Astaxanthin Ameliorates Ischemic-Hypoxic-Induced Neurotrophin Receptor p75 Upregulation in the Endothelial Cells of Neonatal Mouse Brains
Abstract
1. Introduction
2. Results
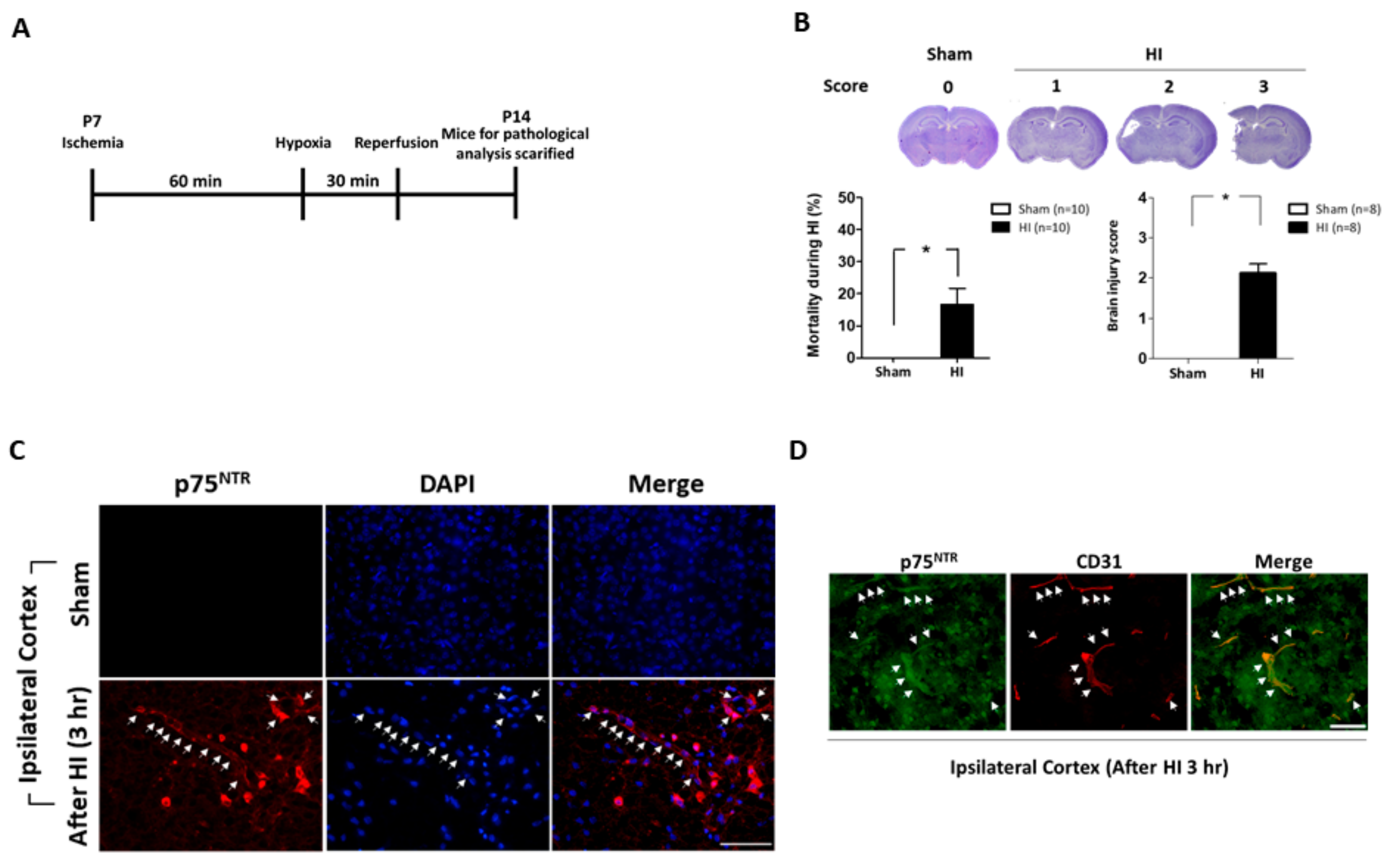
2.1. Neonatal Mice Brains Were Severely Damaged after Ischemic-Hypoxic Reperfusion
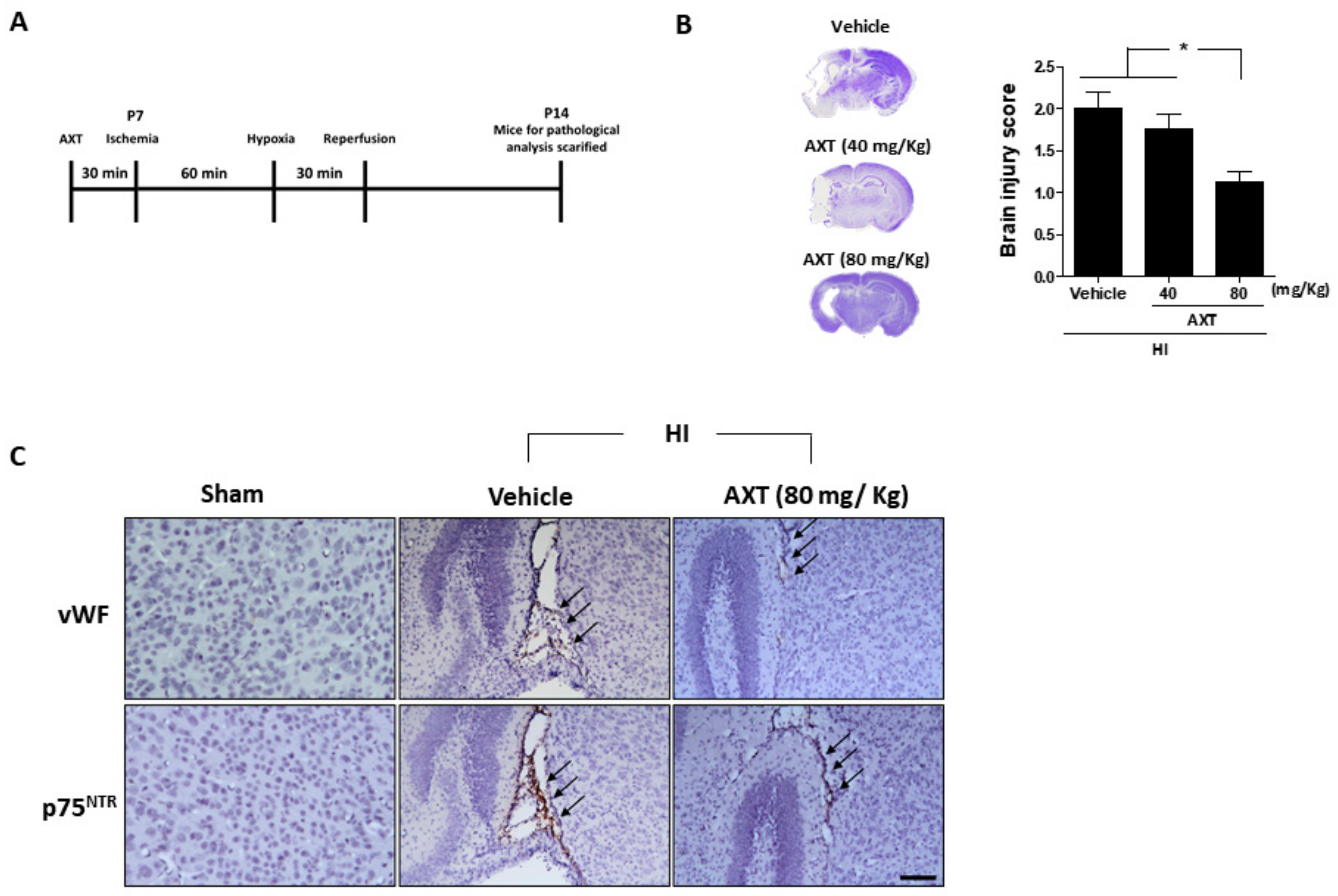
2.2. AXT Treatment Effectively Decreased HI-Induced Brain Injury in Neonatal Mice
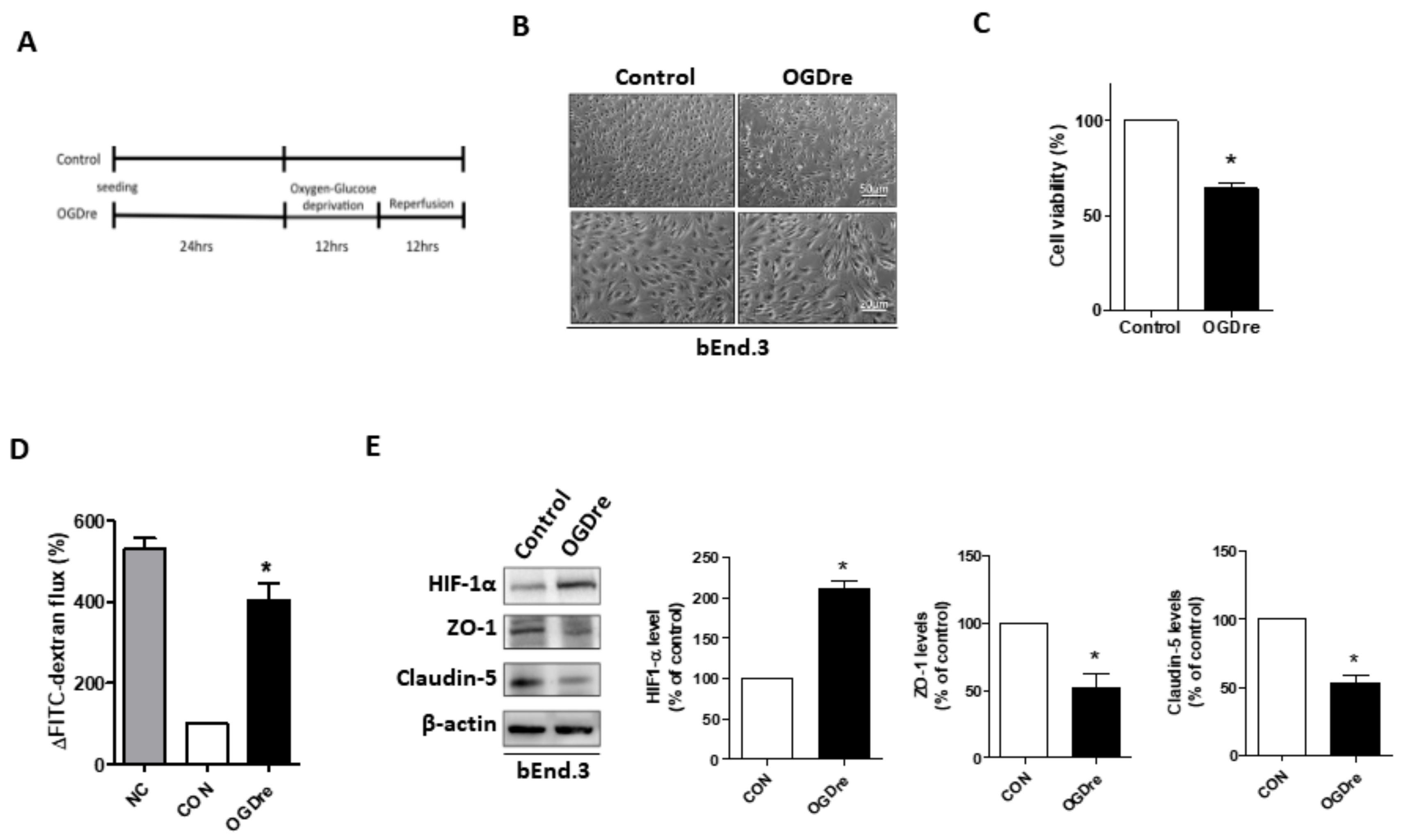
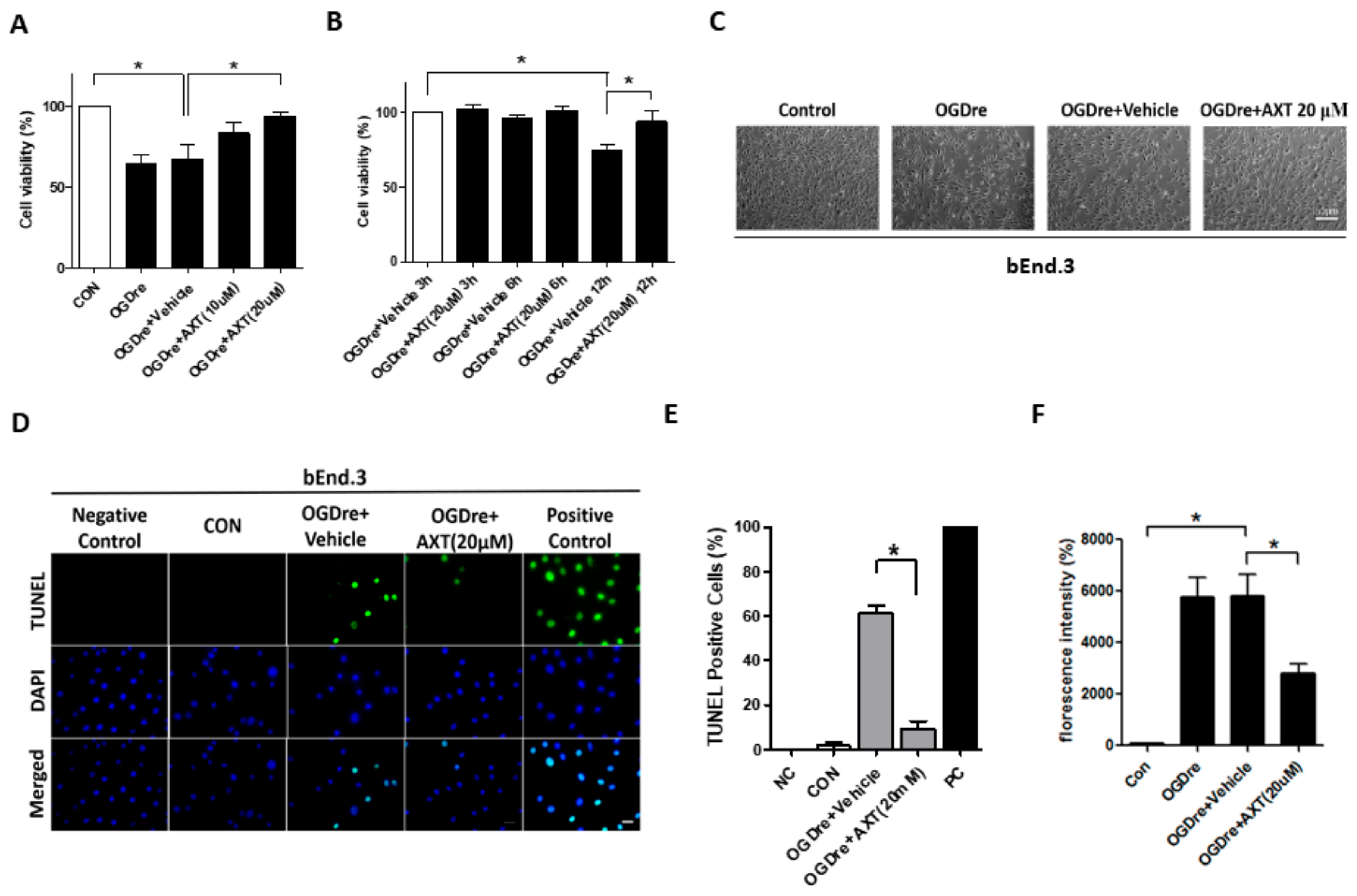
2.3. Oxygen-Glucose Deprivation/Reperfusion Treatment Decreased the Cell Viability and Tight Junction Stability of bEnd.3 Cells
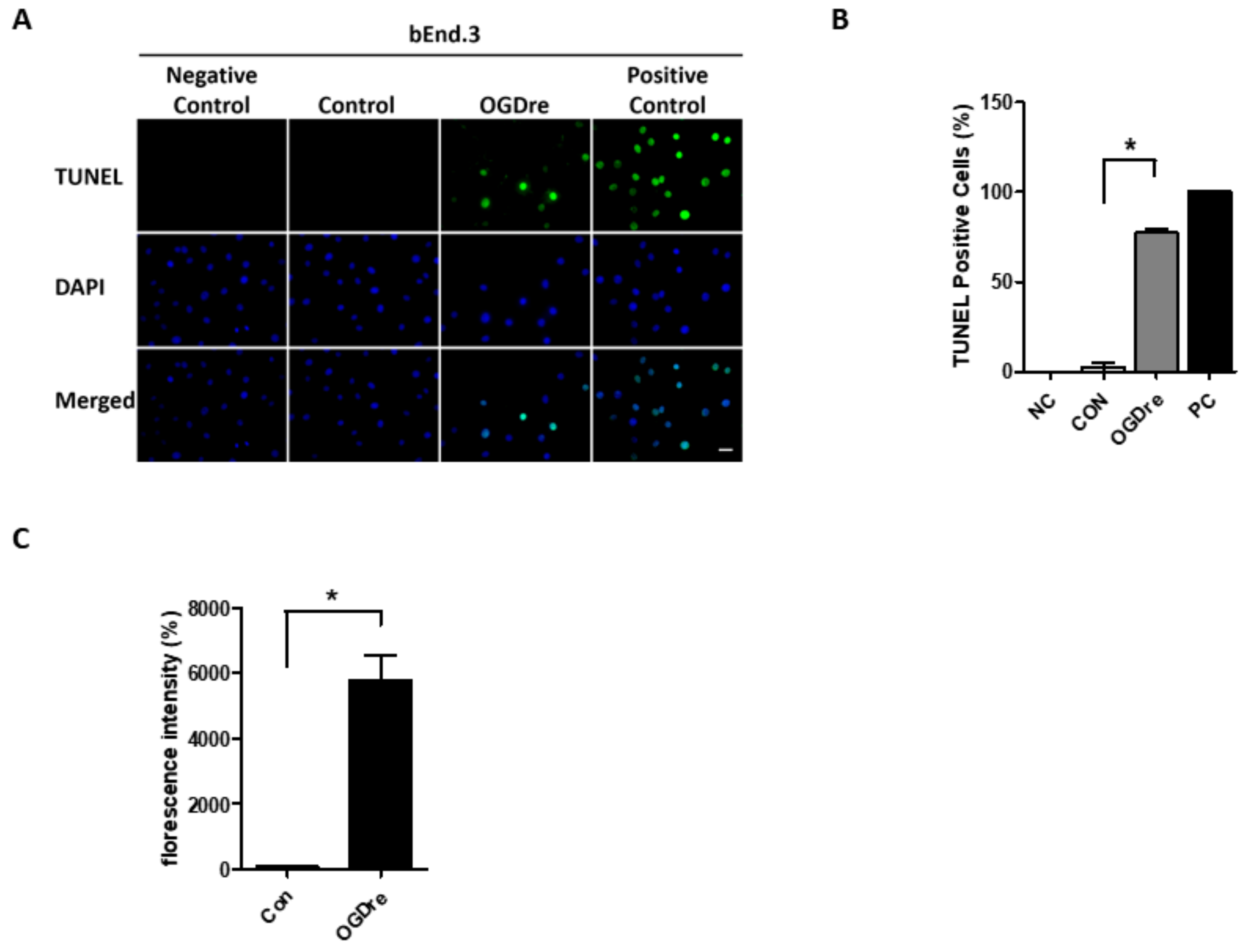
2.4. Apoptosis Was Induced by Oxygen-Glucose Deprivation/Reperfusion Injury
2.5. AXT Attenuates Oxygen-Glucose Deprivation/Reperfusion-Induced Cell Survival and Apoptosis
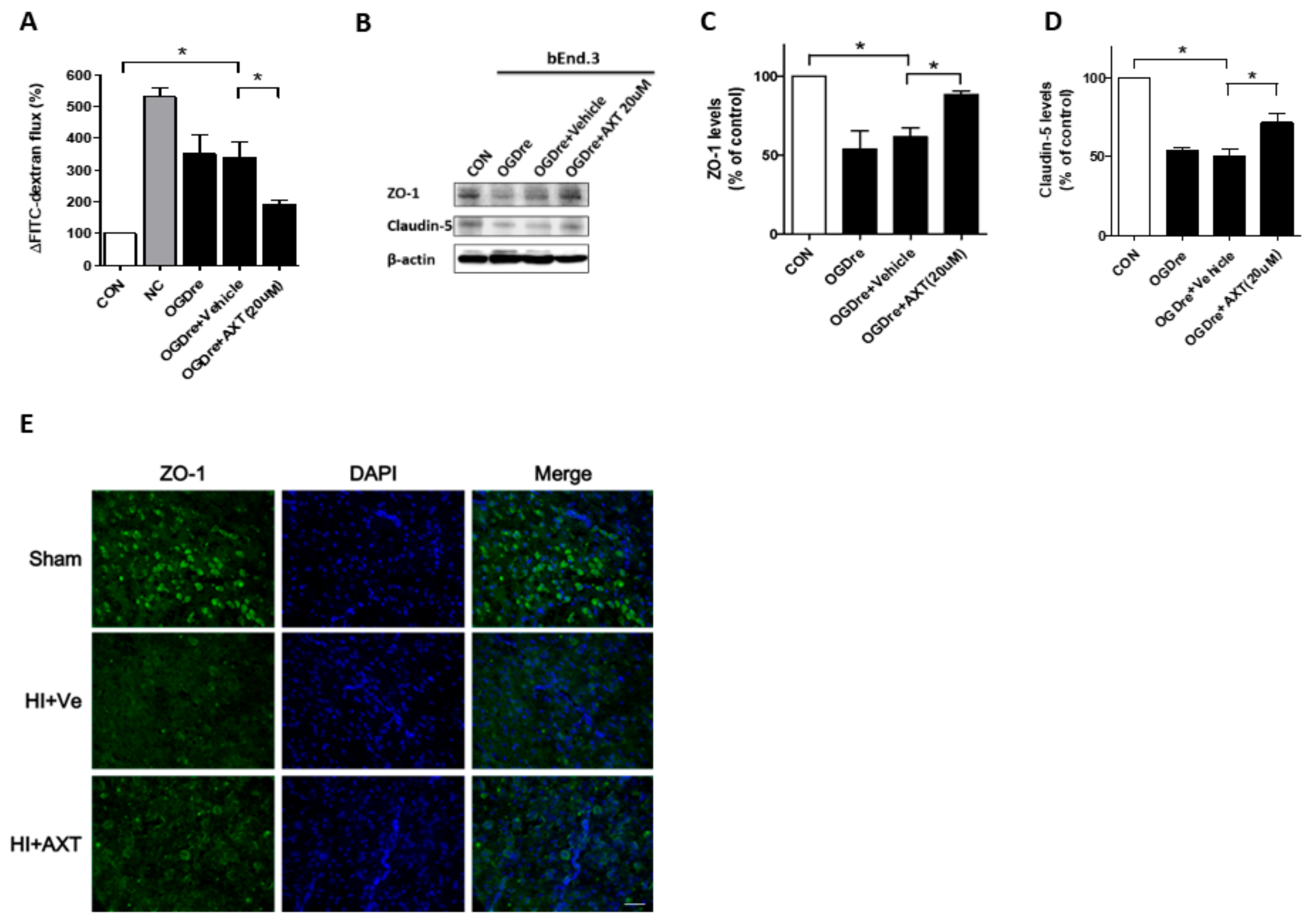
2.6. AXT Maintains the Tight Junction Stability of bEnd.3 Cells
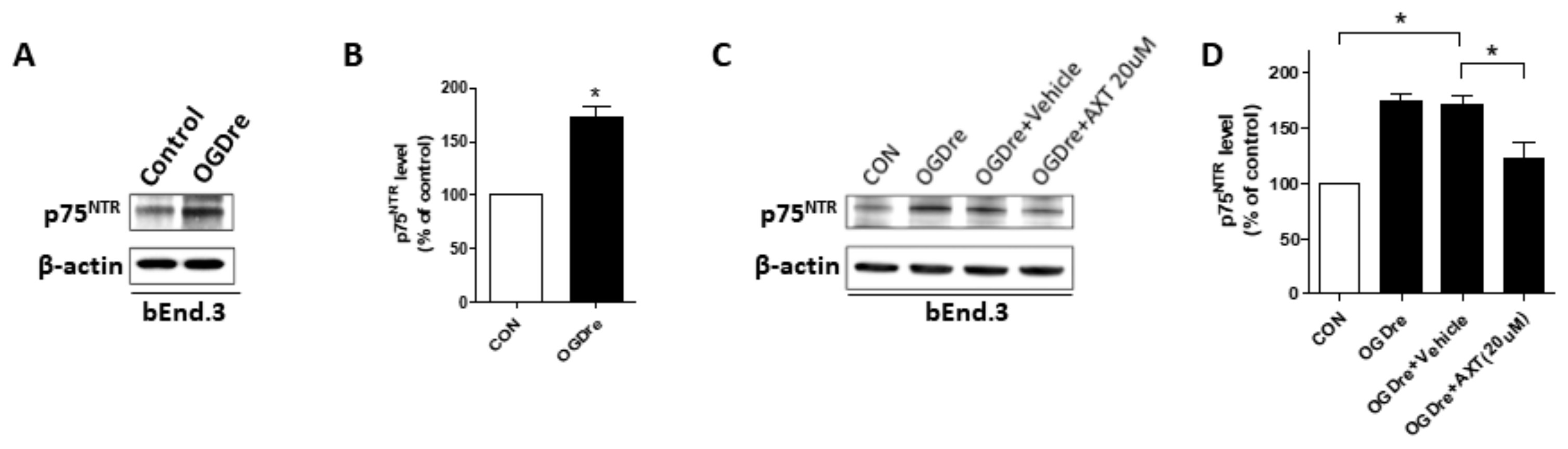
2.7. p75NTR is Expressed in Cerebromicrovascular Endothelial Cells with Enhanced Expression after OGD Reperfusion
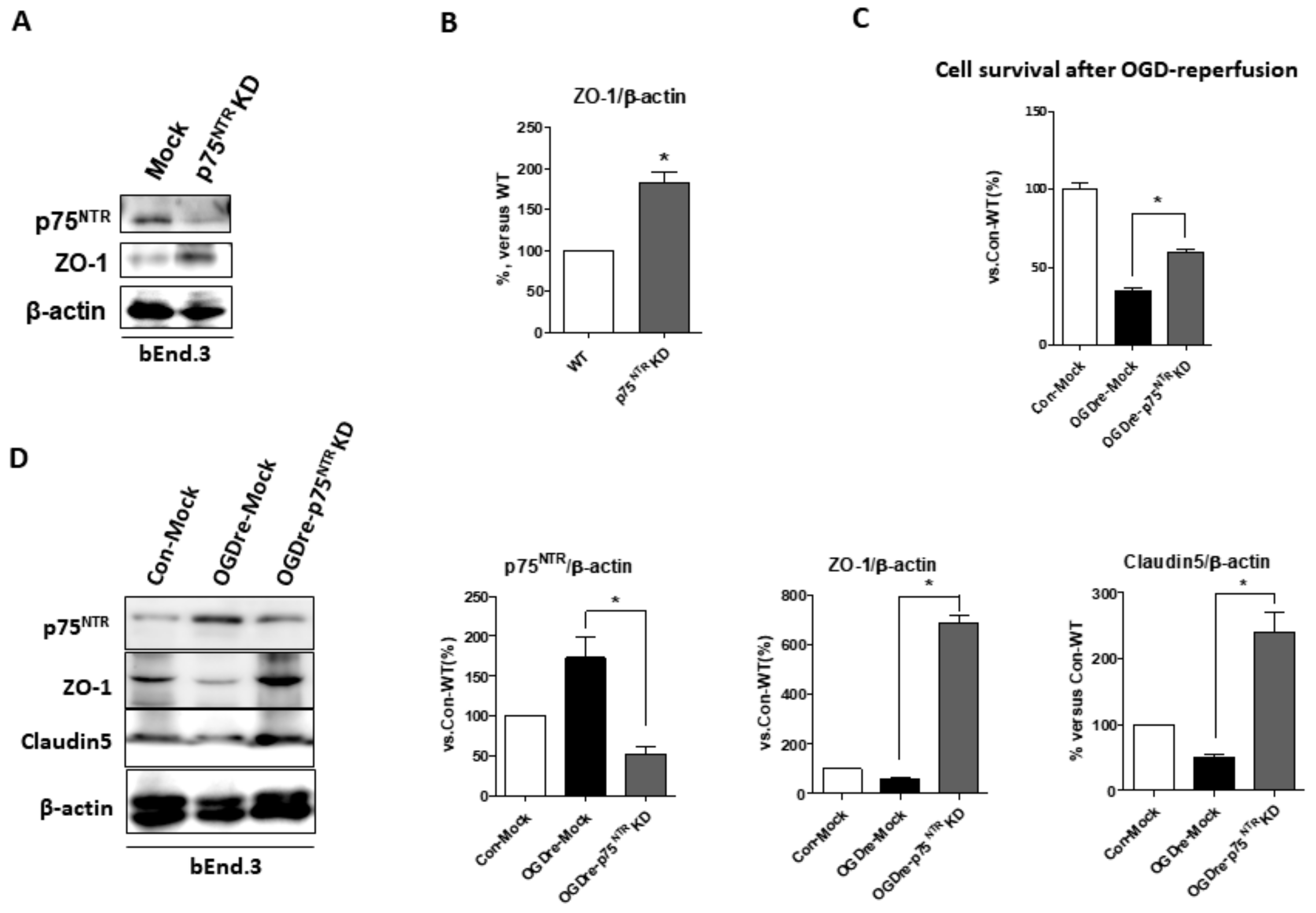
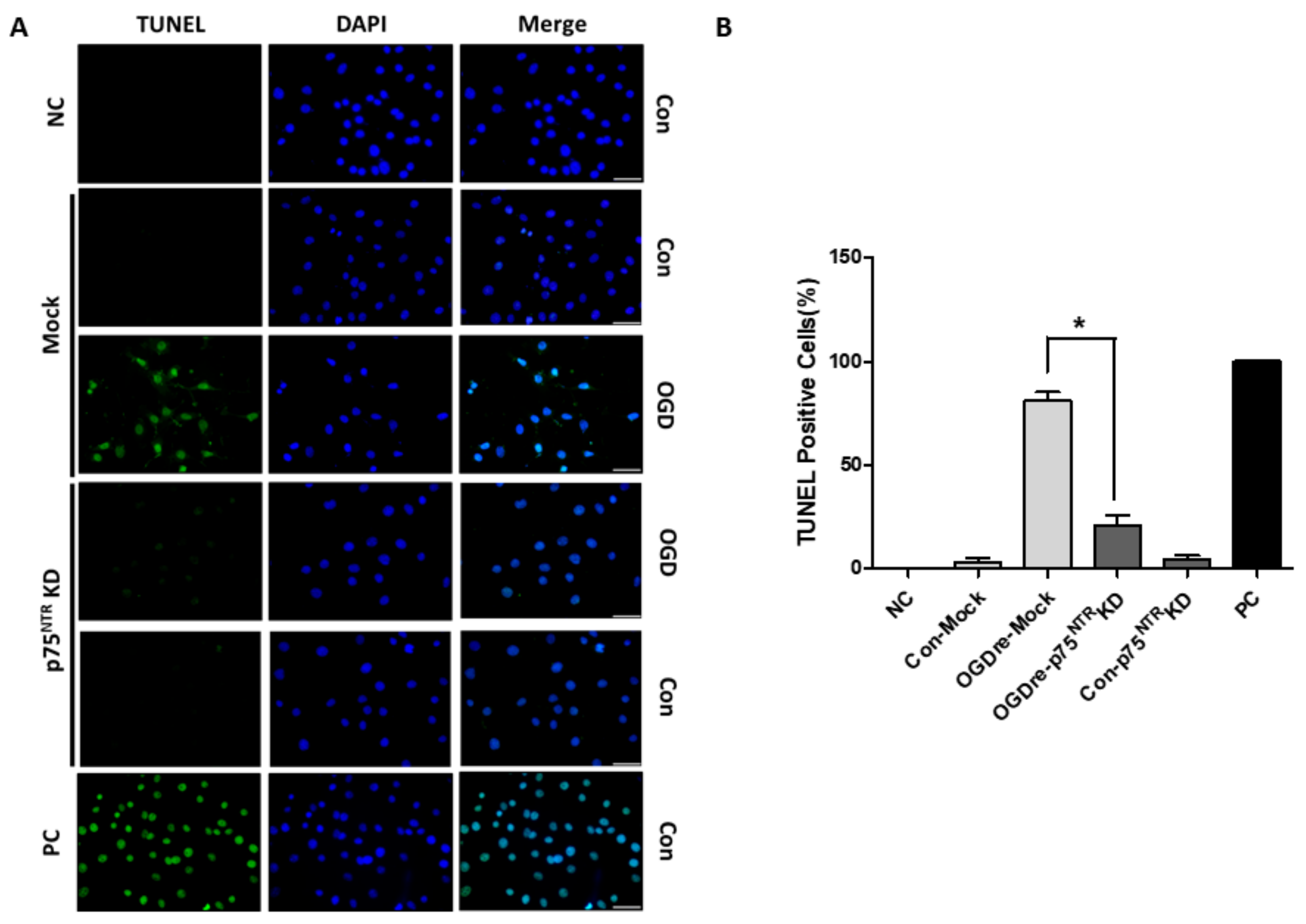
2.8. p75NTR Is Required for Unstable Tight Junction and Survival in OGDre-Induced Injury
3. Discussion
4. Materials and Methods
4.1. Reagents and Assay Kits
4.2. A Mice-Pup HI Model and AXT Treatment
4.3. Cell Culture and OGD Reperfusion Conditions
