A Novel, Tumor-Induced Osteoclastogenesis Pathway Insensitive to Denosumab but Interfered by Cannabidiol
Abstract
1. Introduction
2. Results
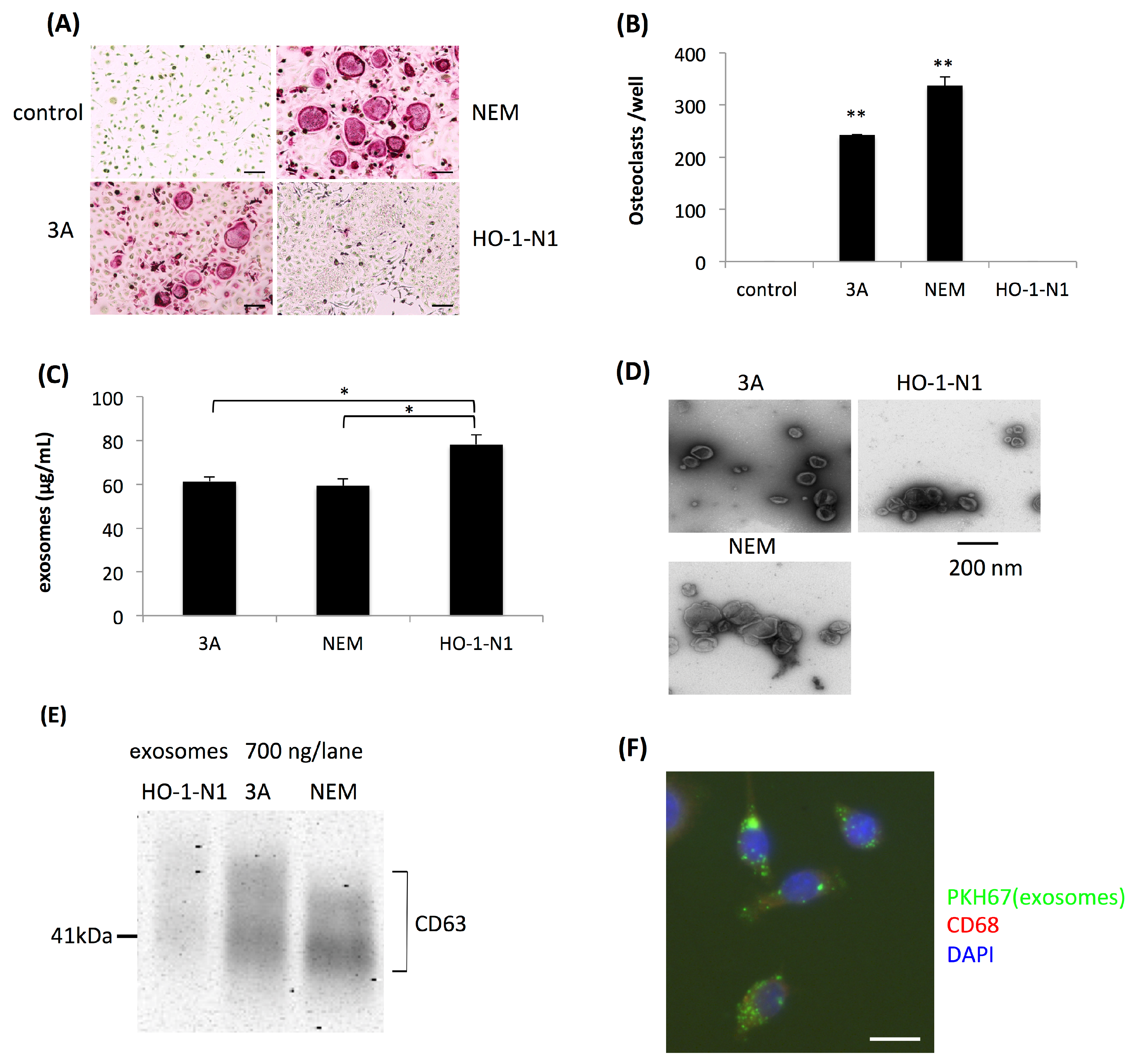
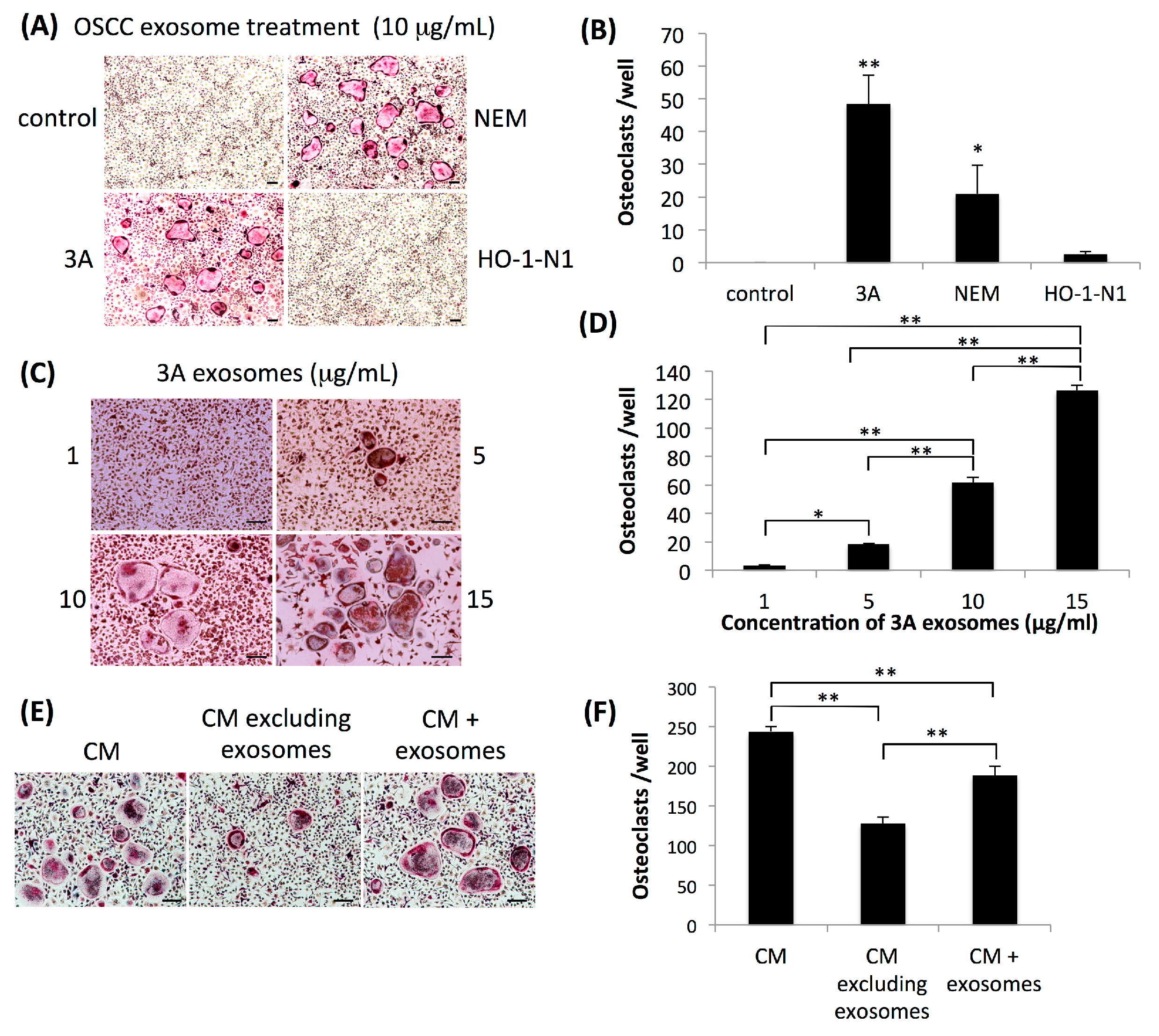
2.1. Exosomes Secreted by OSCC Cells Exhibit Osteoclastogenic Activity
