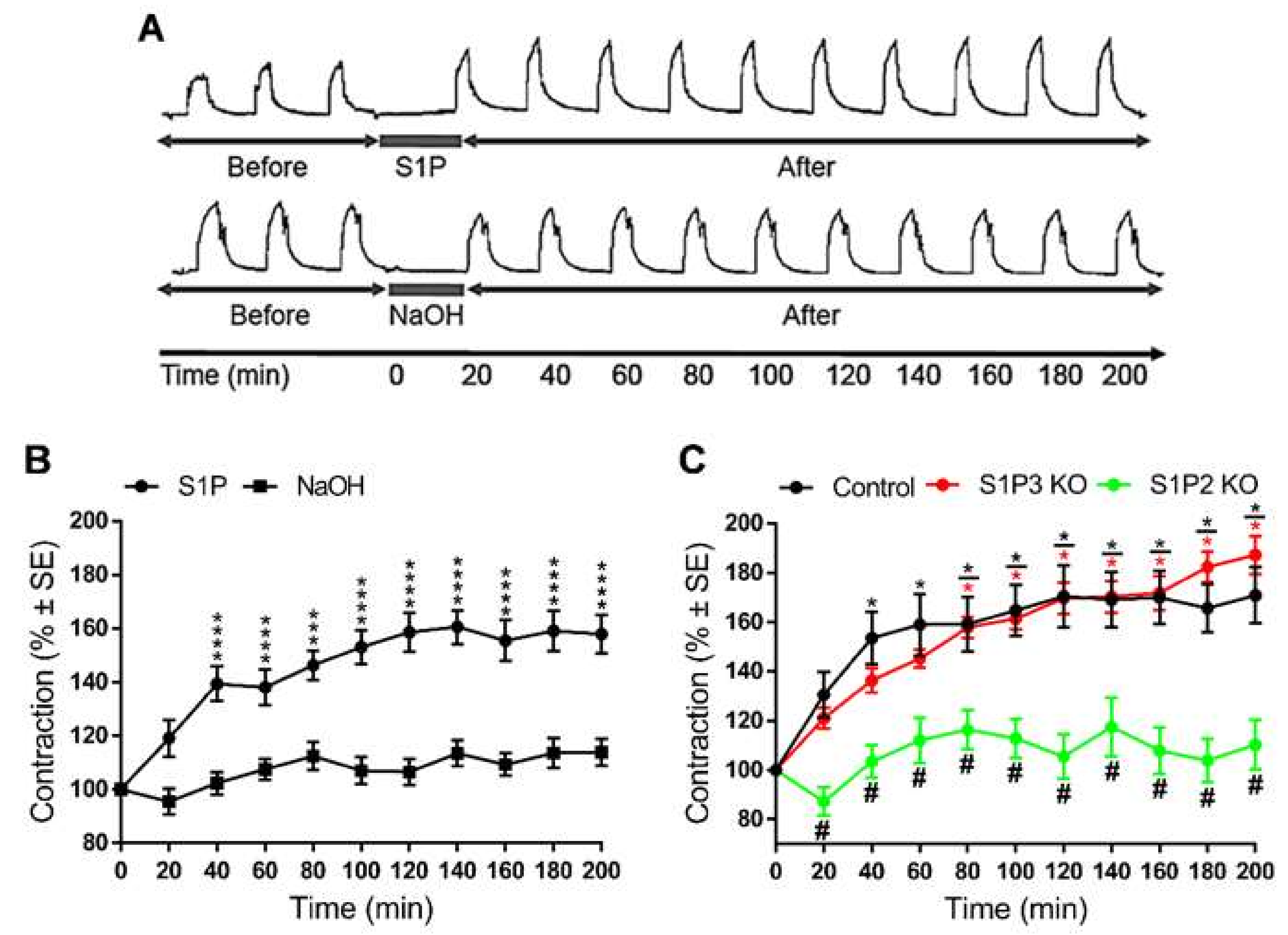
2. Results
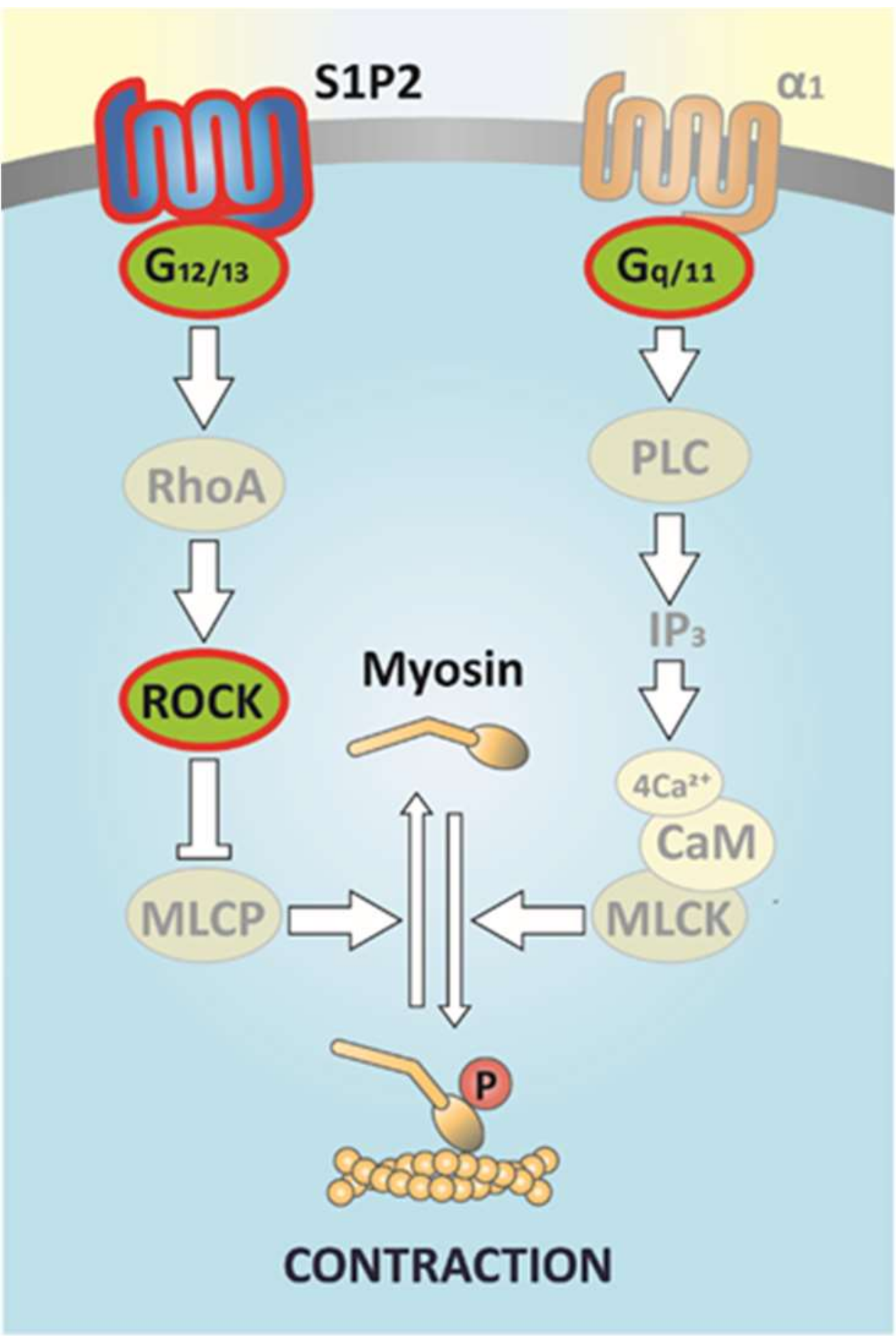
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of Vessels
4.3. General Myography Protocol
4.4. Protocol for Testing the Direct Vasoactive Effect of S1P on Resting Tone and after PE Precontraction
4.5. Protocol for Testing the Short-Term Effect of S1P on α1-Adrenergic Vasoconstriction
4.6. Protocol for Testing the Long-Term Effect of S1P on α1-Adrenergic Vasoconstriction
4.7. Reagents
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| CTRL | Control |
| eNOS | Endothelial nitric oxide synthase |
| HUVEC | Human umbilical vein endothelial cell |
| IP3 | Inositol trisphosphate |
| KO | Knockout |
| MLCP | Myosin light-chain phosphatase |
| MLCK | Myosin light-chain kinase |
| NO | Nitric oxide |
| PC | Precontraction |
| PE | Phenylephrine |
| PLC | Phospholipase C |
| PTX | Pertussis toxin |
| RT | Resting tone |
| ROCK | Rho-associated protein kinase |
| S1P | Sphingosine-1-phosphate |
| S1P1/S1P2/S1P3 | Sphingosine-1-phosphate receptor |
| TA | Thoracic Aorta |
| W | Wash out |
| WT | Wild type |
References
- Kono, M.; Allende, M.L.; Proia, R.L. Sphingosine-1-phosphate regulation of mammalian development. Biochim. Biophys. Acta 2008, 1781, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Mizugishi, K.; Li, C.; Olivera, A.; Bielawski, J.; Bielawska, A.; Deng, C.X.; Proia, R.L. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Investig. 2007, 117, 2993–3006. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Wollny, T.; Watek, M.; Durnas, B.; Niemirowicz, K.; Piktel, E.; Zendzian-Piotrowska, M.; Gozdz, S.; Bucki, R. Sphingosine-1-Phosphate Metabolism and Its Role in the Development of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Cannavo, A.; Liccardo, D.; Komici, K.; Corbi, G.; de Lucia, C.; Femminella, G.D.; Elia, A.; Bencivenga, L.; Ferrara, N.; Koch, W.J.; et al. Sphingosine Kinases and Sphingosine 1-Phosphate Receptors: Signaling and Actions in the Cardiovascular System. Front. Pharmacol. 2017, 8, 556. [Google Scholar] [CrossRef]
- Levkau, B. Cardiovascular effects of sphingosine-1-phosphate (S1P). Handb. Exp. Pharmacol. 2013. [Google Scholar] [CrossRef]
- Arkensteijn, B.W.; Berbee, J.F.; Rensen, P.C.; Nielsen, L.B.; Christoffersen, C. The apolipoprotein m-sphingosine-1-phosphate axis: Biological relevance in lipoprotein metabolism, lipid disorders and atherosclerosis. Int. J. Mol. Sci. 2013, 14, 4419–4431. [Google Scholar] [CrossRef]
- Kerage, D.; Brindley, D.N.; Hemmings, D.G. Review: Novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta 2014, 35, S86–S92. [Google Scholar] [CrossRef]