4.4. AXT Administration in Cell Culture
4.5. Cell Viability
4.6. Western Blot Analysis
4.7. Dextran for Endothelial Permeability Assay
4.8. TUNEL Assay
4.9. Measurement of Intracellular Reactive Oxygen Species (ROS) Levels Assay
4.10. Immunochemistry and Double-Fluorescence Immunocytochemistry
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Falluji, N.; Abou-Chebl, A.; Castro, C.; Mukherjee, D. Reperfusion strategies for acute ischemic stroke. Angiology 2012, 63, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J. Neurology of The Newborn, 5th ed.; Saunders: Philadelphia, PA, USA, 2008. [Google Scholar]
- Sandoval, K.; Witt, K. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Khatri, R.; Mckinney, A.; Swenson, B.; Janardhan, V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012, 25, 79. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, V.; Qureshi, A. Mechanisms of ischemic brain injury. Curr. Cardiol. Rep. 2004, 6, 117–123. [Google Scholar] [CrossRef]
- Chen, G.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Eltzschig, H.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Peters, O.; Back, T.; Lindauer, U.; Busch, C.; Megow, D.; Dreier, J.; Dirnagl, U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow. Metab. 1998, 18, 196–205. [Google Scholar] [CrossRef]
- Olmez, I.; Ozyurt, H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem. Int. 2012, 60, 208–212. [Google Scholar] [CrossRef]
