Osteogenic Differentiation of Human Adipose Tissue-Derived MSCs by Non-Toxic Calcium Poly(ethylene phosphate)s
Abstract
1. Introduction
2. Results and Discussion
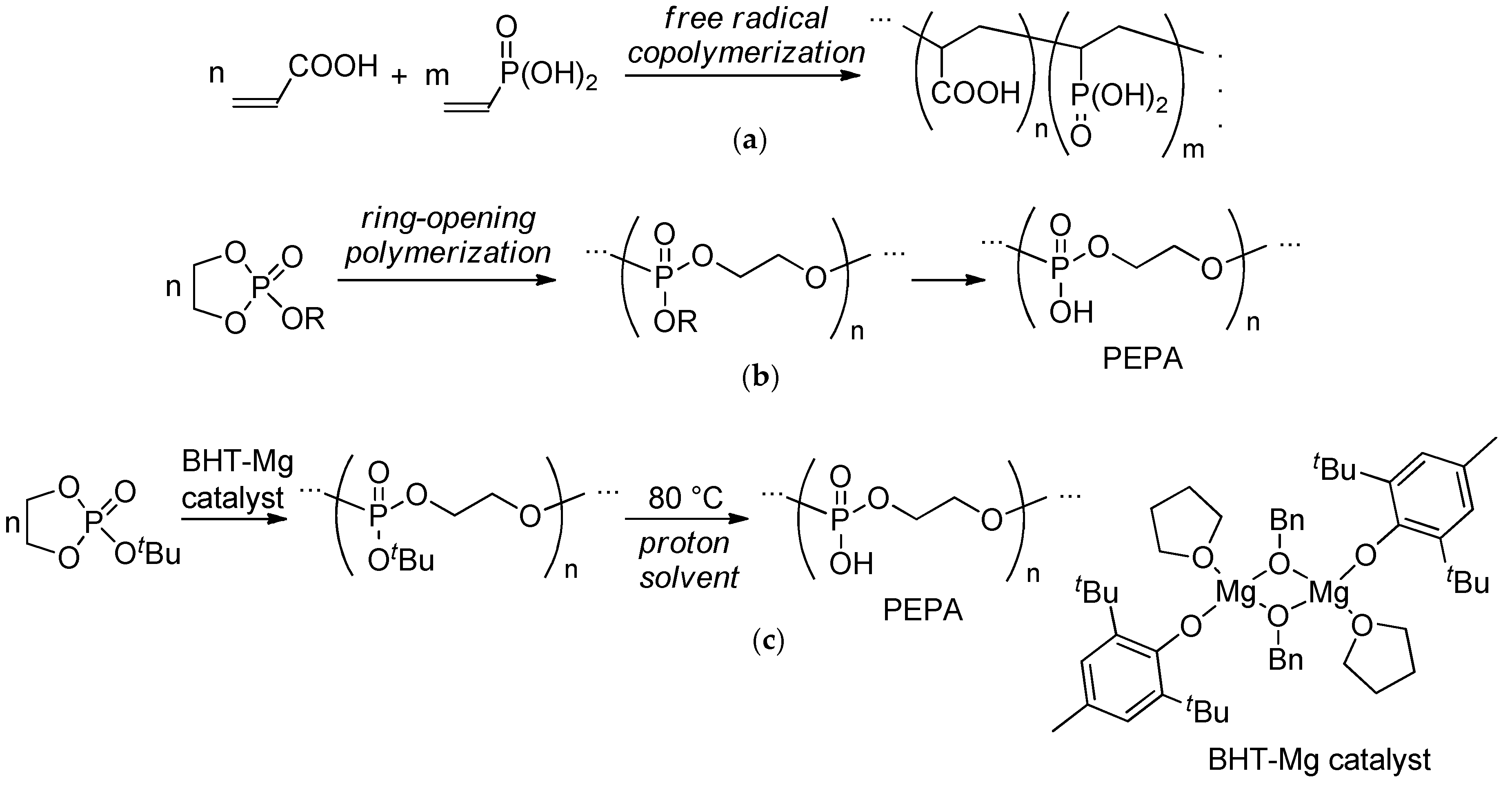
2.1. Preparation of PEPA and PEPA Salts
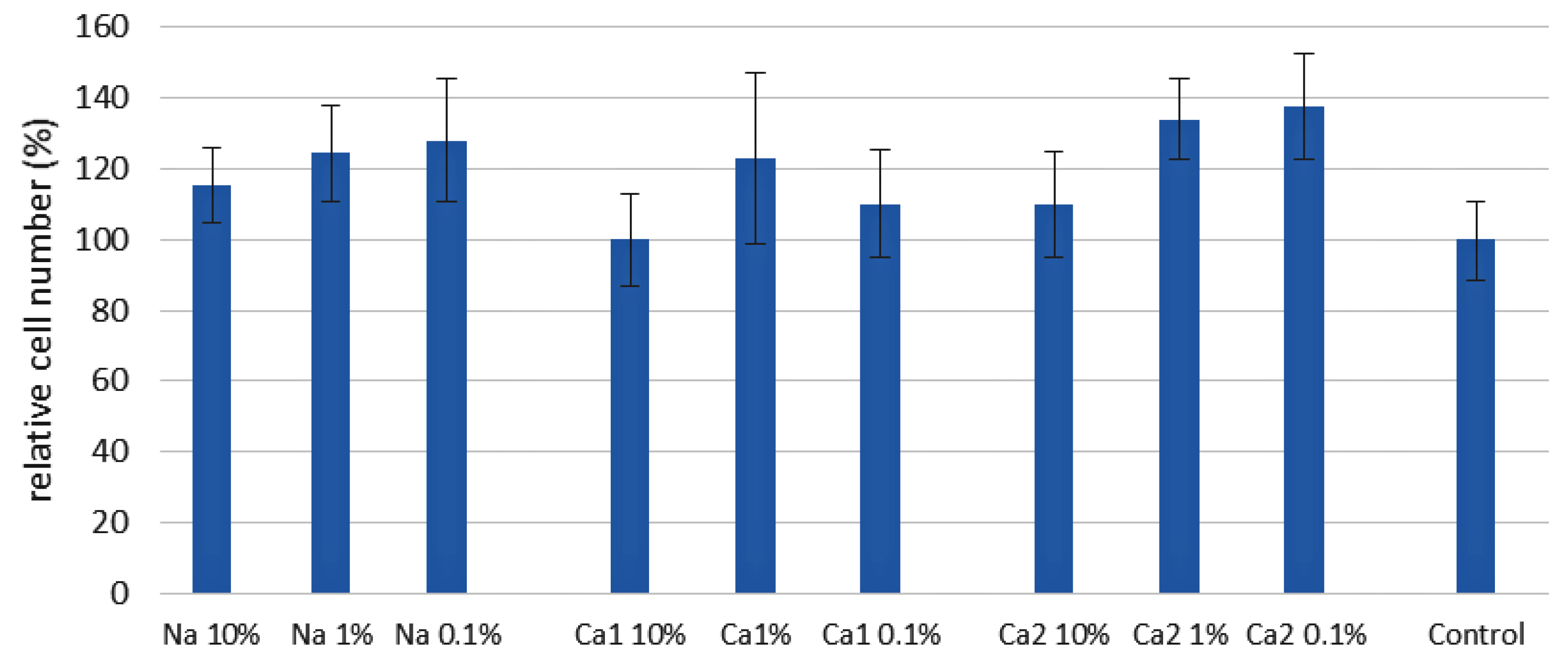
2.2. The Influence of PEPA Salts on ADSCs’ Adhesion and Proliferation
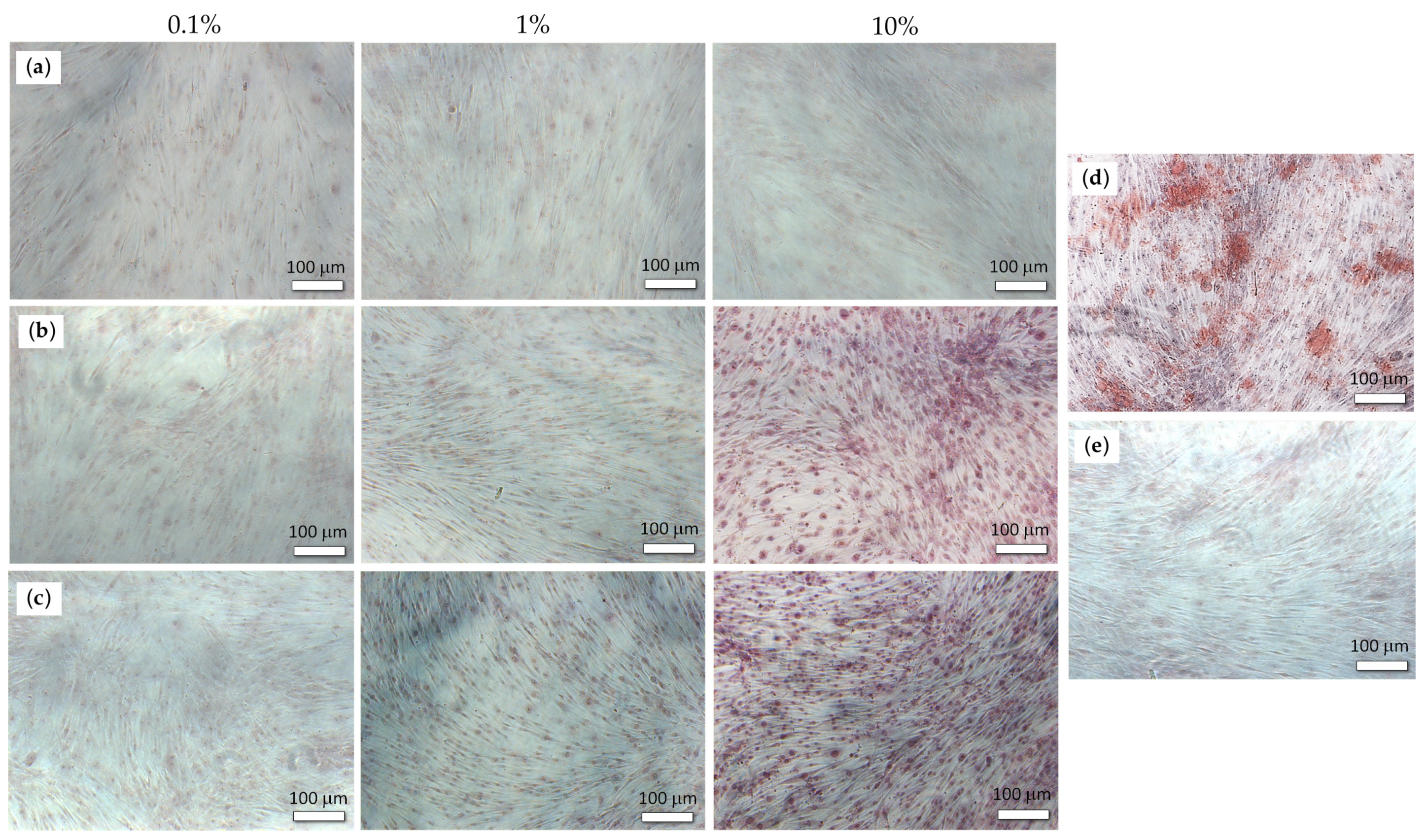
2.3. Osteogenic Potential of the PEPA Salts
3. Materials and Methods
3.1. Synthesis of PEPA and Metal Salts
3.1.1. General Experimental Remarks
3.1.2. Synthesis of Poly(tBuOEP)
3.1.3. Preparation of the PEPA Solution
3.1.4. Preparation of the Solutions of PEPA Metal Salts
3.2. Cultivation of the ADSCs
3.3. The Study of the Influence of PEPA Salts on Cell Adhesion and Proliferation
3.4. The Study of the Osteogenic Potential of PEPA Salts
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | human adipose-tissue-derived stem cells |
| BHT | 2,6-di-tert-butyl-4-methylphenol |
| BMP-2 | bone morphogenetic protein 2 |
| DSC | differential scanning calorimetry |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| MSCs | mesenchymal stem cells |
| PEP | poly(ethylene phosphate) |
| PEPA | poly(ethylene phosphoric acid) |
| PTFE | poly(tetrafluoroethylene) |
| RCMG | Research Centre of Medical Genetics |
| ROP | ring-opening polymerization |
| TGA | thermogravimetric analysis |
References
- Yousefi, A.-M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, G.; de Groot, K. Biomimetic coatings for bone tissue engineering of critical-sized defects. J. R. Soc. Interface 2010, 7, S631–S647. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Patel, D.M.; Shah, J.; Srivastava, A.S. Therapeutic Potential of Mesenchymal Stem Cells in Regenerative Medicine. Stem Cells Int. 2013, 2013, 496218. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Ansari, A.S.; Yazid, M.D.; Sainik, N.Q.A.V.; Razali, R.A.; Saim, A.B.; Idrus, R.B.H. Osteogenic Induction of Wharton’s Jelly-Derived Mesenchymal Stem Cell for Bone Regeneration: A Systematic Review. Stem Cells Int. 2018, 2018, 2406462. [Google Scholar] [CrossRef]