- Hemmings, D.G. Signal transduction underlying the vascular effects of sphingosine 1-phosphate and sphingosylphosphorylcholine. Naunyn Schmiedeberg Arch. Pharmacol. 2006, 373, 18–29. [Google Scholar] [CrossRef]
- Pelletier, D.; Hafler, D.A. Fingolimod for multiple sclerosis. N. Engl. J. Med. 2012, 366, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Pujol-Lereis, L.M. Alteration of Sphingolipids in Biofluids: Implications for Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3564. [Google Scholar] [CrossRef] [PubMed]
- Patmanathan, S.N.; Wang, W.; Yap, L.F.; Herr, D.R.; Paterson, I.C. Mechanisms of sphingosine 1-phosphate receptor signalling in cancer. Cell. Signal. 2017, 34, 66–75. [Google Scholar] [CrossRef]
- Pyne, N.J.; Tonelli, F.; Lim, K.G.; Long, J.S.; Edwards, J.; Pyne, S. Sphingosine 1-phosphate signalling in cancer. Biochem. Soc. Trans. 2012, 40, 94–100. [Google Scholar] [CrossRef]
- Pyne, N.J.; El Buri, A.; Adams, D.R.; Pyne, S. Sphingosine 1-phosphate and cancer. Adv. Biol. Regul. 2018, 68, 97–106. [Google Scholar] [CrossRef]
- Mahajan-Thakur, S.; Bien-Moller, S.; Marx, S.; Schroeder, H.; Rauch, B.H. Sphingosine 1-phosphate (S1P) signaling in glioblastoma multiforme-A systematic review. Int. J. Mol. Sci. 2017, 18, 2448. [Google Scholar] [CrossRef]
- Zhuang, X.P.; Zhu, Q. Sphingosine-1-phosphate/sphingosine-1-phosphate receptor 1 and T cell migration. Acta Pharm. Sin. 2016, 51, 873–878. [Google Scholar]
- Tiper, I.V.; East, J.E.; Subrahmanyam, P.B.; Webb, T.J. Sphingosine 1-phosphate signaling impacts lymphocyte migration, inflammation and infection. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef]
- Paik, J.H.; Chae, S.; Lee, M.J.; Thangada, S.; Hla, T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J. Biol. Chem. 2001, 276, 11830–11837. [Google Scholar] [CrossRef]
- Lemos, J.P.; Smaniotto, S.; Messias, C.V.; Moreira, O.C.; Cotta-de-Almeida, V.; Dardenne, M.; Savino, W.; Mendes-da-Cruz, D.A. Sphingosine-1-Phosphate Receptor 1 Is Involved in Non-Obese Diabetic Mouse Thymocyte Migration Disorders. Int. J. Mol. Sci. 2018, 19, 1446. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, Y.; Du, W.; Qi, X.; Okamoto, Y.; Takuwa, N.; Yoshioka, K. Roles of sphingosine-1-phosphate signaling in angiogenesis. World J. Biol. Chem. 2010, 1, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Mi, Y.; Liu, Y.; Sasaki, T.; Allende, M.L.; Wu, Y.P.; Yamashita, T.; Proia, R.L. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 2004, 279, 29367–29373. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, B.A.; Argraves, K.M. The role of sphingosine-1-phosphate in endothelial barrier function. Biochim. Et Biophys. Acta 2014, 1841, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Hla, T. S1P control of endothelial integrity. Curr. Top. Microbiol. Immunol. 2014, 378, 85–105. [Google Scholar] [CrossRef]