- Vitturi, D.; Patel, R. Current perspectives and challenges in understanding the role of nitrite as an integral player in nitric oxide biology and therapy. Free Radic. Biol. Med. 2011, 51, 805–812. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Schreibelt, G.; Kooij, G.; Reijerkerk, A.; van Doorn, R.; Gringhuis, S.I.; van der Pol, S.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Piontek, J.; et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007, 21, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chang, Y.; Ho, C.; Huang, C. Ischemic preconditioning reduces neurovascular damage after hypoxia-ischemia via the cellular inhibitor of apoptosis 1 in neonatal brain. Stroke 2013, 44, 162–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lawther, B.; Kumar, S.; Krovvidi, H. Blood-brain barrier. Contin. Educ. Anaesth. Crit. Care Pain 2011, 11, 128–132. [Google Scholar] [CrossRef]
- Najjar, S.; Pearlman, D.; Devinsky, O.; Najjar, A.; Zagzag, D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: A review of clinical and experimental evidence. J. Neuroinflamm. 2013, 10, 142. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, H.; Pan, Q.; Zhao, Y.; Chen, J.; Zhao, B.; Chen, Y. Hypoxia/Aglycemia-induced endothelial barrier dysfunction and tight junction protein downregulation can be ameliorated by citicoline. PLoS ONE 2013, 8, e82604. [Google Scholar] [CrossRef]
