Membrane Transporters in Citrus clementina Fruit Juice-Derived Nanovesicles
Abstract
1. Introduction
2. Results and Discussion
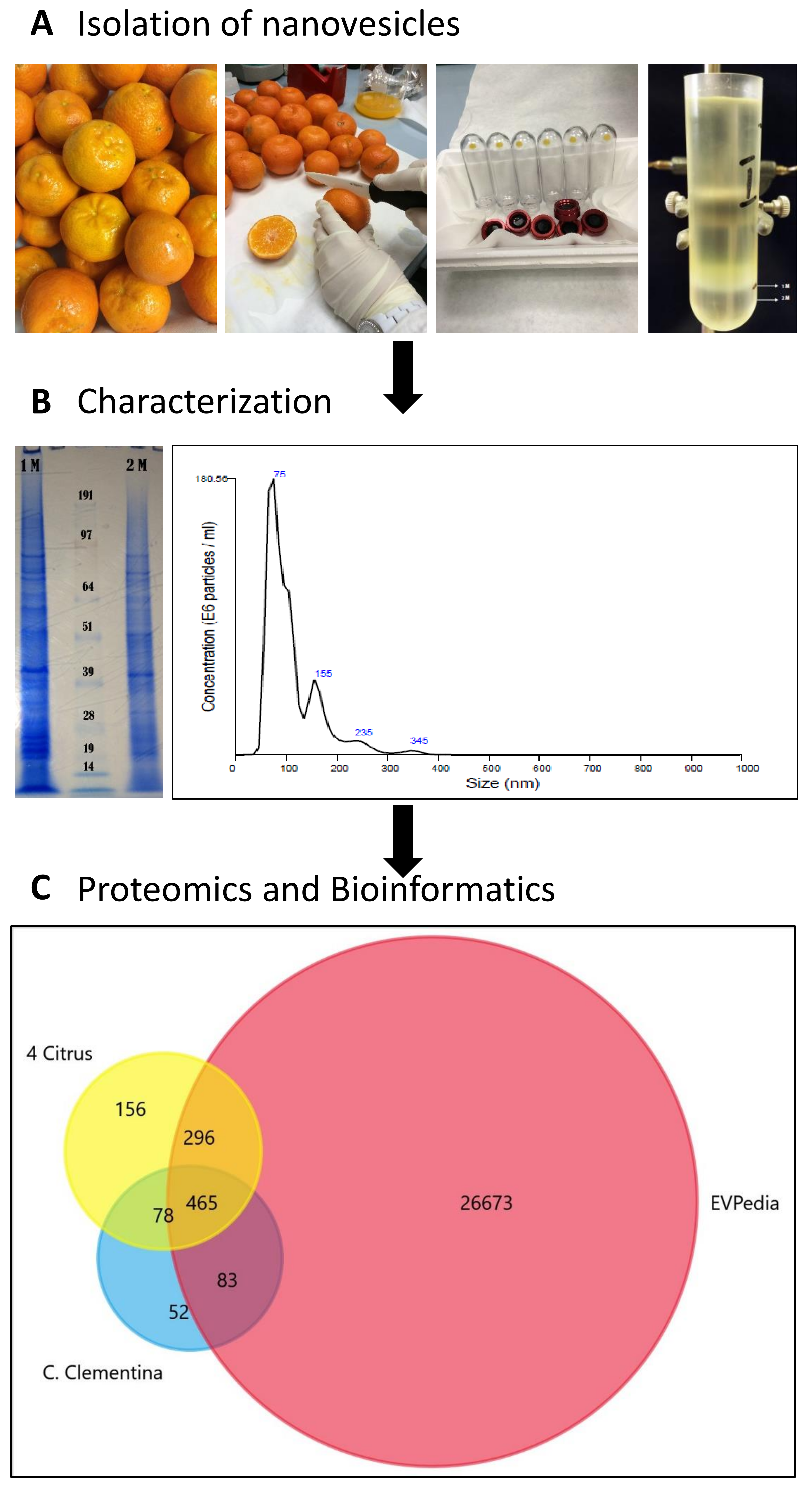
2.1. Isolation of Nanovesicles (NVs) from Clementine Juice
2.2. Characterization of Clementine Nanovesicles
2.3. The Protein Cargo of Citrus clementina Nanovesicles
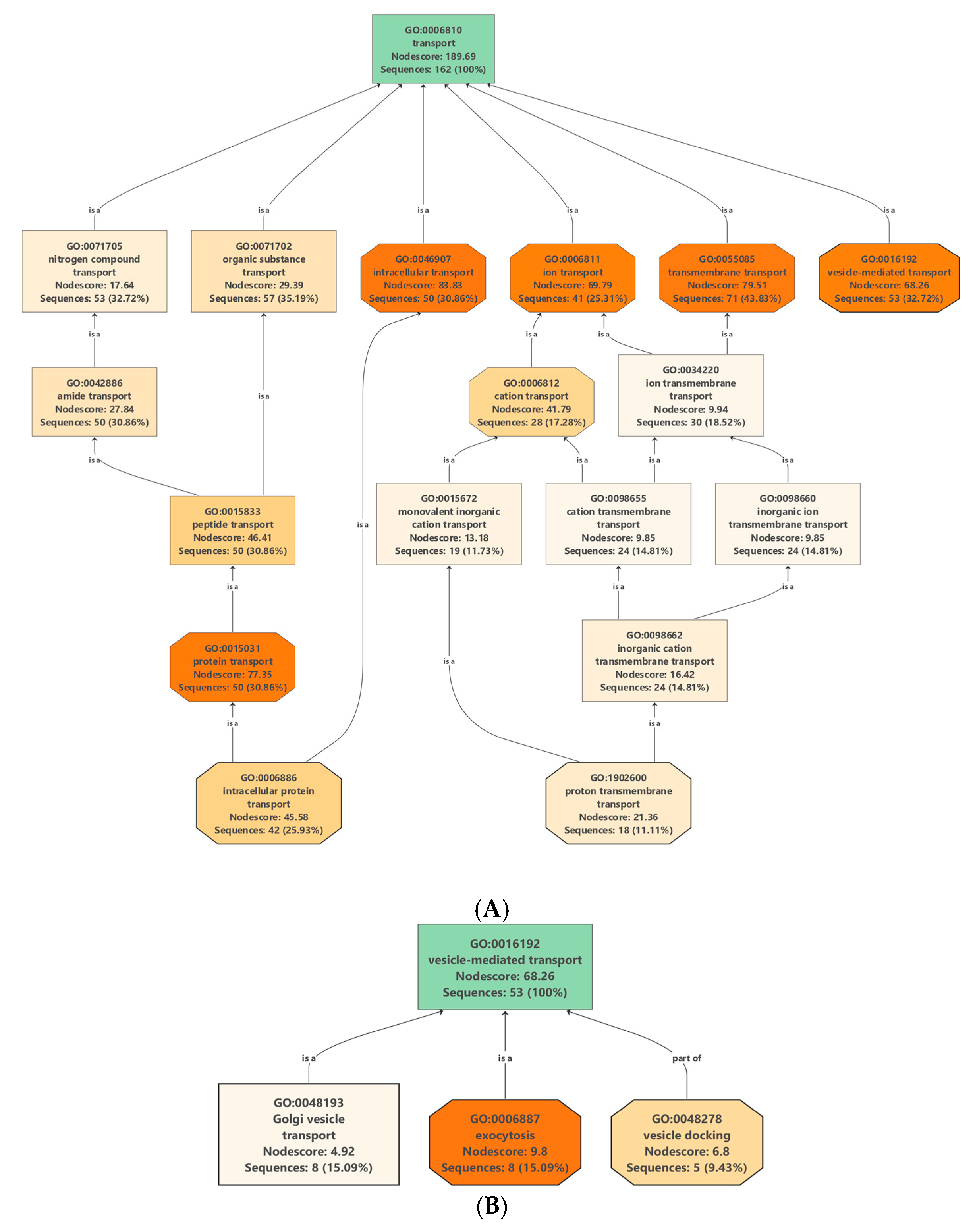
2.4. Proteins Involved in Molecular Transport
2.4.1. Transmembrane Transporters in Clementine Vesicles
2.4.2. Vesicle-Mediated Transport: Clathrin-Coated Vesicle (CCV), Coat Protein Complex I (COPI) and Coat Protein Complex II (COPII) Vesicles
2.4.3. Regulators of Trafficking: The Tetraspanins
3. Materials and Methods
3.1. Fruit Material and Vesicle Isolation
3.2. Physiochemical Characterization of Different Vesicle Populations
3.2.1. Nanoparticle Tracking Analysis
3.2.2. Proteomics and Data analysis
SDS-PAGE Analysis
Lysis of the Vesicles and Shotgun Proteomics Analysis
Nano Liquid Chromatography-Electrospray Ionization–Tandem Mass Spectrometry (NanoLC-ESI-MS/MS)
Protein Identification, Quantification and Statistical Analysis
Bioinformatics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Echeverría, E. Vesicle-mediated solute transport between the vacuole and the plasma membrane. Plant Physiol. 2000, 123, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Robinson, D.G. Transport vesicle formation in plant cells. Curr. Opin. Plant Biol. 2009, 12, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Van de Meene, A.M.L.; Doblin, M.S.; Bacic, A. The plant secretory pathway seen through the lens of the cell wall. Protoplasma 2017, 254, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; Elizalde, M.; de la Canal, L. Apoplastic exosome-like vesicles: A new way of protein secretion in plants? Plant Signal. Behav. 2012, 7, 544–546. [Google Scholar] [CrossRef]