- Sanchez, T.; Skoura, A.; Wu, M.T.; Casserly, B.; Harrington, E.O.; Hla, T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1312–1318. [Google Scholar] [CrossRef]
- Kimura, T.; Sato, K.; Kuwabara, A.; Tomura, H.; Ishiwara, M.; Kobayashi, I.; Ui, M.; Okajima, F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J. Biol. Chem. 2001, 276, 31780–31785. [Google Scholar] [CrossRef]
- Morales-Ruiz, M.; Lee, M.J.; Zollner, S.; Gratton, J.P.; Scotland, R.; Shiojima, I.; Walsh, K.; Hla, T.; Sessa, W.C. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J. Biol. Chem. 2001, 276, 19672–19677. [Google Scholar] [CrossRef]
- Dantas, A.P.; Igarashi, J.; Michel, T. Sphingosine 1-phosphate and control of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2045–H2052. [Google Scholar] [CrossRef]
- Igarashi, J.; Michel, T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. eNOS activation by sphingosine 1-phosphate and the role of caveolin-1 in sphingolipid signal transduction. J. Biol. Chem. 2000, 275, 32363–32370. [Google Scholar] [CrossRef]
- Nofer, J.R.; van der Giet, M.; Tolle, M.; Wolinska, I.; von Wnuck Lipinski, K.; Baba, H.A.; Tietge, U.J.; Godecke, A.; Ishii, I.; Kleuser, B.; et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Investig. 2004, 113, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, J.; Michel, T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc. Res. 2009, 82, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Coussin, F.; Scott, R.H.; Wise, A.; Nixon, G.F. Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: Differential role in vasoconstriction. Circ. Res. 2002, 91, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Czyborra, P.; Fetscher, C.; Meyer Zu Heringdorf, D.; Jakobs, K.H.; Michel, M.C. Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br. J. Pharmacol. 2000, 130, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, T.; Yatomi, Y.; Osada, M.; Kazama, F.; Takafuta, T.; Ikeda, H.; Ozaki, Y. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc. Res. 2003, 58, 170–177. [Google Scholar] [CrossRef]
- Tosaka, M.; Okajima, F.; Hashiba, Y.; Saito, N.; Nagano, T.; Watanabe, T.; Kimura, T.; Sasaki, T. Sphingosine 1-phosphate contracts canine basilar arteries in vitro and in vivo: Possible role in pathogenesis of cerebral vasospasm. Stroke 2001, 32, 2913–2919. [Google Scholar] [CrossRef]
- Bolz, S.S.; Vogel, L.; Sollinger, D.; Derwand, R.; de Wit, C.; Loirand, G.; Pohl, U. Nitric oxide-induced decrease in calcium sensitivity of resistance arteries is attributable to activation of the myosin light chain phosphatase and antagonized by the RhoA/Rho kinase pathway. Circulation 2003, 107, 3081–3087. [Google Scholar] [CrossRef]
