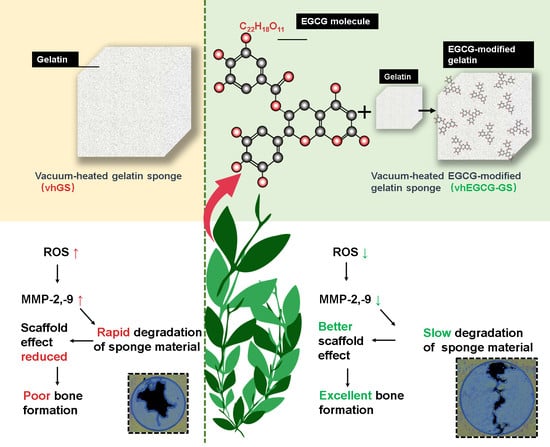
Integration of Epigallocatechin Gallate in Gelatin Sponges Attenuates Matrix Metalloproteinase-Dependent Degradation and Increases Bone Formation
Abstract
1. Introduction
2. Results
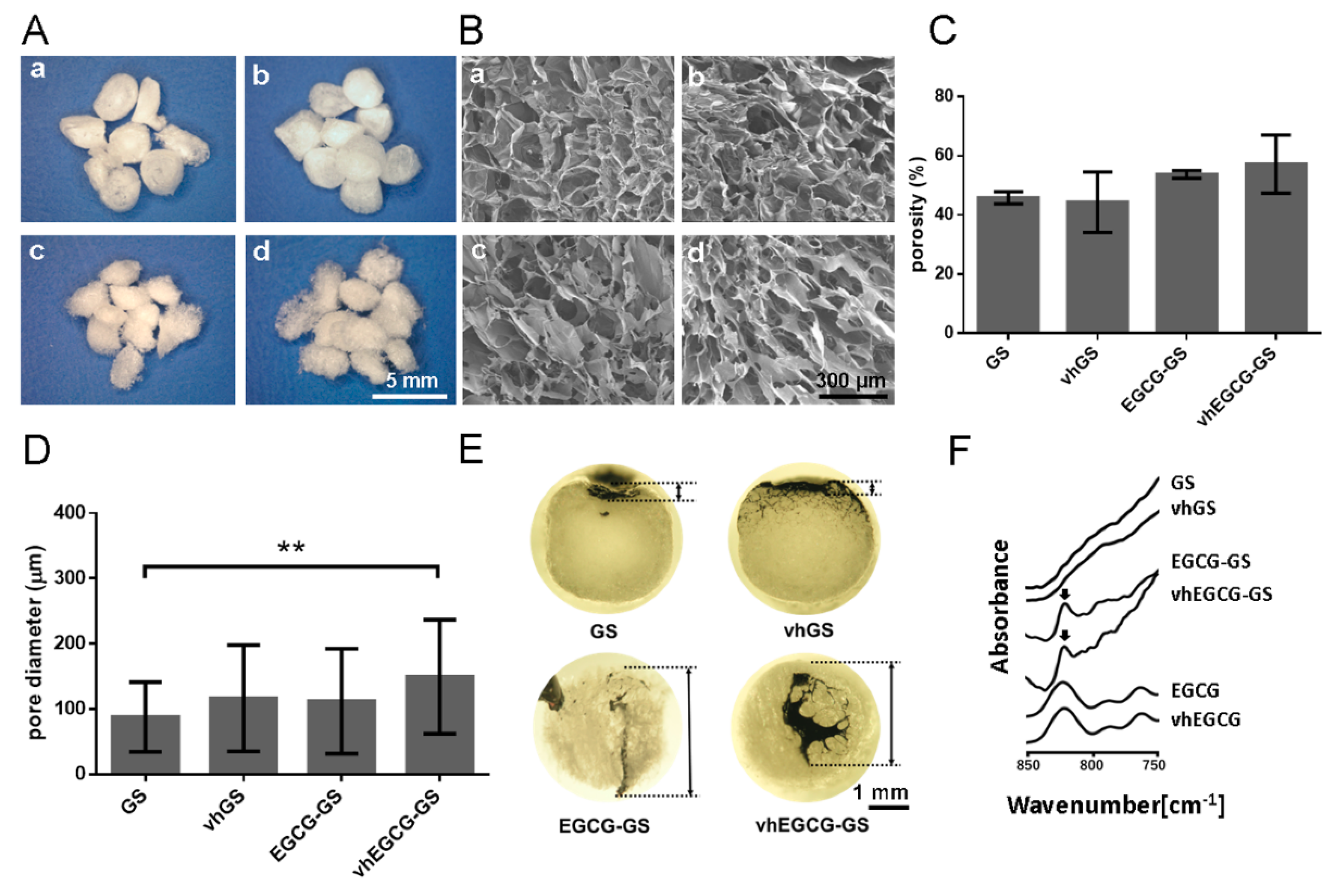
2.1. Characterization of Sponges
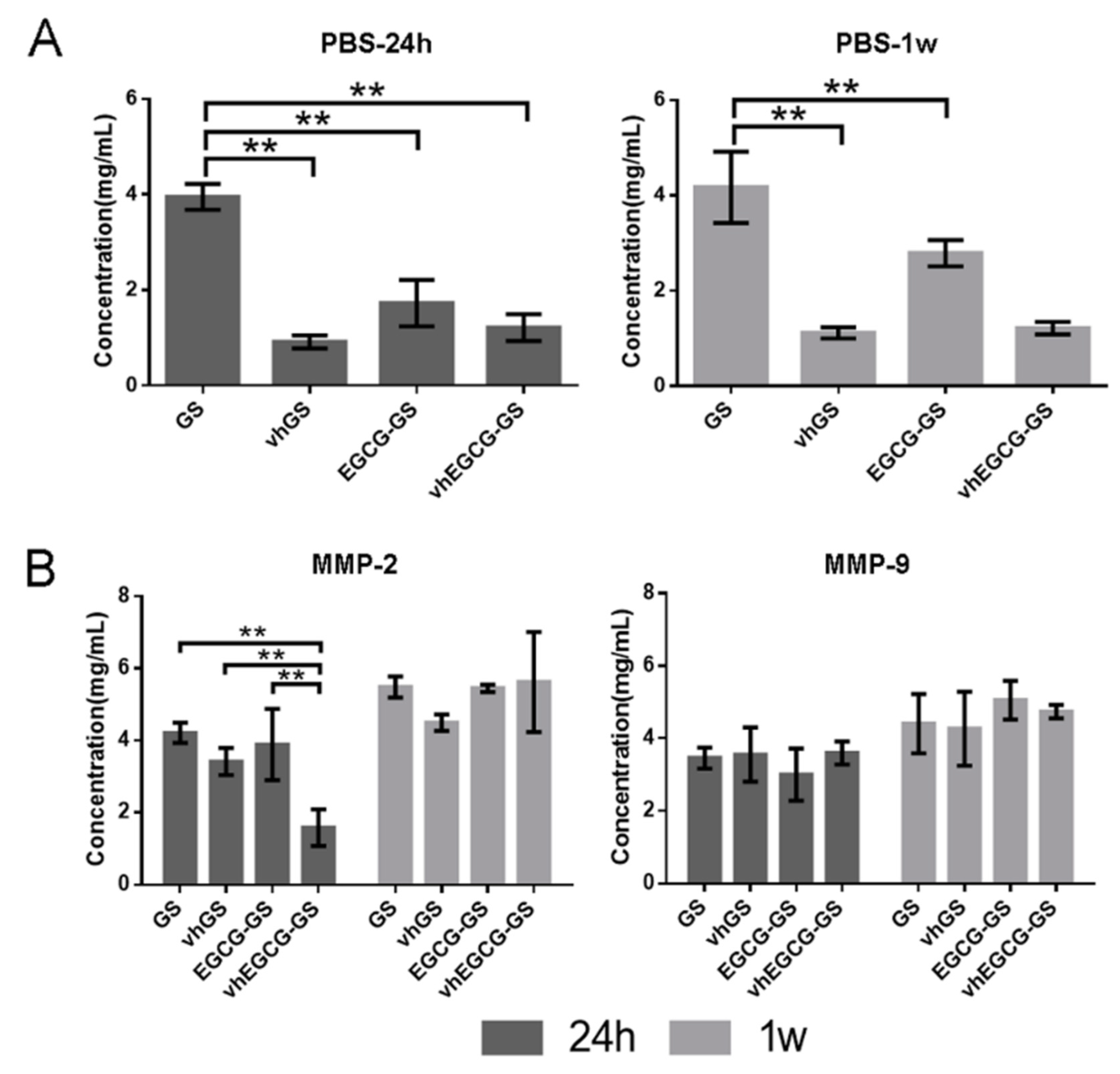
2.2. Degradability of Sponges
2.3. Degradability of Sponges in the Presence of MMPs
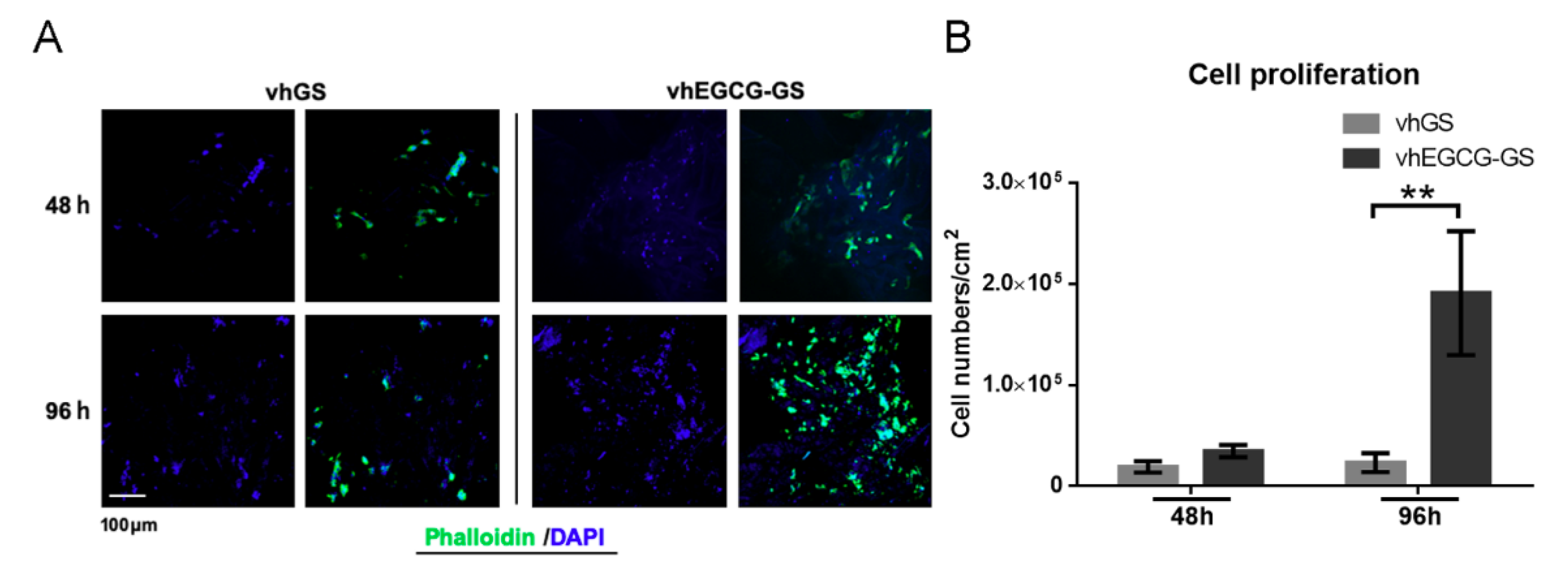
2.4. Cell Behavior on Sponges In Vitro
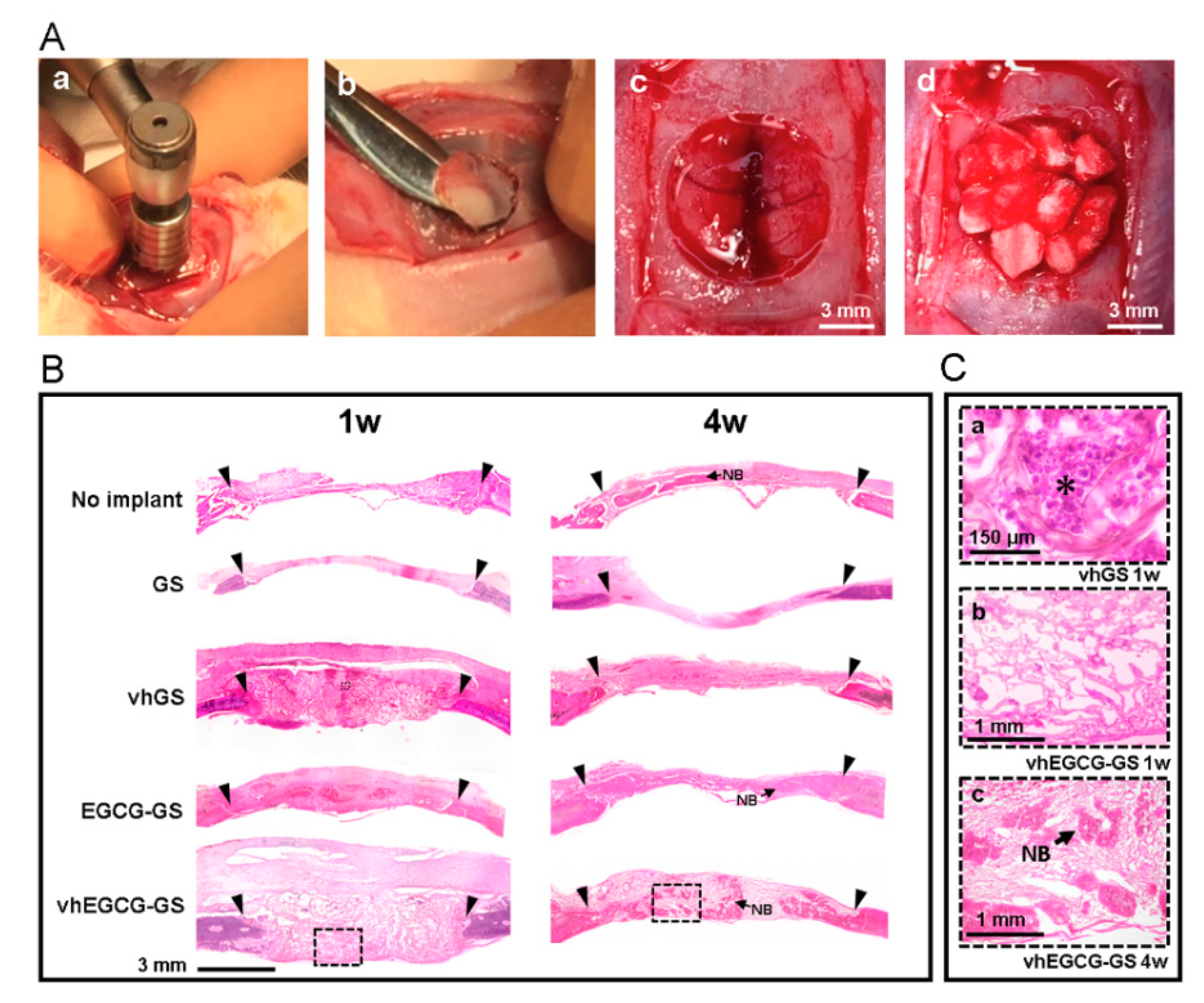
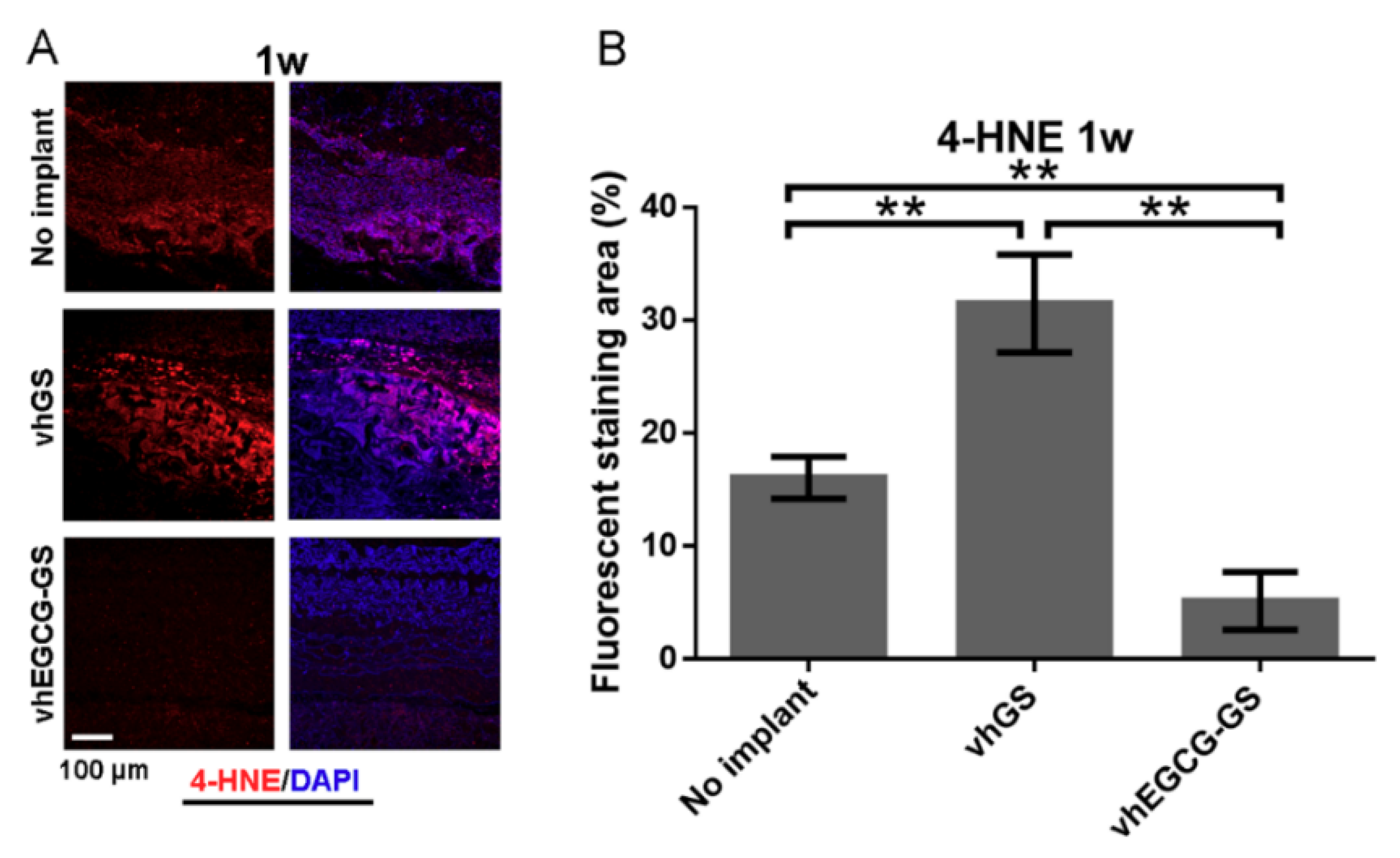
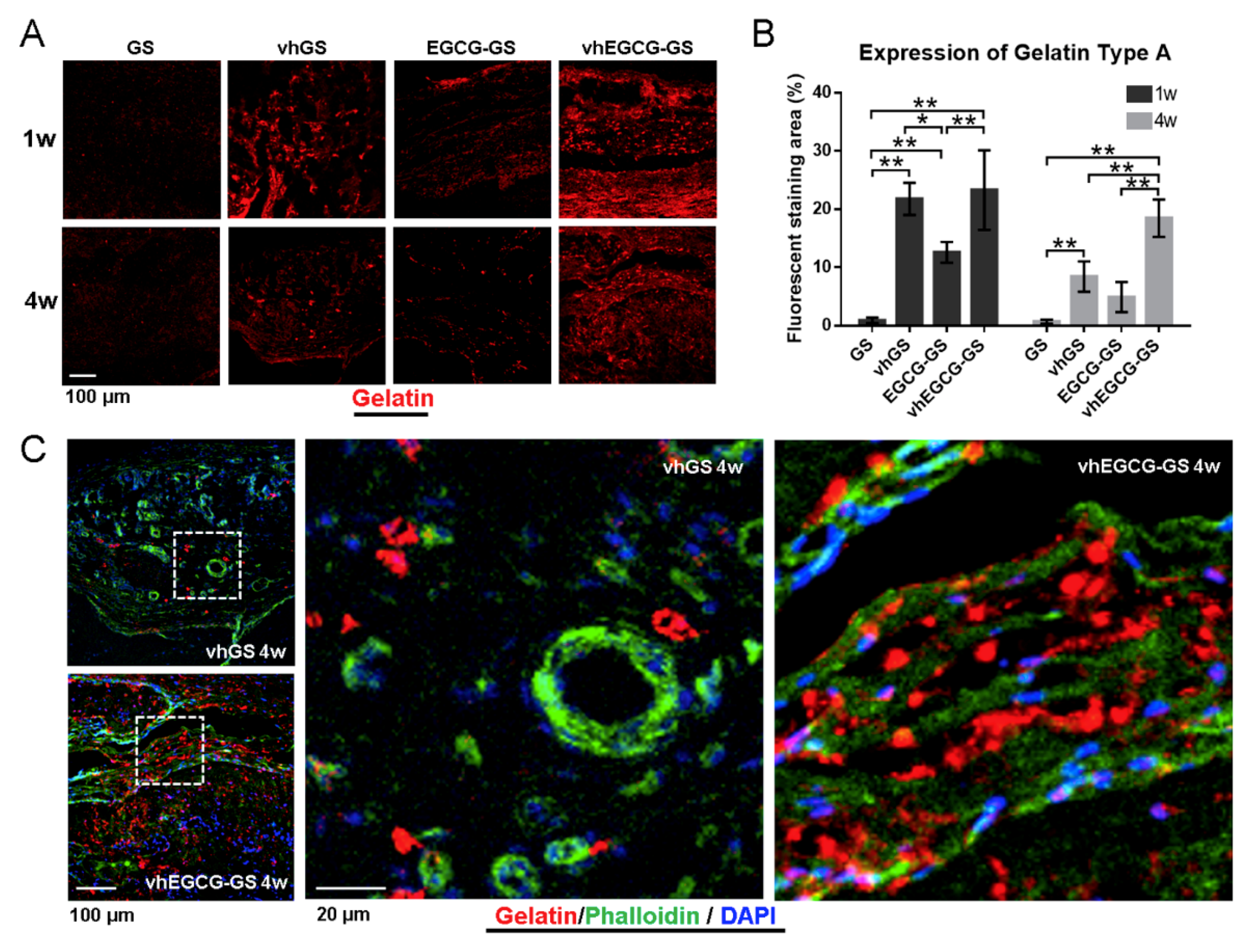
2.5. Histological and Immunohistological Analyses
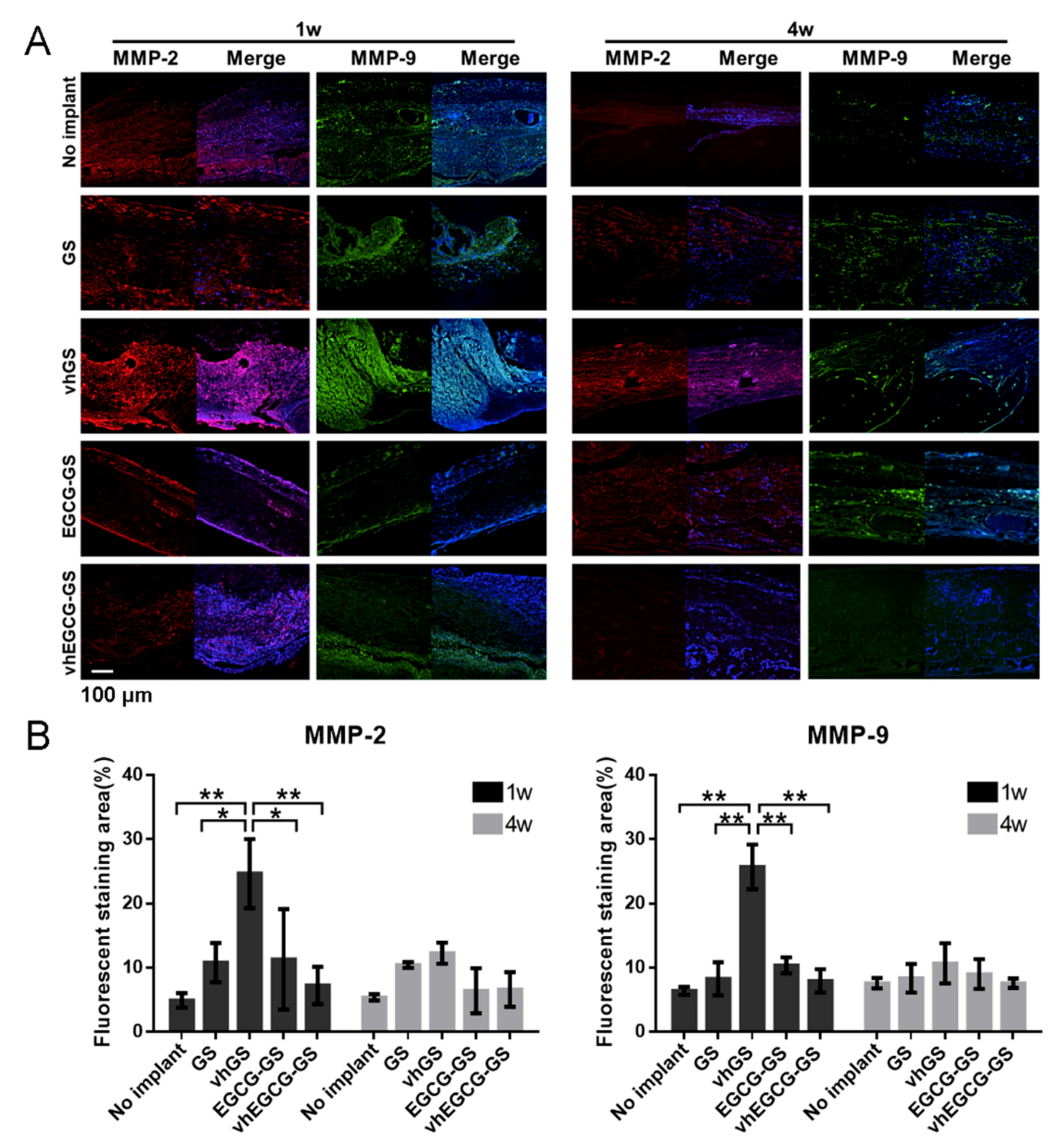
2.6. MMP Expression in Defects Treated with/without Sponges
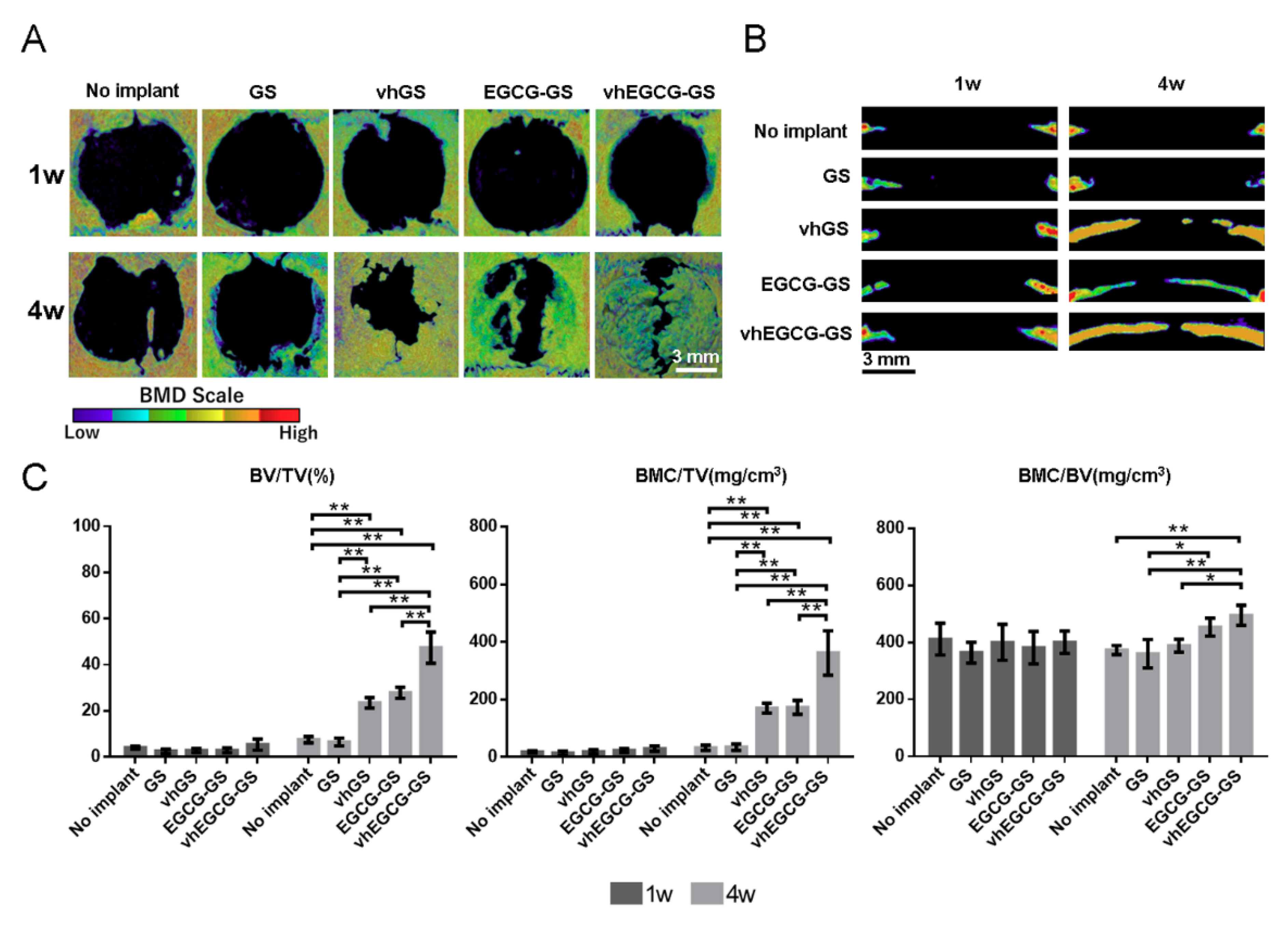
2.7. Bone-forming Capability of Sponges
2.8. Residual Sponges in the Defects
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Sponges
4.2. Degradation Assays
4.3. Cell Maintenance and Staining In Vitro
4.4. Animal Experiments
4.5. Histological and Immunohistological Analyses
4.6. μCT Analysis
4.7. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GS | Gelatin sponge |
| vhGS | Vacuum heated gelatin sponge |
| EGCG | Epigallocatechin gallate |
| EGCG-GS | Epigallocatechin gallate-modified gelatin sponge |