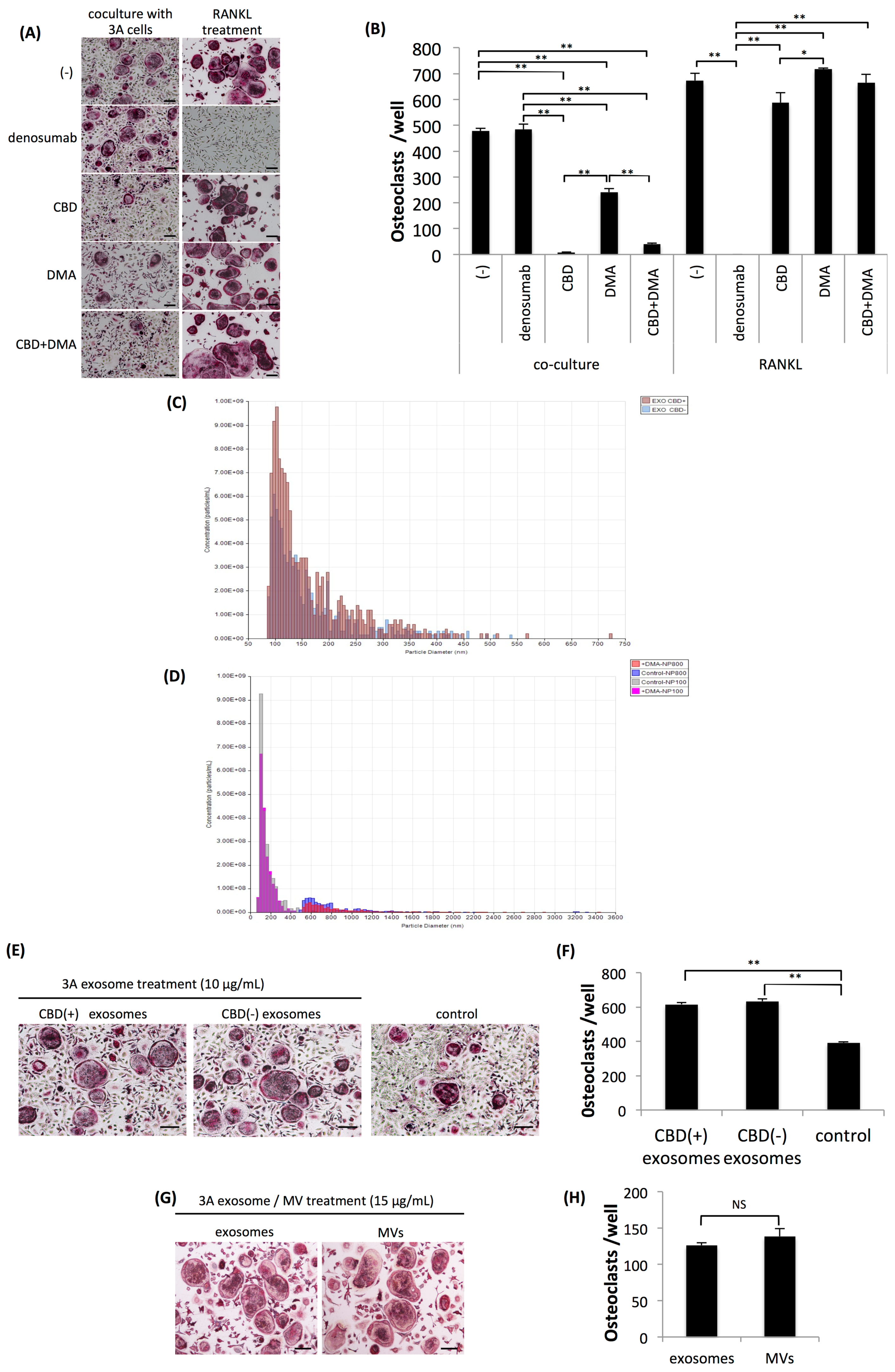
2.2. Osteoclastogenesis Induced by OSCC Cells was Resistant to Denosumab but was Effectively Suppressed by CBD and Dimethyl Amiloride.