- Sugiyama, A.; Yatomi, Y.; Ozaki, Y.; Hashimoto, K. Sphingosine 1-phosphate induces sinus tachycardia and coronary vasoconstriction in the canine heart. Cardiovasc. Res. 2000, 46, 119–125. [Google Scholar] [CrossRef][Green Version]
- Bischoff, A.; Czyborra, P.; Meyer Zu Heringdorf, D.; Jakobs, K.H.; Michel, M.C. Sphingosine-1-phosphate reduces rat renal and mesenteric blood flow in vivo in a pertussis toxin-sensitive manner. Br. J. Pharmacol. 2000, 130, 1878–1883. [Google Scholar] [CrossRef]
- Tolle, M.; Levkau, B.; Kleuser, B.; van der Giet, M. Sphingosine-1-phosphate and FTY720 as anti-atherosclerotic lipid compounds. Eur. J. Clin. Investig. 2007, 37, 171–179. [Google Scholar] [CrossRef]
- Salomone, S.; Yoshimura, S.; Reuter, U.; Foley, M.; Thomas, S.S.; Moskowitz, M.A.; Waeber, C. S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur. J. Pharmacol. 2003, 469, 125–134. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Kurano, M.; Nishikawa, M.; Kano, K.; Dohi, T.; Miyauchi, K.; Daida, H.; Shimizu, T.; Aoki, J.; Yatomi, Y. Vehicle-dependent Effects of Sphingosine 1-phosphate on Plasminogen Activator Inhibitor-1 Expression. J. Atheroscler. Thromb. 2017, 24, 954–969. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Mulders, A.C.; Jongsma, M.; Alewijnse, A.E.; Peters, S.L. Vascular effects of sphingolipids. Acta Paediatr. 2007, 96, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Levkau, B. Sphingosine-1-phosphate in the regulation of vascular tone: A finely tuned integration system of S1P sources, receptors, and vascular responsiveness. Circ. Res. 2008, 103, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Tolle, M.; Levkau, B.; Keul, P.; Brinkmann, V.; Giebing, G.; Schonfelder, G.; Schafers, M.; von Wnuck Lipinski, K.; Jankowski, J.; Jankowski, V.; et al. Immunomodulator FTY720 Induces eNOS-dependent arterial vasodilatation via the lysophospholipid receptor S1P3. Circ. Res. 2005, 96, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Waeber, C. Sphingosine 1-phosphate (S1P) signaling and the vasculature. Lysophospholipid Recept. Signal. Biochem. 2013, 313–347. [Google Scholar] [CrossRef]
- Igarashi, J.; Erwin, P.A.; Dantas, A.P.; Chen, H.; Michel, T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 10664–10669. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Constable, P.D.; Smith, G.W.; Haschek, W.M. Effects of Exogenous Sphinganine, Sphingosine, and Sphingosine-1-Phosphate on Relaxation and Contraction of Porcine Thoracic Aortic and Pulmonary Arterial Rings. Toxicol. Sci. 2005, 86, 194–199. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, P.; Proia, R.L.; Hla, T. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J. Clin. Investig. 2014, 124, 4823–4828. [Google Scholar] [CrossRef]