| vhEGCG-GS | Vacuum heated EGCG-GS |
References
- Szpalski, C.; Barr, J.; Wetterau, M.; Saadeh, P.B.; Warren, S.M. Cranial bone defects: Current and future strategies. Neurosurg. Focus 2010, 29, E8. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K. Growth factors in oral and maxillofacial surgery: Potentials and challenges. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.B.; Pacelli, S.; Haj, A.J.E.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F. Gelatin-based materials in ocular tissue engineering. Materials (Basel) 2014, 7, 3106–3135. [Google Scholar] [CrossRef]
- Fang, X.; Xie, J.; Zhong, L.; Li, J.; Rong, D.; Li, X.; Ouyang, J. Biomimetic gelatin methacrylamide hydrogel scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1070–1080. [Google Scholar] [CrossRef]
- Honda, Y.; Takeda, Y.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; Uemura, N.; Ueda, M.; Hashimoto, M.; Arita, K.; et al. Epigallocatechin gallate-modified gelatin sponges treated by vacuum heating as a novel scaffold for bone tissue engineering. Molecules 2018, 23, 876. [Google Scholar] [CrossRef]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control Release 2014, 190, 210–218. [Google Scholar] [CrossRef]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering—A mini review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef]
- Di Filippo, M.F.; Amadori, S.; Casolari, S.; Bigi, A.; Dolci, L.S.; Panzavolta, S. Cylindrical layered bone scaffolds with anisotropic mechanical properties as potential drug delivery systems. Molecules 2019, 24, 1931. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Demitri, C.; Ambrosio, L. Bioactivation routes of gelatin-based scaffolds to enhance at nanoscale level bone tissue regeneration. Front. Bioeng. Biotechnol. 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Kokol, V. Collagen- Vs. Gelatine-based biomaterials and their biocompatibility: Review and perspectives. In Biomaterials Applications for Nanomedicine; Rosario, P., Ed.; InTech Europe: Rijeka, Croatia, 2011; pp. 17–52. [Google Scholar]
- Gross, J.; Lapiere, C. Collagenolytic activity in amphibian tissues: A tissue culture assay. PNAS 1962, 15, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Kurzepa, J.; Baran, M.; Watroba, S.; Barud, M.; Babula, D. Collagenases and gelatinases in bone healing. The focus on mandibular fractures. Curr. Issues Pharm. Med. Sci. 2014, 27, 121–126. [Google Scholar] [CrossRef]