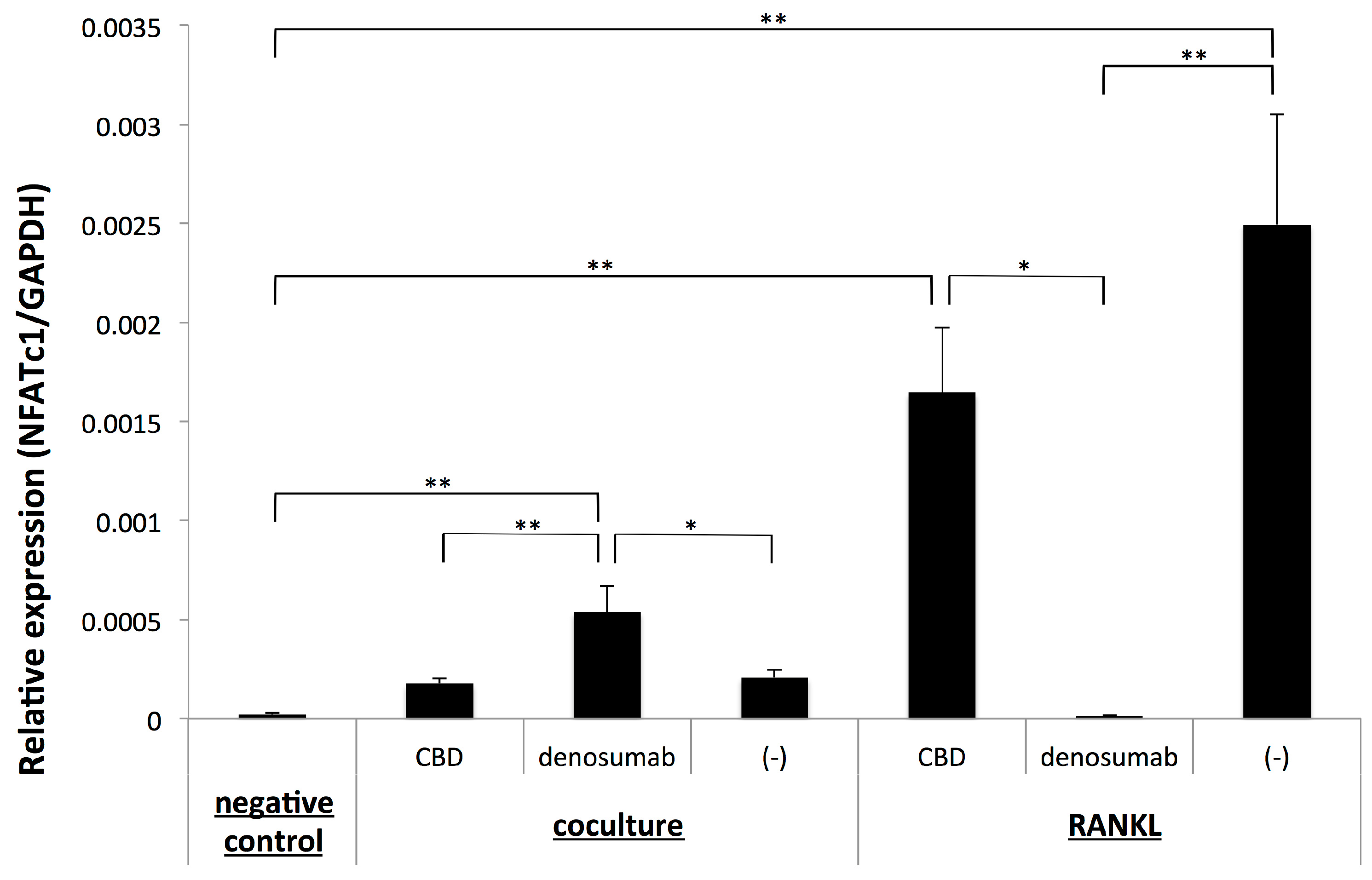
2.3. Osteoclastogenesis Induced by OSCC Cells was not Dependent on the Up-Regulation of NFATc1
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Mouse Bone Marrow Macrophages and OPCs
4.3. In Vitro Osteoclastogenesis Induced by OSCC Cells or RANKL
4.4. Preparation of Exosomes and MVs
4.5. Detection of the Uptake of Exosomes into OPCs
4.6. In Vitro Osteoclastogenesis Induced by Exosomes
4.7. Quantitative RT-PCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| TNF-α | Tumor necrosis factor-alpha |
| OPCs | Osteoclast precursor cells |
| OSCC | Oral squamous cell carcinoma |
| NFATc1 | Nuclear factor of T cell c1 |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| PTHrP | Parathyroid hormone related peptide |
| BRONJ | Bisphosphonate-related osteonecrosis of the jaw |
| MRONJ | Medication related osteonecrosis of the jaw |
| OPG | Osteoprotegerin |
| CBD | Cannabidiol |
| DMA | Dimethyl amiloride |
| MVs | Microvesicles |
| 2-AG | 2-arachidonoylglycerol |
| CB1 | Cannabinoid receptor type 1 |
| CB2 | Cannabinoid receptor type 2 |
| TRPV1 | Transient receptor potential vanilloid type 1 |
| TRAP | Tartrate-resistant acid phosphatase |
References
- Reinhardt, R.A.; Payne, J.B.; Maze, C.A.; Patil, K.D.; Gallagher, S.J.; Mattson, J.S. Influence of estrogen and osteopenia/osteoporosis on clinical periodontitis in postmenopausal women. J. Periodontol. 1999, 70, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Khandani, A.; Camirand, A.; Harrison, R.E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 2011, 49, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kwan Tat, S.; Padrines, M.; Theoleyre, S.; Heymann, D.; Fortun, Y. IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [PubMed]
- Kayamori, K.; Sakamoto, K.; Nakashima, T.; Takayanagi, H.; Morita, K.; Omura, K.; Nguyen, S.T.; Miki, Y.; Iimura, T.; Himeno, A.; et al. Roles of interleukin-6 and parathyroid hormone-related peptide in osteoclast formation associated with oral cancers: Significance of interleukin-6 synthesized by stromal cells in response to cancer cells. Am. J. Pathol. 2010, 176, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Kawai, V.K.; Stein, C.M.; Perrien, D.S.; Griffin, M.R. Effects of anti-tumor necrosis factor alpha agents on bone. Curr. Opin. Rheumatol. 2012, 24, 576–585. [Google Scholar] [CrossRef]
- Suva, L.J.; Washam, C.; Nicholas, R.W.; Griffin, R.J. Bone metastasis: Mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011, 7, 208–218. [Google Scholar] [CrossRef]
- Guise, T.A.; Mundy, G.R. Cancer and bone. Endocr. Rev. 1998, 19, 18–54. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Dougall, W.C. Molecular pathways: Osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin. Cancer Res. 2012, 18, 326–335. [Google Scholar] [CrossRef]