- Humbert, P.; Brennan, M.Á.; Davison, N.; Rosset, P.; Trichet, V.; Blanchard, F.; Layrolle, P. Immune Modulation by Transplanted Calcium Phosphate Biomaterials and Human Mesenchymal Stromal Cells in Bone Regeneration. Front. Immunol. 2019, 10, 663. [Google Scholar] [CrossRef]
- Liang, X.; Duan, P.; Gao, J.; Guo, R.; Qu, Z.; Li, X.; He, Y.; Yao, H.; Ding, J. Bilayered PLGA/PLGA-HAp Composite Scaffold for Osteochondral Tissue Engineering and Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3506–3521. [Google Scholar] [CrossRef]
- Kunisch, E.; Gunnella, F.; Wagner, S.; Dees, F.; Maenz, S.; Bossert, J.; Jandt, K.D.; Kinne, R.W. The poly (l-lactid-co-glycolide; PLGA) fiber component of brushiteforming calcium phosphate cement induces the osteogenic differentiation of human adipose tissue-derived stem cells. Biomed. Mater. 2019, 14, 055012. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, R.; Jiang, X.; Xie, J.; Cai, P.; Chen, H.; Zhan, X.; Lei, D.; Zhao, J.; Zheng, L. Electrospun PLGA/PCL/OCP nanofiber membranes promote osteogenic differentiation of mesenchymal stem cells (MSCs). Mater. Sci. Eng. C 2019, 104, 109796. [Google Scholar] [CrossRef]
- Hasan, A.; Byambaa, B.; Morshed, M.; Cheikh, M.I.; Shakoor, R.A.; Mustafy, T.; Marei, H.E. Advances in osteobiologic materials for bone substitutes. Tissue Eng. Regen. Med. 2018, 12, 1448–1468. [Google Scholar] [CrossRef]