- Soumyarani, V.S.; Jayakumari, N. Oxidatively modified high density lipoprotein promotes inflammatory response in human monocytes-macrophages by enhanced production of ROS, TNF-alpha, MMP-9, and MMP-2. Mol. Cell Biochem. 2012, 366, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, N.; Khuth, S.; Murat, A.; Chalon, A.; Giraudon, P.; Belin, M.; Ghersi-Egea, J. Pro-inflammatory cytokines modulate matrix metalloproteinase secretion and organic anion transport at the blood-cerebrospinal fluid barrier. J. Neuropathol. Exp. Neurol. 2003, 62, 1254–1264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, B.; Yan, L.; Tsang, P.C.; Moses, M.A. Matrix metalloproteinase-2 (MMP-2) expression and regulation by tumor necrosis factor alpha (TNFalpha) in the bovine corpus luteum. Mol. Reprod. Dev. 2005, 70, 122–132. [Google Scholar] [CrossRef]
- Portela, V.; Veiga, A.; Price, C. Regulation of MMP2 and MMP9 metalloproteinases by FSH and growth factors in bovine granulosa cells. Genet. Mol. Biol. 2009, 32, 516–520. [Google Scholar] [CrossRef]
- Xu, P.; Sefton, M.V. Expression of matrix metalloproteinase-2 and -9 in exudates associated with polydimethyl siloxane and gelatin tubes implanted in mice. J. Biomed. Mater. Res. A 2004, 71, 226–232. [Google Scholar] [CrossRef]
- Ahmed, S.; Pakozdi, A.; Koch, A.E. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis. Rheum. 2006, 54, 2393–2401. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Min, K.J.