- Croucher, P.I.; McDonald, M.M.; Martin, T.J. Bone metastasis: The importance of the neighbourhood. Nat. Rev. Cancer 2016, 16, 373–386. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Green, J.R. Bisphosphonates: Preclinical review. Oncologist 2004, 9, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L.; Burdett, S.; Rydzewska, L.H.M.; Albiges, L.; Clarke, N.W.; Fisher, D.; Fizazi, K.; Gravis, G.; James, N.D.; Mason, M.D.; et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: A systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016, 17, 243–256. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Aghaloo, T.L.; Felsenfeld, A.L.; Tetradis, S. Osteonecrosis of the jaw in a patient on Denosumab. J. Oral Maxillofac. Surg. 2010, 68, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Tempesta, A.; Limongelli, L.; Crincoli, V.; Iannone, F.; Lapadula, G.; Maiorano, E. A Case of Osteonecrosis of the Jaw in a Patient with Crohn’s Disease Treated with Infliximab. Am. J. Case Rep. 2017, 18, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Wada, A.; Tsuchiya, M.; Ozaki-Honda, Y.; Kayamori, K.; Sakamoto, K.; Yamaguchi, A.; Ikeda, T. A new osteoclastogenesis pathway induced by cancer cells targeting osteoclast precursor cells. Biochem. Biophys. Res. Commun. 2019, 509, 108–113. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Muto, A.; Udagawa, N.; Arai, A.; Yamashita, T.; Hosoya, A.; Ninomiya, T.; Nakamura, H.; Yamamoto, Y.; Kinugawa, S.; et al. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. J. Cell Biol. 2009, 184, 541–554. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Mould, R.; Henley, A.B.; Nunn, A.V.; Guy, G.W.; Thomas, E.L.; Inal, J.M.; Bell, J.D.; Lange, S. Cannabidiol (CBD) Is a Novel Inhibitor for Exosome and Microvesicle (EMV) Release in Cancer. Front. Pharmacol. 2018, 9, 889. [Google Scholar] [CrossRef]
- Wilson, C.M.; Naves, T.; Vincent, F.; Melloni, B.; Bonnaud, F.; Lalloue, F.; Jauberteau, M.O. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J. Cell Sci. 2014, 127, 3983–3997. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yoshioka, Y.; Ochiya, T. Extracellular vesicle transfer of cancer pathogenic components. Cancer Sci. 2016, 107, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Sun, R.; Wu, C.; Wang, L.; Zhang, C. Exosome: A Novel Approach to Stimulate Bone Regeneration through Regulation of Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2016, 17, 712. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Lundholm, M.; Widmark, A.; Persson, E. Tumor Cell-Derived Exosomes from the Prostate Cancer Cell Line TRAMP-C1 Impair Osteoclast Formation and Differentiation. PLoS ONE 2016, 11, e0166284. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, X.; Wang, H.; Li, J.; Dai, L.; Li, J.; Dong, C. Lung adenocarcinoma cell-derived exosomal miR-21 facilitates osteoclastogenesis. Gene 2018, 666, 116–122. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Ji, X.; Chen, X.; Yu, X. MicroRNAs in Osteoclastogenesis and Function: Potential Therapeutic Targets for Osteoporosis. Int. J. Mol. Sci. 2016, 17, 349. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshioka, Y.; Yamamoto, Y.; Ochiya, T. How cancer cells dictate their microenvironment: Present roles of extracellular vesicles. Cell. Mol. Life Sci. 2017, 74, 697–713. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and cancer: Therapeutic implication. Br. J. Pharmacol. 2011, 163, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Ralston, S.H. Cannabinoids and bone: Friend or foe? Calcif. Tissue Int. 2010, 87, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; van’t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar] [CrossRef]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA 2009, 0902743106. [Google Scholar] [CrossRef]