- Ikeda, H.; Nagashima, K.; Yanase, M.; Tomiya, T.; Arai, M.; Inoue, Y.; Tejima, K.; Nishikawa, T.; Watanabe, N.; Omata, M.; et al. Sphingosine 1-phosphate enhances portal pressure in isolated perfused liver via S1P2 with Rho activation. Biochem. Biophys. Res. Commun. 2004, 320, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, B.A.; Grass, G.D.; Wing, S.B.; Argraves, W.S.; Argraves, K.M. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: High density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J. Biol. Chem. 2012, 287, 44645–44653. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Hara, M.; Tsuneyama, K.; Sakoda, H.; Shimizu, T.; Tsukamoto, K.; Ikeda, H.; Yatomi, Y. Induction of insulin secretion by apolipoprotein M, a carrier for sphingosine 1-phosphate. Biochim. Biophys. Acta 2014, 1841, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Blaho, V.A.; Galvani, S.; Engelbrecht, E.; Liu, C.; Swendeman, S.L.; Kono, M.; Proia, R.L.; Steinman, L.; Han, M.H.; Hla, T. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 2015, 523, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Galvani, S.; Sanson, M.; Blaho, V.A.; Swendeman, S.L.; Obinata, H.; Conger, H.; Dahlback, B.; Kono, M.; Proia, R.L.; Smith, J.D.; et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 2015, 8, ra79. [Google Scholar] [CrossRef]
- Hanel, P.; Andreani, P.; Graler, M.H. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007, 21, 1202–1209. [Google Scholar] [CrossRef]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef]
- Pappu, R.; Schwab, S.R.; Cornelissen, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.W.; Huang, Y.; Cyster, J.G.; et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007, 316, 295–298. [Google Scholar] [CrossRef]
- Camerer, E.; Regard, J.B.; Cornelissen, I.; Srinivasan, Y.; Duong, D.N.; Palmer, D.; Pham, T.H.; Wong, J.S.; Pappu, R.; Coughlin, S.R. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Investig. 2009, 119, 1871–1879. [Google Scholar] [CrossRef]
- Yatomi, Y.; Ozaki, Y.; Ohmori, T.; Igarashi, Y. Sphingosine 1-phosphate: Synthesis and release. Prostaglandins Other Lipid Mediat. 2001, 64, 107–122. [Google Scholar] [CrossRef]
- Venkataraman, K.; Lee, Y.M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Szasz, T.; Webb, R.C. Rho-Mancing to Sensitize Calcium Signaling for Contraction in the Vasculature: Role of Rho Kinase. Adv. Pharmacol. 2017, 78, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Khalil, R.A. Evolving mechanisms of vascular smooth muscle contraction highlight key targets in vascular disease. Biochem. Pharmacol. 2018, 153, 91–122. [Google Scholar] [CrossRef] [PubMed]