- Krämer-Albers, E.-M.; Hill, A.F. Extracellular vesicles: Interneural shuttles of complex messages. Curr. Opin. Neurobiol. 2016, 39, 101–107. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.-B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Girbardt, M. Über die Substruktur von Polystictus versicolor L. Arch. Für Mikrobiol. 1957, 28, 255–269. [Google Scholar] [CrossRef]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef]
- Rome, S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019, 10, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, M.; Merlin, D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Lee, J.; Kim, S.R.; Choi, D.-S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. EVpedia: A community web portal for extracellular vesicles research. Bioinformatics 2015, 31, 933–939. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Forslund, K.; Cook, H.; Heller, D.; Walter, M.C.; Rattei, T.; Mende, D.R.; Sunagawa, S.; Kuhn, M.; et al. eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016, 44, D286–D293. [Google Scholar] [CrossRef]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2018, 43, 273–303. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Garcia-Ibañez, P.; Yepes-Molina, L.; Rios, J.J.; Carvajal, M. The expanding role of vesicles containing aquaporins. Cells 2018, 7, 179. [Google Scholar] [CrossRef]
- Maxson, M.E.; Grinstein, S. The vacuolar-type H+-ATPase at a glance—more than a proton pump. J. Cell Sci. 2014, 127, 4987–4993. [Google Scholar] [CrossRef]
- Trentmann, O.; Haferkamp, I. Current progress in tonoplast proteomics reveals insights into the function of the large central vacuole. Front. Plant Sci. 2013, 4, 34. [Google Scholar] [CrossRef]
- Kang, J.; Park, J.; Choi, H.; Burla, B.; Kretzschmar, T.; Lee, Y.; Martinoia, E. Plant ABC transporters. Arabidopsis Book 2011, 9, e0153. [Google Scholar] [CrossRef]
- Jimenez-Jimenez, S.; Hashimoto, K.; Santana, O.; Aguirre, J.; Kuchitsu, K.; Cárdenas, L. Emerging roles of tetraspanins in plant inter-cellular and inter-kingdom communication. Plant Signal. Behav. 2019, 14, e1581559. [Google Scholar] [CrossRef]