- Inamura, A.; Adachi, Y.; Inoue, T.; He, Y.; Tokuda, N.; Nawata, T.; Shirao, S.; Nomura, S.; Fujii, M.; Ikeda, E.; et al. Cooling treatment transiently increases the permeability of brain capillary endothelial cells through translocation of claudin-5. Neurochem. Res. 2013, 38, 1641–1647. [Google Scholar] [CrossRef]
- Xia, Y.; He, Q.; Li, Y.; Chen, S.; Huang, M.; Wang, Y.; Gao, Y.; Huang, Y.; Wang, M.-D.; Mao, L.; et al. Recombinant human sonic hedgehog protein regulates the expression of ZO-1 and occludin by activating angiopoietin-1 in stroke damage. PLoS ONE 2013, 8, e68891. [Google Scholar] [CrossRef]
- Freund, V.; Frossard, N. Expression of nerve growth factor in the airways and its possible role in asthma. Prog. Brain Res. 2004, 146, 335–346. [Google Scholar]
- Fiore, M.; Chaldakov, G.; Aloe, L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev. Neurosci. 2009, 20, 133–145. [Google Scholar] [CrossRef]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef]
- Lee, D.; Kim, C.; Lee, Y. Astaxanthin protects against MPTP/MPP -induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011, 49, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kuo, C.C.; Chou, J.; Delvolve, A.; Jackson, S.N.; Post, J.; Woods, A.S.; Hoffer, B.J.; Wang, Y.; Harvey, B.K. Astaxanthin reduces ischemic brain injury in adult rats. Faseb J. 2009, 23, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, Y.J.; Kwon, K.H. Neuroprotective effects of astaxanthin in oxygen-glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. J. Clin. Biochem. Nutr. 