Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities
Abstract
1. Introduction
2. Results
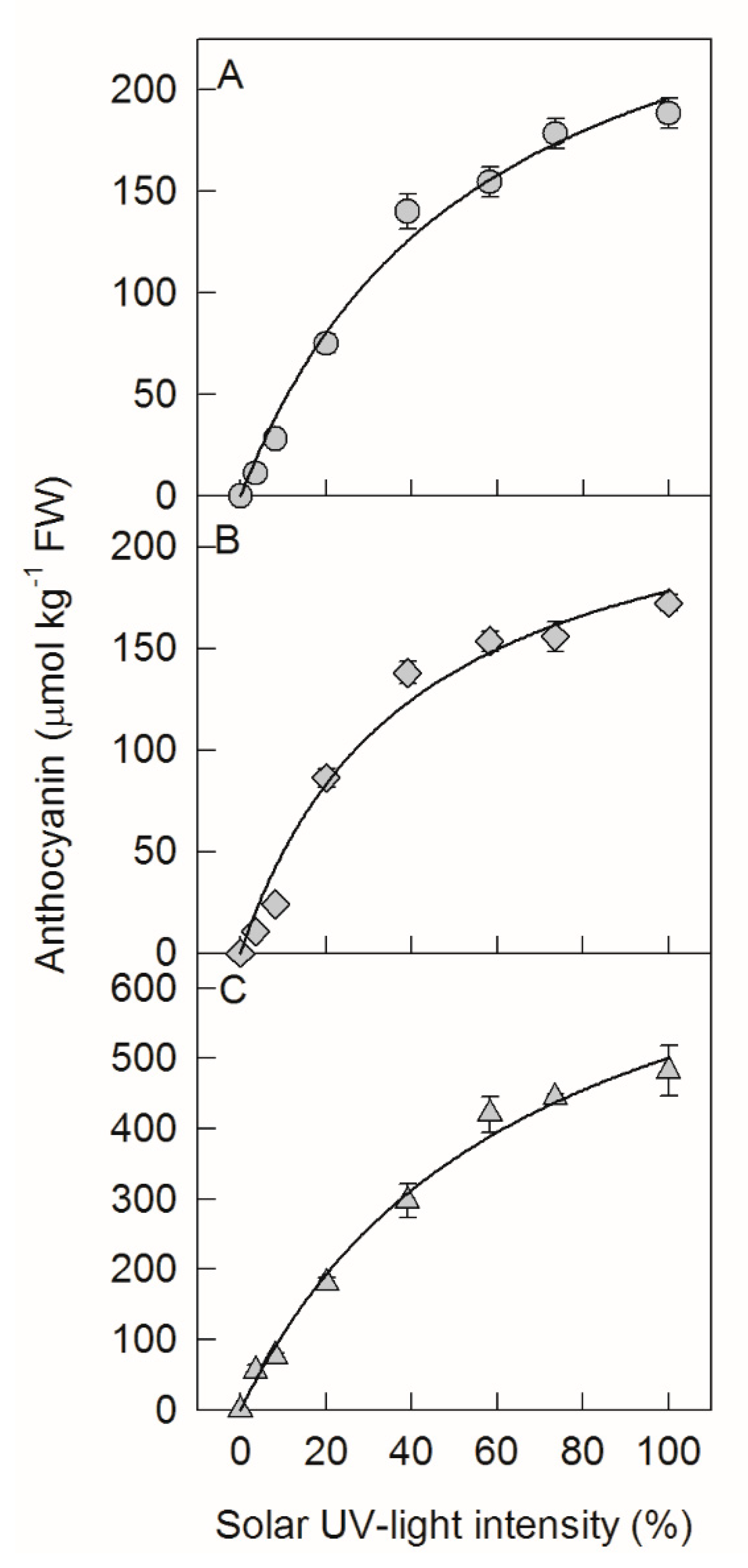
2.1. Anthocyanin Concentrations under Different Sunlight Intensities Fit with Michaelis–Menten Equation
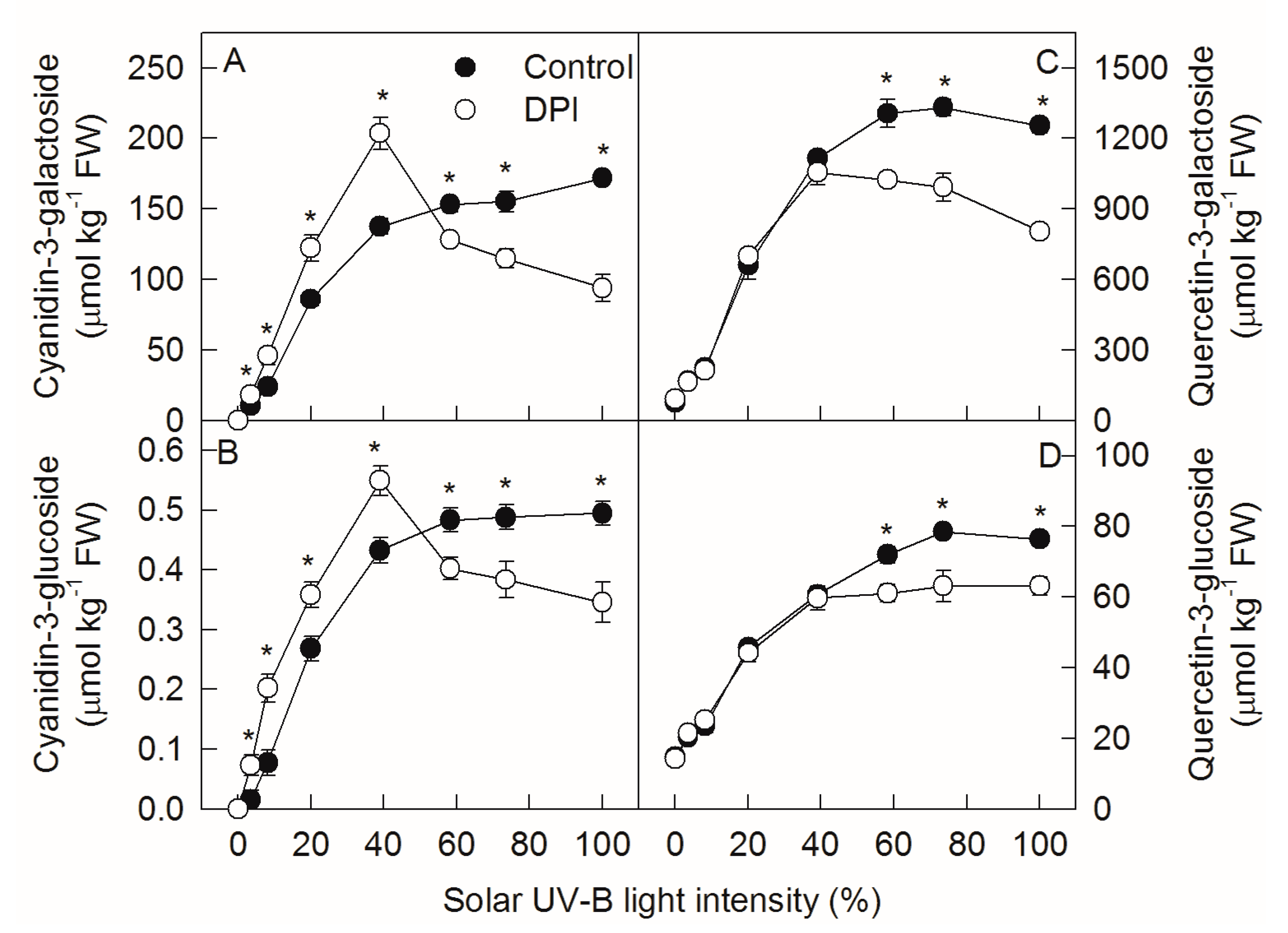
2.2. Analysis of Anthocyanin and Flavonol Concentrations under Different Sunlight Intensities
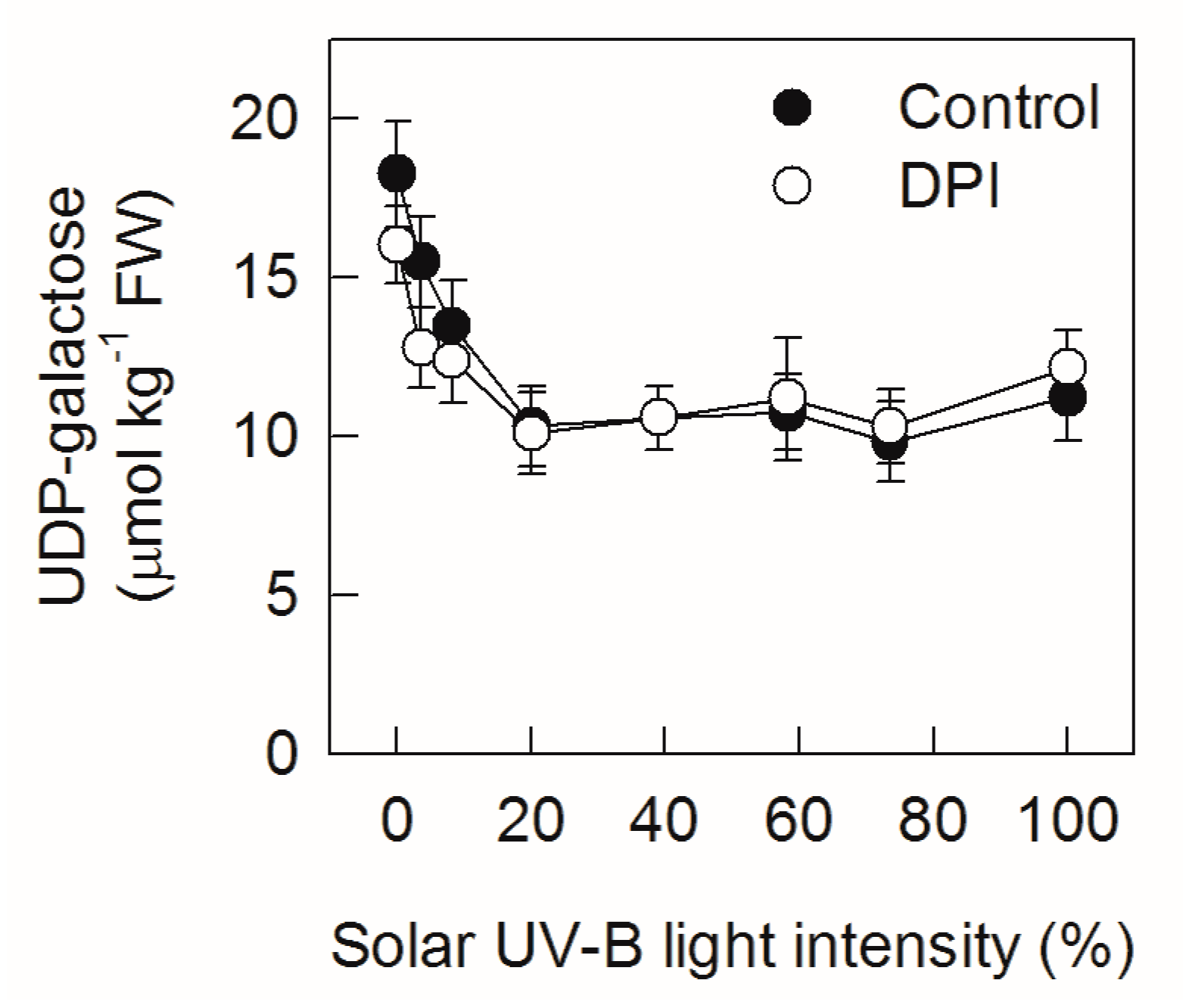
2.3. Analysis of Nucleotide Sugar Concentrations under Different Sunlight Intensities
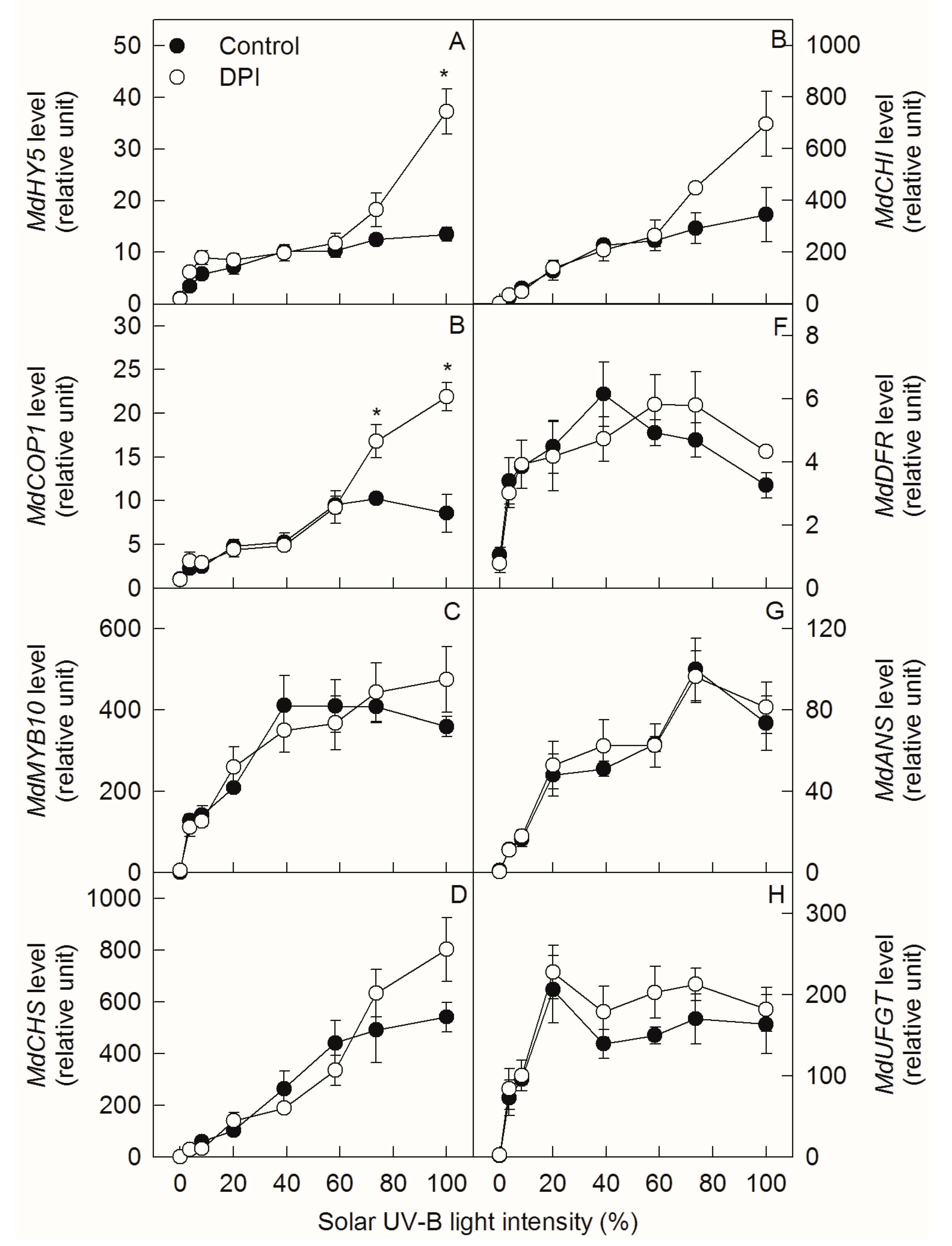
2.4. Analyze Gene Expression Levels under Different Sunlight Intensities
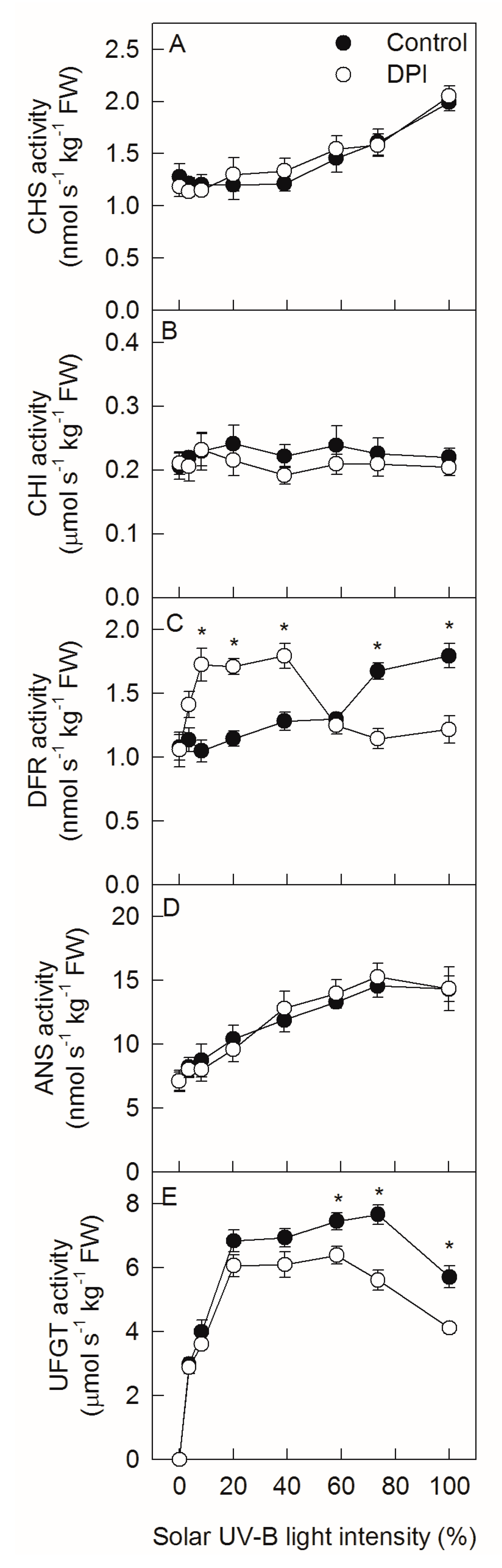
2.5. Analysis of Enzyme Activities under Different Sunlight Intensities
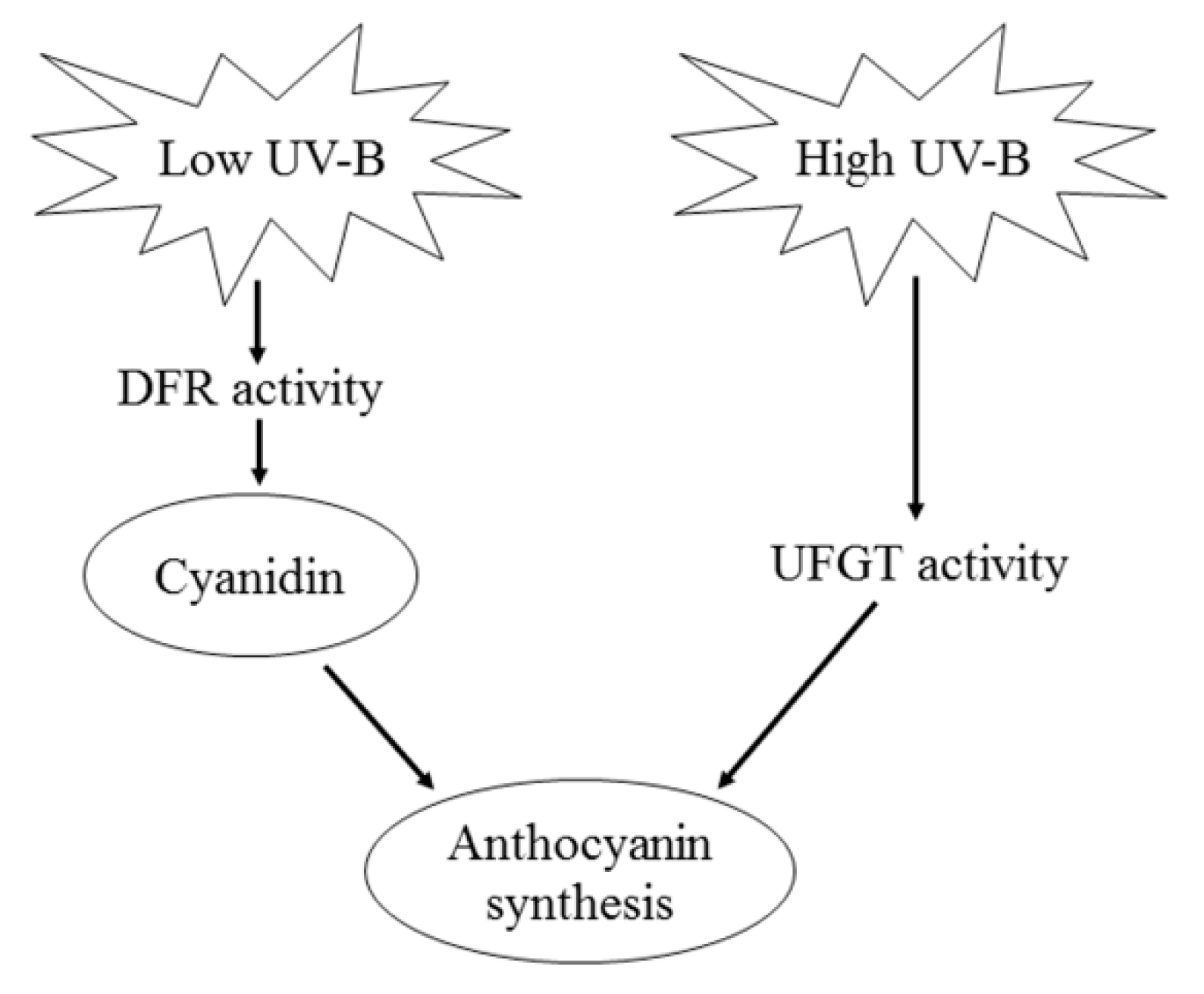
3. Discussion
4. Materials and Methods
4.1. Plant Materials
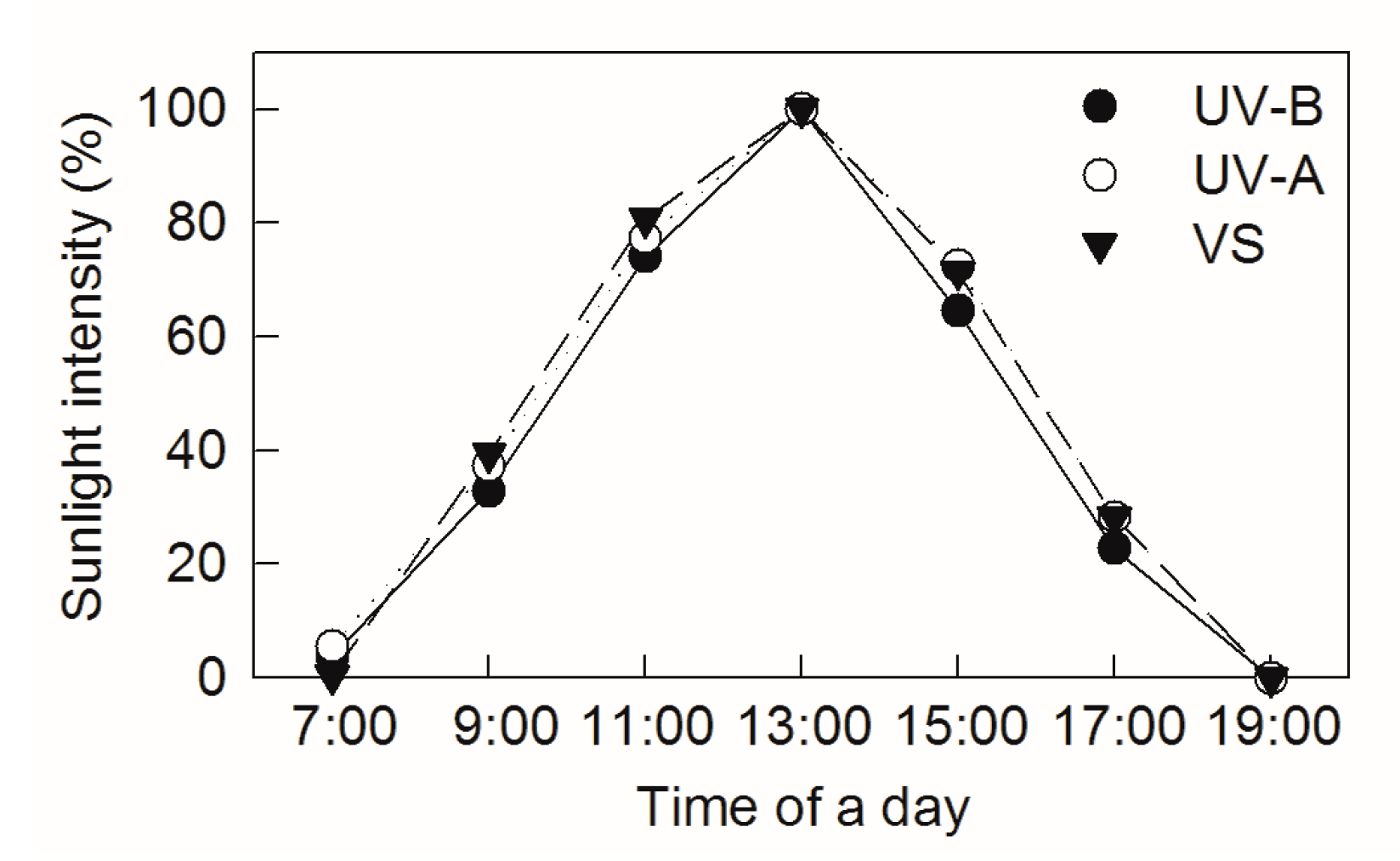
4.2. Chemical and Diverse Intensities Solar UV-B Light Exposure Treatments
4.3. Analysis of Flavonoid Compounds
4.4. Analysis of Nucleotide Sugar
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Expression Analysis
4.6. Analysis of Key Enzyme Activities in Anthocyanin Synthesis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CHS | chalcone synthase |
| CHI | chalcone isomerase |
| F3H | flavanone 3-hydroxylase |
| DFR | dihydroflavonol 4-reductase |
| ANS | anthocyanidin synthase |