- Naira, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Vert, M. Aliphatic Polyesters: Great Degradable Polymers That Cannot Do Everything. Biomacromolecules 2005, 6, 538–546. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Ghavimi, S.A.A.; Allen, B.N.; Stromsdorfer, J.L.; Kramer, J.S.; Li, X.; Ulery, B.D. Calcium and phosphate ions as simple signaling molecules with versatile osteoinductivity. Biomed. Mater. 2018, 13, 055005. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Muneta, T.; Tsuji, K.; Ichinose, S.; Hakamatsuka, Y.; Koga, H.; Sekiya, I. β-Tricalcium Phosphate Micron Particles Enhance Calcification of Human Mesenchymal Stem Cells In Vitro. J. Nanomater. 2013, 426786. [Google Scholar] [CrossRef]
- Dey, R.E.; Zhong, X.; Youle, P.J.; Wang, Q.G.; Wimpenny, I.; Downes, S.; Hoyland, J.A.; Watts, D.C.; Gough, J.E.; Budd, P.M. Synthesis and Characterization of Poly(vinylphosphonic acid-co-acrylic acid) Copolymers for Application in Bone Tissue Scaffolds. Macromolecules 2016, 49, 2656–2662. [Google Scholar] [CrossRef]
- Dey, R.E.; Wimpenny, I.; Gough, J.E.; Watts, D.C.; Budd, P.M. Poly(vinylphosphonic acid-co-acrylic acid) hydrogels: The effect of copolymer composition on osteoblast adhesion and proliferation. J. Biomed. Mater. Res. 2018, 106, 255–264. [Google Scholar] [CrossRef]
- Steinbach, T.; Wurm, F.R. Poly(phosphoester)s: A new platform for degradable polymers. Angew. Chem. Int. Ed. 2015, 54, 6098–6108. [Google Scholar] [CrossRef]
- Yilmaz, Z.E.; Jérôme, C. Polyphosphoesters: New trends in synthesis and drug delivery applications. Macromol. Biosci. 2016, 16, 1745–1761. [Google Scholar] [CrossRef]
- Bauer, K.N.; Tee, H.T.; Velencoso, M.M.; Wurm, F.R. Main-chain poly(phosphoester)s: History, syntheses, degradation, bio-and flame-retardant applications. Prog. Polym. Sci. 2017, 73, 61–122. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Kosarev, M.A.; Tavtorkin, A.N.; Minyaev, M.E.; Roznyatovsky, V.A.; Ivchenko, P.V. Controlled ring-opening polymerisation of cyclic phosphates, phosphonates and phosphoramidates catalysed by hereroleptic BHT-alkoxy magnesium complexes. Polym. Chem. 2017, 8, 6806–6816. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Karchevsky, S.; Ivchenko, P. Mechanistic Insights of BHT-Mg-Catalyzed Ethylene Phosphate’s Coordination Ring-Opening Polymerization: DFT Modeling and Experimental Data. Polymers 2018, 10, 1105. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Libisowski, J.; Penczek, S. A New Class of Synthetic Polyelectrolytes. Acidic Polyesters of Phosphoric Acid (Poly(hydroxyalkylene phosphates)). Macromolecules 1976, 9, 365–367. [Google Scholar] [CrossRef]
- Penczek, S.; Biela, T.; Klosinski, P.; Lapienis, G. Polymerization of phosphorus containing cyclic monomers: Synthesis of polymers related to biopolymers. Makromol. Chem. Macromol. Symp. 1986, 6, 123–153. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Mao, H.-Q.; Wang, S.; Phua, S.H.; Lee, G.P.; Pan, J.; Lu, S.; Wang, J.; Leong, K.W. Poly(phosphoester) ionomers as tissue-engineering scaffolds. J. Biomed. Mater. Res. 2004, 70B, 91–102. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Kawakita, T.; Yusa, S. Thermoresponsive Polyphosphoesters Bearing Enzyme-cleavable Side Chains. Chem. Lett. 2009, 38, 1054–1055. [Google Scholar] [CrossRef]
- Ergul Yilmaz, Z.; Debuigne, A.; Calvignac, B.; Boury, F.; Jerome, C. Double hydrophilic polyphosphoester containing copolymers as efficient templating agents for calcium carbonate microparticles. J. Mater. Chem. B 2015, 3, 7227–7236. [Google Scholar] [CrossRef]
- Hirano, Y.; Iwasaki, Y. Bone-specific poly(ethylene sodium phosphate)-bearing biodegradable nanoparticles. Coll. Surf. B 2017, 153, 104–110. [Google Scholar] [CrossRef]
- Otaka, A.; Iwasaki, Y. Endocytosis of poly(ethylene sodium phosphate) by macrophages and the effect of polymer length on cellular uptake. J. Ind. Eng. Chem. 2019, 75, 115–122. [Google Scholar] [CrossRef]