2010, 47, 121–129. [Google Scholar] [CrossRef]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Wang, M.; Liu, W.; Gu, X.; Lv, C. Astaxanthin induces mitochondria-mediated apoptosis in rat hepatocellular carcinoma CBRH-7919 cells. Biol. Pharm. Bull. 2011, 34, 839–844. [Google Scholar] [CrossRef]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Kimelberg, H. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia 2005, 50, 389–397. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharm. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- DeFreitas, M.F.; McQuillen, P.S.; Shatz, C.J. A novel p75NTR signaling pathway promotes survival, not death, of immunopurified neocortical subplate neurons. J. Neurosci. 2001, 21, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Gorman, A.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, F.S.; Quann, E.J.; Pattabiraman, N.; Wynne, S.; Djakiew, D. Carprofen induction of p75NTR-dependent apoptosis via the p38 mitogen-activated protein kinase pathway in prostate cancer cells. Mol. Cancer 2008, 7, 3539–3545. [Google Scholar] [CrossRef] [PubMed]
- Gamaley, I.; Klyubin, I. Roles of reactive oxygen species: Signaling and regulation of cellular functions. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 1999; pp. 203–255. [Google Scholar]
- Mi, Z.; Rogers, D.A.; Mirnics, Z.K.; Schor, N.F. p75NTR-dependent modulation of cellular handling of reactive oxygen species. J. Neurochem. 2009, 110, 295–306. [Google Scholar] [CrossRef]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Morita, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999, 147, 185–194. [Google Scholar] [CrossRef]
- Tam, S.J.; Watts, R.J. Connecting vascular and nervous system development: Angiogenesis and the blood-brain barrier. Annu. Rev. Neurosci. 2010, 33, 379–408. [Google Scholar] [CrossRef]
- Bojarski, C.; Weiske, J.; Schöneberg, T.; Schröder, W.; Mankertz, J.; Schulzke, J.D.; Florian, P.; Fromm, M.; Tauber, R.; Huber, O. The specific fates of tight junction proteins in apoptotic epithelial cells. J. Cell Sci. 2004, 117, 2097–2107. [Google Scholar] [CrossRef]
- Riccioni, G.; D’Orazio, N.; Salvatore, C.; Franceschelli, S.; Pesce, M.; Speranza, L. Carotenoids and vitamins C and E in the prevention of cardiovascular disease. Int. J. Vitam. Nutr. Res. 2012, 82, 15–26. [Google Scholar] [CrossRef]
- Riccioni, G.; Speranza, L.; Pesce, M.; Cusenza, S.; D’Orazio, N.; Glade, M.J. Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition 2012, 28, 605–610. [Google Scholar] [CrossRef]
- Hosomi, N.; Ban, C.R.; Naya, T.; Takahashi, T.; Guo, P.; Song, X.Y.; Kohno, M. Tumor necrosis factor-α neutralization reduced cerebral edema through inhibition of matrix metalloproteinase production after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2005, 25, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Osawa, T. Astaxanthin