- Leijon, F.; Melzer, M.; Zhou, Q.; Srivastava, V.; Bulone, V. Proteomic analysis of plasmodesmata from populus cell suspension cultures in relation with callose biosynthesis. Front. Plant Sci. 2018, 9, 1681. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Fiume, I.; Capasso, G.; Pocsfalvi, G. Isolation of exosome-like vesicles from plants by ultracentrifugation on sucrose/Deuterium Oxide (D2O) density cushions. In Unconventional Protein Secretion: Methods and Protocols; Pompa, A., De Marchis, F., Eds.; Springer: New York, NY, USA, 2016; pp. 259–269. ISBN 978-1-4939-3804-9. [Google Scholar]
- Käll, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
| Accession UniProt | UniProt Description | MW [kDa] | pI | Scores | COG | EggNOG Description | EVpedia | 4 Citrus |
|---|---|---|---|---|---|---|---|---|
| V4V9V7 | Clathrin heavy chain | 192.8 | 5.3 | 814.0 | ENOG410XPH1 | Clathrin heavy chain | 451 | Y |
| V4T403 | Uncharacterized protein | 64.6 | 4.9 | 906.9 | ENOG410XRSQ | Carrier protein, Patellin-3 like | 44 | Y |
| V4TVM6 | Uncharacterized protein | 101.2 | 5.8 | 740.5 | COG0542 | Heat shock protein | 61 | Y |
| V4VRS8 | Uncharacterized protein | 110.9 | 6.1 | 646.6 | COG2352 | Phosphoenolpyruvate carboxylase | 10 | N |
| V4UFD6 | Uncharacterized protein | 71.0 | 5.1 | 860.1 | COG0443 | Heat shock cognate 70 kDa | 608 | Y |
| V4SV61 | Uncharacterized protein | 61.0 | 5.7 | 880.4 | COG0696 | Phosphoglycerate mutase | 15 | Y |
| V4SQM8 | Uncharacterized protein | 154.8 | 4.7 | 621.9 | n.d. | Unknown | n.d. | N |
| V4WGV2 | Uncharacterized protein | 80.0 | 5.0 | 730.9 | COG0326 | Heat shock protein | 557 | Y |
| V4SQ92 | Uncharacterized protein | 41.7 | 5.3 | 757.5 | COG5277 | Actins | 592 | Y |
| V4ULL2 | Uncharacterized protein | 94.1 | 5.1 | 659.3 | COG0443 | Heat-shock 70 kDa protein | 608 | Y |
| V4SU87 | Plasma membrane ATPase | 105.2 | 6.1 | 810.6 | COG0474 | Plasma membrane ATPase | 378 | Y |