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Peairs, A.; Dai, R.; Gan, L.; Shimp, S.; Rylander, M.N.; Li, L.; Reilly, C.M. Epigallocatechin-3-gallate (EGCG) attenuates inflammation in MRL/lpr mouse mesangial cells. Cell Mol. Immunol. 2010, 7, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, Y.; Kanzaki, H.; Honda, Y.; Tanaka, T.; Yamaguchi, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Narimiya, T.; Wada, S.; et al. Single local injection of epigallocatechin gallate-modified gelatin attenuates bone resorption and orthodontic tooth movement in mice. Polymers 2018, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Pang, E.K.; Kim, C.S.; Yoo, Y.J.; Cho, K.S.; Chai, J.K.; Kim, C.K.; Choi, S.H. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J. Periodontal Res. 2004, 39, 300–307. [Google Scholar] [CrossRef]
- Deng, Y.T.; Lin, J.K. EGCG inhibits the invasion of highly invasive CL1-5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/M arrest. J. Agric. Food Chem. 2011, 59, 13318–13327. [Google Scholar] [CrossRef]
- Morin, M.P.; Grenier, D. Regulation of matrix metalloproteinase secretion by green tea catechins in a three-dimensional co-culture model of macrophages and gingival fibroblasts. Arch. Oral Biol. 2017, 75, 89–99. [Google Scholar] [CrossRef]
- Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local controlled release of polyphenol conjugated with gelatin facilitates bone formation. Int. J. Mol. Sci. 2015, 16, 14143–14157. [Google Scholar] [CrossRef]
- Sasayama, S.; Hara, T.; Tanaka, T.; Honda, Y.; Baba, S. Osteogenesis of multipotent progenitor cells using the epigallocatechin gallate-modified gelatin sponge scaffold in the rat congenital cleft-jaw model. Int. J. Mol. Sci. 2018, 19, 3803. [Google Scholar] [CrossRef]
- Hannink, G.; Arts, J.J. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42 (Suppl. 2), S22–S25. [Google Scholar] [CrossRef]
- Xie, H.; Sun, J.; Chen, Y.; Zong, M.; Li, S.; Wang, Y. EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the notch pathway. Oxid. Med. Cell Longev. 2015, 2015, 214836. [Google Scholar] [CrossRef]
- Chowdhury, A.; Nandy, S.K.; Sarkar, J.; Chakraborti, T.; Chakraborti, S. Inhibition of pro-/active MMP-2 by green tea catechins and prediction of their interaction by molecular docking studies. Mol. Cell Biochem. 2017, 427, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Grigoriou, V.; Shapiro, I.M.; Cavalcanti-Adam, E.A.; Composto, R.J.; Ducheyne, P.; Adams, C.S. Apoptosis and survival of osteoblast-like cells are regulated by surface attachment. J. Biol. Chem. 2005, 280, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Jiang, H.; Zeng, K.; Liang, Y.; Wu, Y.; Yang, Y. Removal