| UFGT | UDP-glycose: flavonoid 3-O-glycosyltransferase |
| UVR8 | UV RESISTANCE LOCUS 8 |
| COP1 | E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC1 |
| HY5 | bZIP transcription factor ELONGATED HYPOGOTYL5 |
| MBW | MYB-bHLH-WD40 |
| MAPKs | mitogen-activated protein kinases |
| DPI | diphenyleneiodonium chloride |
| PVPP | polyvinylpolypyrrolidone |
| BSA | bovine serum albumin |
| DTT | dithiothreitol |
| HPLC | high-performance liquid chromatography |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| UV-B | ultraviolet-B |
| ROS | reactive oxygen species |
| VS | visible sight |
References
- Jimenez-Garcia, S.N.; Guevara-Gonzalez, R.G.; Miranda-Lopez, R.; Feregrino-Perez, A.A.; Torres-Pacheco, I.; Vazquez-Cruz, M.A. Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology, and genomics. Food Res. Int. 2013, 54, 1195–1207. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, B.; Alkan, N. Increased anthocyanin and flavonoids in mango fruit peel are associated with cold and pathogen resistance. Postharvest Biol. Technol. 2015, 111, 132–139. [Google Scholar] [CrossRef]
- Jezek, M.; Zörb, C.; Merkt, N.; Geilfus, C.M. Anthocyanin management in fruits by fertilization. J. Agric. Food Chem. 2018, 66, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Venail, J.; Mackay, S.; Bailey, P.C.; Schwinn, K.E.; Jameson, P.E.; Martin, C.R.; Davies, K.M. The molecular basis for venation patterning of pigmentation and its effect on pollinator attraction in flowers of Antirrhinum. New Phytol. 