protects neuronal cells against oxidative damage and is a potent candidate for brain food. Forum. Nutr. 2009, 61, 129–135. [Google Scholar] [PubMed]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar] [PubMed]
- Yoshihisa, Y.; Rehman, M.U.; Shimizu, T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp. Derm. 2014, 23, 178–183. [Google Scholar] [CrossRef]
- Dong, L.Y.; Jin, J.; Lu, G.; Kang, X.L. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar. Drugs 2013, 11, 960–974. [Google Scholar] [CrossRef]
- Chiou, M.H. A Strain of Genetically Reengineered Escherichia Coli for Biosynthesis of High Yield Carotenoids after Mutation Screening. U.S. Patent EP2088199A1, 12 August 2009. [Google Scholar]
- Lin, Y.J.; Lin, J.Y.; Wang, D.S.; Chen, C.H.; Chiou, M.H. Safety assessment of astaxanthin derived from engineered Escherichia coli K-12 using a 13-week repeated dose oral toxicity study and a prenatal developmental toxicity study in rats. Regul. Toxicol. Pharmacol. 2017, 87, 95–105. [Google Scholar] [CrossRef]
- Zhao, J.; Meng, M.; Zhang, J.; Li, L.; Zhu, X.; Zhang, L.; Wang, C.; Gao, M. Astaxanthin ameliorates renal interstitial fibrosis and peritubular capillary rarefaction in unilateral ureteral obstruction. Mol. Med. Rep. 2019, 19, 3168–3178. [Google Scholar] [CrossRef]