- Bolz, S.S.; Vogel, L.; Sollinger, D.; Derwand, R.; Boer, C.; Pitson, S.M.; Spiegel, S.; Pohl, U. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation 2003, 108, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, D.G.; Hudson, N.K.; Halliday, D.; O’Hara, M.; Baker, P.N.; Davidge, S.T.; Taggart, M.J. Sphingosine-1-phosphate acts via rho-associated kinase and nitric oxide to regulate human placental vascular tone. Biol. Reprod. 2006, 74, 88–94. [Google Scholar] [CrossRef]
- Szczepaniak, W.S.; Pitt, B.R.; McVerry, B.J. S1P2 receptor-dependent Rho-kinase activation mediates vasoconstriction in the murine pulmonary circulation induced by sphingosine 1-phosphate. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L137–L145. [Google Scholar] [CrossRef][Green Version]
- Lorenz, J.N.; Arend, L.J.; Robitz, R.; Paul, R.J.; MacLennan, A.J. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R440–R446. [Google Scholar] [CrossRef]
- Wirth, A.; Benyo, Z.; Lukasova, M.; Leutgeb, B.; Wettschureck, N.; Gorbey, S.; Orsy, P.; Horvath, B.; Maser-Gluth, C.; Greiner, E.; et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 2008, 14, 64–68. [Google Scholar] [CrossRef]
- Horvath, B.; Orsy, P.; Benyo, Z. Endothelial NOS-mediated relaxations of isolated thoracic aorta of the C57BL/6J mouse: A methodological study. J. Cardiovasc. Pharmacol. 2005, 45, 225–231. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panta, C.R.; Ruisanchez, É.; Móré, D.; Dancs, P.T.; Balogh, A.; Fülöp, Á.; Kerék, M.; Proia, R.L.; Offermanns, S.; Tigyi, G.J.; et al. Sphingosine-1-Phosphate Enhances α1-Adrenergic Vasoconstriction via S1P2–G12/13–ROCK Mediated Signaling. Int. J. Mol. Sci. 2019, 20, 6361. https://doi.org/10.3390/ijms20246361
Panta CR, Ruisanchez É, Móré D, Dancs PT, Balogh A, Fülöp Á, Kerék M, Proia RL, Offermanns S, Tigyi GJ, et al. Sphingosine-1-Phosphate Enhances α1-Adrenergic Vasoconstriction via S1P2–G12/13–ROCK Mediated Signaling. International Journal of Molecular Sciences. 2019; 20(24):6361. https://doi.org/10.3390/ijms20246361
Chicago/Turabian StylePanta, Cecília R., Éva Ruisanchez, Dorottya Móré, Péter T. Dancs, Andrea Balogh, Ágnes Fülöp, Margit Kerék, Richard L. Proia, Stefan Offermanns, Gábor J. Tigyi, and et al. 2019. "Sphingosine-1-Phosphate Enhances α1-Adrenergic Vasoconstriction via S1P2–G12/13–ROCK Mediated Signaling" International Journal of Molecular Sciences 20, no. 24: 6361. https://doi.org/10.3390/ijms20246361
APA StylePanta, C. R., Ruisanchez, É., Móré, D., Dancs, P. T., Balogh, A., Fülöp, Á., Kerék, M., Proia, R. L., Offermanns, S., Tigyi, G. J., & Benyó, Z. (2019). Sphingosine-1-Phosphate Enhances α1-Adrenergic Vasoconstriction via S1P2–G12/13–ROCK Mediated Signaling. International Journal of Molecular Sciences, 20(24), 6361. https://doi.org/10.3390/ijms20246361