2011, 189, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The effect of anthocyanin-rich foods or extracts on vascular function in adults: a systematic review and meta-analysis of randomised controlled trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef]
- Xiao, J.B.; Hogger, P. Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: a review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin absorption and metabolism by human intestinal Caco-2 cells—A review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Zhang, D.; Li, H.; Li, P.; Ma, F. Reactive oxygen species produced via plasma membrane NADPH oxidase regulate anthocyanin synthesis in apple peel. Planta 2014, 240, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Dai, Z.; Wu, B.H.; Wu, J.; Merlin, I.; Hilbert, G.; Renaud, C.; Gomès, E.; Edwards, E.; Li, S.H.; et al. Anthocyanin biosynthesis is differentially regulated by light in the skin and flesh of white-fleshed and teinturier grape berries. Planta 2016, 243, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.G.; Sun, C.H.; Zhang, Q.Y.; An, J.P.; You, C.X.; Hao, Y.J. Glucose sensor MdHXK1 phosphorylates and stabilizes MdbHLH3 to promote anthocyanin biosynthesis in apple. PLoS Genet. 2016, 12, e1006273. [Google Scholar] [CrossRef] [PubMed]
- Henry-Kirk, R.A.; Plunkett, B.; Hall, M.; McGhie, T.; Allan, A.C.; Wargent, J.J.; Espley, R.V. Solar UV light regulates flavonoid metabolism in apple (Malus x domestica). Plant Cell Environ. 2018, 41, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, Y.H.; Li, B.; Li, D.N.; Li, L.; Liu, X.; Han, J.; Meng, X. Comparative transcriptome analysis of genes involved in anthocyanin synthesis in blueberry. Plant Physiol. Biochem. 2018, 127, 561–572. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.; Xu, H.; Jiang, S.; Fang, H.; Zhang, T.; Su, M.; Zhang, Z.; Chen, X. Nitrogen affects anthocyanin biosynthesis by regulating MdLOB52 downstream of MdARF19 in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). J. Plant Growth Regul. 2018, 37, 719–729. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Yin, R.; Skvortsova, M.Y.; Loubéry, S.; Ulm, R. COP1 is required for UV-B-induced nuclear accumulation of the UVR8 photoreceptor. Proc. Natl. Acad. Sci. USA 2016, 113, E4415–E4422. [Google Scholar] [CrossRef]
- Li, Y.Y.; Mao, K.; Zhao, C.; Zhao, X.Y.; Zhang, H.L.; Shu, H.R.; Hao, Y.J. MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol. 2012, 160, 1011–1022. [Google Scholar] [CrossRef]