from adherent culture contributes to apoptosis in human bone marrow mesenchymal stem cells. Mol. Med. Rep. 2017, 15, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Grove, C.; Jerram, D.A. jPOR: An ImageJ macro to quantify total optical porosity from blue-stained thin sections. Comput. Geosci. 2011, 37, 1850–1859. [Google Scholar]
- Kanda, N.; Anada, T.; Handa, T.; Kobayashi, K.; Ezoe, Y.; Takahashi, T.; Suzuki, O. Orthotopic osteogenecity enhanced by a porous gelatin sponge in a critical-sized rat calvaria defect. Macromol. Biosci. 2015, 15, 1647–1655. [Google Scholar] [CrossRef]
- Forrest, S.; Ng, K.; Findlay, D.; Michelangeli, V.; Livesey, S.; Partridge, N.; Zajac, J.; Martin, T. Characterization of an osteoblast-like clonal cell line which responds to both parathyroid hormone and calcitonin. Calcif. Tissue Int. 1985, 37, 51–56. [Google Scholar] [CrossRef]
- Hara, E.; Honda, Y.; Suzuki, O.; Tanaka, T.; Matsumoto, N. Epigallocatechin gallate-modified gelatins with different compositions alter the quality of regenerated bones. Int. J. Mol. Sci. 2018, 19, 3232. [Google Scholar] [CrossRef]