- Yasuda, H.; Sumitani, M.; Nakamura, A. Novel Synthesis of Acidic Polyesters of Phosphoric Acid by Thermal Elimination of Isobutylene from Poly(alkylene tert-butyl phosphates). Macromolecules 1981, 14, 458–460. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Tavtorkin, A.N.; Minyaev, M.E.; Kosarev, M.A.; Ivchenko, P.V. Synthesis in aqueous media of poly(ethylene phosphoric acids) by mild thermolysis of homopolymers and block copolymers based on tert-butyl ethylene phosphate. Eur. Polym. J. 2018, 106, 249–256. [Google Scholar] [CrossRef]
- Wang, Q.G.; Wimpenny, I.; Dey, R.E.; Zhong, X.; Youle, P.J.; Downes, S.; Watts, D.C.; Budd, P.M.; Hoyland, J.A.; Gough, J.E. The unique calcium chelation property of poly(vinyl phosphonic acid-co-acrylic acid) and effects on osteogenesis in vitro. J. Biomed. Mater. Res. 2018, 106, 168–179. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.; Kaluzynski, K. Poly(alkylene phosphates): From Synthetic Models of Biomacromolecules and Biomembranes toward Polymer−Inorganic Hybrids (Mimicking Biomineralization). Biomacromolecules 2005, 6, 547–551. [Google Scholar] [CrossRef]
- Wang, D.A.; Williams, C.G.; Yang, F.; Cher, N.; Lee, H.; Elisseeff, J.H. Bioresponsive phosphoester hydrogels for bone tissue engineering. Tissue Eng. 2005, 11, 201–213. [Google Scholar] [CrossRef]
- Du, J.-Z.; Du, X.-J.; Mao, C.-Q.; Wang, J. Tailor-made dual pH-sensitive polymer–doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Yuan, Y.-Y.; Du, J.-Z.; Yang, X.-Z.; Wang, J. Recent progress in polyphosphoesters: From controlled synthesis to biomedical applications. Macromol. Biosci. 2009, 9, 1154–1164. [Google Scholar] [CrossRef]
- Zhai, X.; Huang, W.; Liu, J.; Pang, Y.; Zhu, X.; Zhou, Y.; Yan, D. Micelles from amphiphilic block copolyphosphates for drug delivery. Macromol. Biosci. 2011, 11, 1603–1610. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.B.; Kaluzynski, K.; Lapienis, G. Polymers with Esters of Phosphoric Acid Units: From Synthesis, Models of Biopolymers to Polymer—Inorganic Hybrids. Isr. J. Chem. 2012, 52, 306–319. [Google Scholar] [CrossRef]