- Peng, T.; Saito, T.; Honda, C.; Ban, Y.; Kondo, S.; Liu, J.H.; Hatsuyama, Y.; Moriguchi, T. Screening of UV-B-induced genes from apple peels by SSH: possible involvement of MdCOP1-mediated signaling cascade genes in anthocyanin accumulation. Physiol. Plant 2013, 148, 432–444. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Qu, F.J.; Yao, J.F.; Wang, X.N.; You, C.X.; Wang, X.F.; Hao, Y.J. The bZIP transcription factor MdHY5 regulates anthocyanin accumulation and nitrate assimilation in apple. Hortic. Res. 2017, 4, 17023. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.C.; Hellens, R.P.; Laing, W.A. MYB transcription factors that colour our fruit. Trends Plant Sci. 2008, 13, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Transcriptional control of flavonoid biosynthesis: fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal. Behav. 2014, 9, e27522. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.B.; Li, S.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.X.; You, C.X.; Zhang, X.S.; Hao, Y.J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012, 35, 1884–1897. [Google Scholar] [CrossRef]
- Ju, Z.G.; Yuan, Y.B.; Liou, C.L.; Xin, S.H. Relationships among phenylalanine ammonia-Iyase activity, simple phenol concentrations and anthocyanin accumulation in apple. Sci. Hortic. 1995, 61, 215–226. [Google Scholar] [CrossRef]
- Ju, Z.; Liu, C.; Yuan, Y. Activities of chalcone synthase and UDPGal: flavonoid-3-o-glycosyltransferase in relation to anthocyanin synthesis in apple. Sci. Hortic. 1995, 63, 175–185. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zheng, X.T.; Sun, B.Y.; Peng, C.L.; Chow, W.S. Over-expression of the CHS gene enhances resistance of Arabidopsis, leaves to high light. Environ. Exp. Bot. 2017, 154, 33–43. [Google Scholar] [CrossRef]