| V4TDB5 | Uncharacterized protein | 68.6 | 5.3 | 710.6 | COG1155 | V-type proton ATPase catalytic subunit | 21 | Y |
| V4U840 | Uncharacterized protein | 93.8 | 5.9 | 759.8 | COG0480 | Elongation factor | 500 | Y |
| V4T1U7 | Uncharacterized protein | 47.1 | 6.0 | 898.2 | COG0446 | Monodehydroascorbate reductase | 112 | Y |
| V4W5U2 | Uncharacterized protein | 63.3 | 5.5 | 696.8 | COG0033 | Phosphoglucomutase | 217 | Y |
| V4RUA3 | Alpha-1,4 glucan phosphorylase | 95.2 | 7.0 | 780.2 | COG0058 | Phosphorylase | 297 | Y |
| V4TVW5 | Sucrose synthase | 92.6 | 6.0 | 660.8 | COG0438 | Sucrose-cleaving enzyme | 154 | Y |
| V4UJC4 | Uncharacterized protein | 90.2 | 6.3 | 649.7 | COG0339 | Oligopeptidase | 142 | Y |
| V4TCA6 | Uncharacterized protein | 252.7 | 6.0 | 601.6 | COG0439 | Acetyl-CoA Carboxylase | 159 | Y |
| V4SL33 | Uncharacterized protein | 89.4 | 5.1 | 575.2 | COG0464 | Cell-division cycle protein 48 | 479 | Y |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanly, C.; Moubarak, M.; Fiume, I.; Turiák, L.; Pocsfalvi, G. Membrane Transporters in Citrus clementina Fruit Juice-Derived Nanovesicles. Int. J. Mol. Sci. 2019, 20, 6205. https://doi.org/10.3390/ijms20246205
Stanly C, Moubarak M, Fiume I, Turiák L, Pocsfalvi G. Membrane Transporters in Citrus clementina Fruit Juice-Derived Nanovesicles. International Journal of Molecular Sciences. 2019; 20(24):6205. https://doi.org/10.3390/ijms20246205
Chicago/Turabian StyleStanly, Christopher, Maneea Moubarak, Immacolata Fiume, Lilla Turiák, and Gabriella Pocsfalvi. 2019. "Membrane Transporters in Citrus clementina Fruit Juice-Derived Nanovesicles" International Journal of Molecular Sciences 20, no. 24: 6205. https://doi.org/10.3390/ijms20246205
APA StyleStanly, C., Moubarak, M., Fiume, I., Turiák, L., & Pocsfalvi, G. (2019). Membrane Transporters in Citrus clementina Fruit Juice-Derived Nanovesicles. International Journal of Molecular Sciences, 20(24), 6205. https://doi.org/10.3390/ijms20246205