- Lee, H.T.; Chang, Y.C.; Tu, Y.F.; Huang, C.C. VEGF-A/VEGFR-2 signaling leading to cAMP response element-binding protein phosphorylation is a shared pathway underlying the protective effect of preconditioning on neurons and endothelial cells. J. Neurosci. 2009, 29, 4356–4368. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, M.-H.; Lee, H.-F.; Tu, Y.-F.; Lin, L.-H.; Cheng, Y.-Y.; Lee, H.-T. Astaxanthin Ameliorates Ischemic-Hypoxic-Induced Neurotrophin Receptor p75 Upregulation in the Endothelial Cells of Neonatal Mouse Brains. Int. J. Mol. Sci. 2019, 20, 6168. https://doi.org/10.3390/ijms20246168
Kuo M-H, Lee H-F, Tu Y-F, Lin L-H, Cheng Y-Y, Lee H-T. Astaxanthin Ameliorates Ischemic-Hypoxic-Induced Neurotrophin Receptor p75 Upregulation in the Endothelial Cells of Neonatal Mouse Brains. International Journal of Molecular Sciences. 2019; 20(24):6168. https://doi.org/10.3390/ijms20246168
Chicago/Turabian StyleKuo, Min-Hsun, Hung-Fu Lee, Yi-Fang Tu, Li-Hsuan Lin, Ya-Yun Cheng, and Hsueh-Te Lee. 2019. "Astaxanthin Ameliorates Ischemic-Hypoxic-Induced Neurotrophin Receptor p75 Upregulation in the Endothelial Cells of Neonatal Mouse Brains" International Journal of Molecular Sciences 20, no. 24: 6168. https://doi.org/10.3390/ijms20246168
APA StyleKuo, M.-H., Lee, H.-F., Tu, Y.-F., Lin, L.-H., Cheng, Y.-Y., & Lee, H.-T. (2019). Astaxanthin Ameliorates Ischemic-Hypoxic-Induced Neurotrophin Receptor p75 Upregulation in the Endothelial Cells of Neonatal Mouse Brains. International Journal of Molecular Sciences, 20(24), 6168. https://doi.org/10.3390/ijms20246168