- McKinlay, C.J.; Waymouth, R.M.; Wender, P.A. Cell-Penetrating, Guanidinium-Rich Oligophosphoesters: Effective and Versatile Molecular Transporters for Drug and Probe Delivery. J. Am. Chem. Soc. 2016, 138, 3510–3517. [Google Scholar] [CrossRef] [PubMed]
- Pranantyo, D.; Xu, L.Q.; Kang, E.-T.; Mya, M.K.; Chan-Park, M.B. Conjugation of polyphosphoester and antimicrobial peptide for enhanced bactericidal activity and biocompatibility. Biomacromolecules 2016, 17, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, S.; Pollack, S.F.; Li, R.; Gonzalez, A.M.; Fan, J.; Zou, J.; Leininger, S.E.; Pavía-Sanders, A.; Johnson, R.; et al. Improving paclitaxel delivery: In vitro and in vivo characterization of PEGylated polyphosphoester-based nanocarriers. J. Am. Chem. Soc. 2015, 137, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotech. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Becker, G.; Wurm, F.R. Breathing air as oxidant: Optimization of 2-chloro-2-oxo-1,3,2-dioxaphospholane synthesis as a precursor for phosphoryl choline derivatives and cyclic phosphate monomers. Tetrahedron 2017, 73, 3536–3540. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Minyaev, M.E.; Churakov, A.V.; Karchevsky, S.G.; Birin, K.P.; Ivchenko, P.V. Mono-BHT heteroleptic magnesium complexes: Synthesis, molecular structure and catalytic behavior in the ring-opening polymerization of cyclic esters. Dalton Trans. 2017, 46, 12132–12146. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. J. Quant. Cell Sci. 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Lamanna, R.; Corti, A.; Iorio, M.; Nocchi, F.; Urciuoli, P.; Lapi, S.; Scatena, F.; Franzini, M.; Vanacore, R.; Lorenzini, E.; et al. Are standard cell culture conditions adequate for human umbilical cord blood mesenchymal stem cells? Blood Transfus. 2014, 12, s375–s377. [Google Scholar] [CrossRef]