- Ikeda, T.; Kasai, M.; Suzuki, J.; Kuroyama, H.; Seki, S.; Utsuyama, M.; Hirokawa, K. Multimerization of the receptor activator of nuclear factor-kappaB ligand (RANKL) isoforms and regulation of osteoclastogenesis. J. Biol. Chem. 2003, 278, 47217–47222. [Google Scholar] [CrossRef]
- Gupta, S.; Knowlton, A.A. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3052–H3056. [Google Scholar] [CrossRef]
- Komaki, M.; Numata, Y.; Morioka, C.; Honda, I.; Tooi, M.; Yokoyama, N.; Ayame, H.; Iwasaki, K.; Taki, A.; Oshima, N.; et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res. Ther. 2017, 8, 219. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In Current Protocols in Cell Biology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006; Volume 30, pp. 3–22. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Kannan, R.; Kitamura, M.; Spee, C.; Barron, E.; Ryan, S.J.; Hinton, D.R. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS ONE 2010, 5, e12578. [Google Scholar] [CrossRef]
- Sakha, S.; Muramatsu, T.; Ueda, K.; Inazawa, J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci. Rep. 2016, 6, 38750. [Google Scholar] [CrossRef]
- Ohata, Y.; Tsuchiya, M.; Hirai, H.; Yamaguchi, S.; Akashi, T.; Sakamoto, K.; Yamaguchi, A.; Ikeda, T.; Kayamori, K. Leukemia inhibitory factor produced by fibroblasts within tumor stroma participates in invasion of oral squamous cell carcinoma. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuchiya, M.; Kayamori, K.; Wada, A.; Komaki, M.; Ohata, Y.; Hamagaki, M.; Sakamoto, K.; Ikeda, T. A Novel, Tumor-Induced Osteoclastogenesis Pathway Insensitive to Denosumab but Interfered by Cannabidiol. Int. J. Mol. Sci. 2019, 20, 6211. https://doi.org/10.3390/ijms20246211
Tsuchiya M, Kayamori K, Wada A, Komaki M, Ohata Y, Hamagaki M, Sakamoto K, Ikeda T. A Novel, Tumor-Induced Osteoclastogenesis Pathway Insensitive to Denosumab but Interfered by Cannabidiol. International Journal of Molecular Sciences. 2019; 20(24):6211. https://doi.org/10.3390/ijms20246211
Chicago/Turabian StyleTsuchiya, Maiko, Kou Kayamori, Akane Wada, Motohiro Komaki, Yae Ohata, Miwako Hamagaki, Kei Sakamoto, and Tohru Ikeda. 2019. "A Novel, Tumor-Induced Osteoclastogenesis Pathway Insensitive to Denosumab but Interfered by Cannabidiol" International Journal of Molecular Sciences 20, no. 24: 6211. https://doi.org/10.3390/ijms20246211
APA StyleTsuchiya, M., Kayamori, K., Wada, A., Komaki, M., Ohata, Y., Hamagaki, M., Sakamoto, K., & Ikeda, T. (2019). A Novel, Tumor-Induced Osteoclastogenesis Pathway Insensitive to Denosumab but Interfered by Cannabidiol. International Journal of Molecular Sciences, 20(24), 6211. https://doi.org/10.3390/ijms20246211