- Li, P.; Ma, F.; Cheng, L. Primary and secondary metabolism in the sun-exposed peel and the shaded peel of apple fruit. Physiol. Plant 2013, 148, 9–24. [Google Scholar] [CrossRef]
- Johnson, E.T.; Ryu, S.; Yi, H.; Shin, B.; Cheong, H.; Choi, G. Alteration of a single amino acid changes the substrate specificity of dihydroflavonol 4-reductase. Plant J. 2001, 25, 325–333. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, M.G.; Kang, C.S.; Park, C.S.; Choi, S.B.; Park, Y.I. Overexpressing the wheat dihydroflavonol 4-reductase gene TaDFR increases anthocyanin accumulation in an Arabidopsis dfr mutant. Genes Genom. 2015, 38, 333–340. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, H.; Yang, R.; Ran, M. Identification of DFR as a promoter of anthocyanin accumulation in poinsettia (Euphorbia pulcherrima, willd. ex Klotzsch) bracts under short-day conditions. Sci. Hortic. 2018, 236, 158–165. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Ban, Y.; Honda, C.; Hatsuyama, Y.; Igarashi, M.; Bessho, H.; Moriguchi, T. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 2007, 48, 958–970. [Google Scholar] [CrossRef]
- Plunkett, B.J.; Espley, R.V.; Dare, A.P.; Warren, B.A.W.; Grierson, E.R.P.; Cordiner, S.; Turner, J.L.; Allan, A.C.; Albert, N.W.; Davies, K.M.; et al. MYBA from blueberry (Vaccinium Section Cyanococcus) is a subgroup 6 type R2R3MYB transcription factor that activates anthocyanin production. Front. Plant Sci. 2018, 9, 1300. [Google Scholar] [CrossRef]
- Hideg, É.; Jansen, M.A.; Strid, Å. UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Wargent, J.J.; Jordan, B.R. From ozone depletion to agriculture: understanding the role of UV radiation in sustainable crop production. New Phytol. 2013, 197, 1058–1076. [Google Scholar] [CrossRef]
- Chen, C.S.; Zhang, D.; Wang, Y.Q.; Li, P.M.; Ma, F.W. Effects of fruit bagging on the contents of phenolic compounds in the peel and flesh of ‘Golden Delicious’, ‘Red Delicious’, and ‘Royal Gala’ apples. Sci. Hortic. 2012, 142, 68–73. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, J.; Duan, Y.; Zhang, M.; Liu, J.; Li, P.; Ma, F. Photoprotection mechanism in the ‘Fuji’ apple peel at different levels of photooxidative sunburn. Physiol. Plant 2015, 154, 54–65. [Google Scholar] [CrossRef]
- Lister, C.E.; Lancaster, J.E.; Sutton, K.H.; Walker, J.R.L. Aglycone and glycoside specificity of apple skin flavonoid glycosyltransferase. J. Sci. Food Agric. 1997, 75, 378–382. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, J.; Chen, C.; Zhang, D.; Li, P.; Ma, F. Anthocyanin contributes more to hydrogen peroxide scavenging than other phenolics in apple peel. Food Chem. 2014, 152, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cheng, L. The elevated anthocyanin level in the shaded peel of ‘Anjou’ pear enhances its tolerance to high temperature under high light. Plant Sci. 2009, 177, 418–426. [Google Scholar] [CrossRef]
- Malnoy, M.; Reynoird, J.P.; Mourgues, F.; Chevreau, E.; Simoneau, P. A method for isolating total RNA from pear leaves. Plant Mol. Biol. Rep. 2001, 19, 69. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, M.; Zhang, G.; Li, P.; Ma, F. Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities. Int. J. Mol. Sci. 2019, 20, 6060. https://doi.org/10.3390/ijms20236060
Chen W, Zhang M, Zhang G, Li P, Ma F. Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities. International Journal of Molecular Sciences. 2019; 20(23):6060. https://doi.org/10.3390/ijms20236060
Chicago/Turabian StyleChen, Weifeng, Mengxia Zhang, Guojing Zhang, Pengmin Li, and Fengwang Ma. 2019. "Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities" International Journal of Molecular Sciences 20, no. 23: 6060. https://doi.org/10.3390/ijms20236060
APA StyleChen, W., Zhang, M., Zhang, G., Li, P., & Ma, F. (2019). Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities. International Journal of Molecular Sciences, 20(23), 6060. https://doi.org/10.3390/ijms20236060