- Kawamoto, T.; Kawamoto, K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using kawamot’s film method (2012). In Skeletal Development and Repair; Hilton, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2014; Volume 1130, pp. 149–164. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, A.; Honda, Y.; Li, P.; Tanaka, T.; Baba, S. Integration of Epigallocatechin Gallate in Gelatin Sponges Attenuates Matrix Metalloproteinase-Dependent Degradation and Increases Bone Formation. Int. J. Mol. Sci. 2019, 20, 6042. https://doi.org/10.3390/ijms20236042
Huang A, Honda Y, Li P, Tanaka T, Baba S. Integration of Epigallocatechin Gallate in Gelatin Sponges Attenuates Matrix Metalloproteinase-Dependent Degradation and Increases Bone Formation. International Journal of Molecular Sciences. 2019; 20(23):6042. https://doi.org/10.3390/ijms20236042
Chicago/Turabian StyleHuang, Anqi, Yoshitomo Honda, Peiqi Li, Tomonari Tanaka, and Shunsuke Baba. 2019. "Integration of Epigallocatechin Gallate in Gelatin Sponges Attenuates Matrix Metalloproteinase-Dependent Degradation and Increases Bone Formation" International Journal of Molecular Sciences 20, no. 23: 6042. https://doi.org/10.3390/ijms20236042
APA StyleHuang, A., Honda, Y., Li, P., Tanaka, T., & Baba, S. (2019). Integration of Epigallocatechin Gallate in Gelatin Sponges Attenuates Matrix Metalloproteinase-Dependent Degradation and Increases Bone Formation. International Journal of Molecular Sciences, 20(23), 6042. https://doi.org/10.3390/ijms20236042