- Baer, P.C.; Koch, B.; Hickmann, E.; Schubert, R.; Cinatl, J., Jr.; Hauser, I.A.; Geiger, H. Isolation, Characterization, Differentiation and Immunomodulatory Capacity of Mesenchymal Stromal/Stem Cells from Human Perirenal Adipose Tissue. Cells 2019, 8, 1346. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Bukharova, T.; Dyakonov, A.; Goldshtein, D.; Galitsyna, E.; Kosarev, M.; Shlyakhtin, A.; Gavrilov, D.; Ivchenko, P. Osteogenic Differentiation of Human Adipose Tissue-Derived MSCs by Non-Toxic Calcium Poly(ethylene phosphate)s. Int. J. Mol. Sci. 2019, 20, 6242. https://doi.org/10.3390/ijms20246242
Nifant’ev I, Bukharova T, Dyakonov A, Goldshtein D, Galitsyna E, Kosarev M, Shlyakhtin A, Gavrilov D, Ivchenko P. Osteogenic Differentiation of Human Adipose Tissue-Derived MSCs by Non-Toxic Calcium Poly(ethylene phosphate)s. International Journal of Molecular Sciences. 2019; 20(24):6242. https://doi.org/10.3390/ijms20246242
Chicago/Turabian StyleNifant’ev, Ilya, Tatiana Bukharova, Alexander Dyakonov, Dmitry Goldshtein, Elena Galitsyna, Maxim Kosarev, Andrey Shlyakhtin, Dmitry Gavrilov, and Pavel Ivchenko. 2019. "Osteogenic Differentiation of Human Adipose Tissue-Derived MSCs by Non-Toxic Calcium Poly(ethylene phosphate)s" International Journal of Molecular Sciences 20, no. 24: 6242. https://doi.org/10.3390/ijms20246242
APA StyleNifant’ev, I., Bukharova, T., Dyakonov, A., Goldshtein, D., Galitsyna, E., Kosarev, M., Shlyakhtin, A., Gavrilov, D., & Ivchenko, P. (2019). Osteogenic Differentiation of Human Adipose Tissue-Derived MSCs by Non-Toxic Calcium Poly(ethylene phosphate)s. International Journal of Molecular Sciences, 20(24), 6242. https://doi.org/10.3390/ijms20246242







