Waste Autochthonous Tuscan Olive Leaves (Olea europaea var. Olivastra seggianese) as Antioxidant Source for Biomedicine
Abstract
1. Introduction
2. Results
2.1. Characterization of OLE from Tuscan Olea Europaea
2.2. Scaffold Characterization
2.3. Dose- and Time-Dependent Effect of OLE on 2D Culture Model
2.4. Antioxidant Activity of OLE in 2D Culture Model
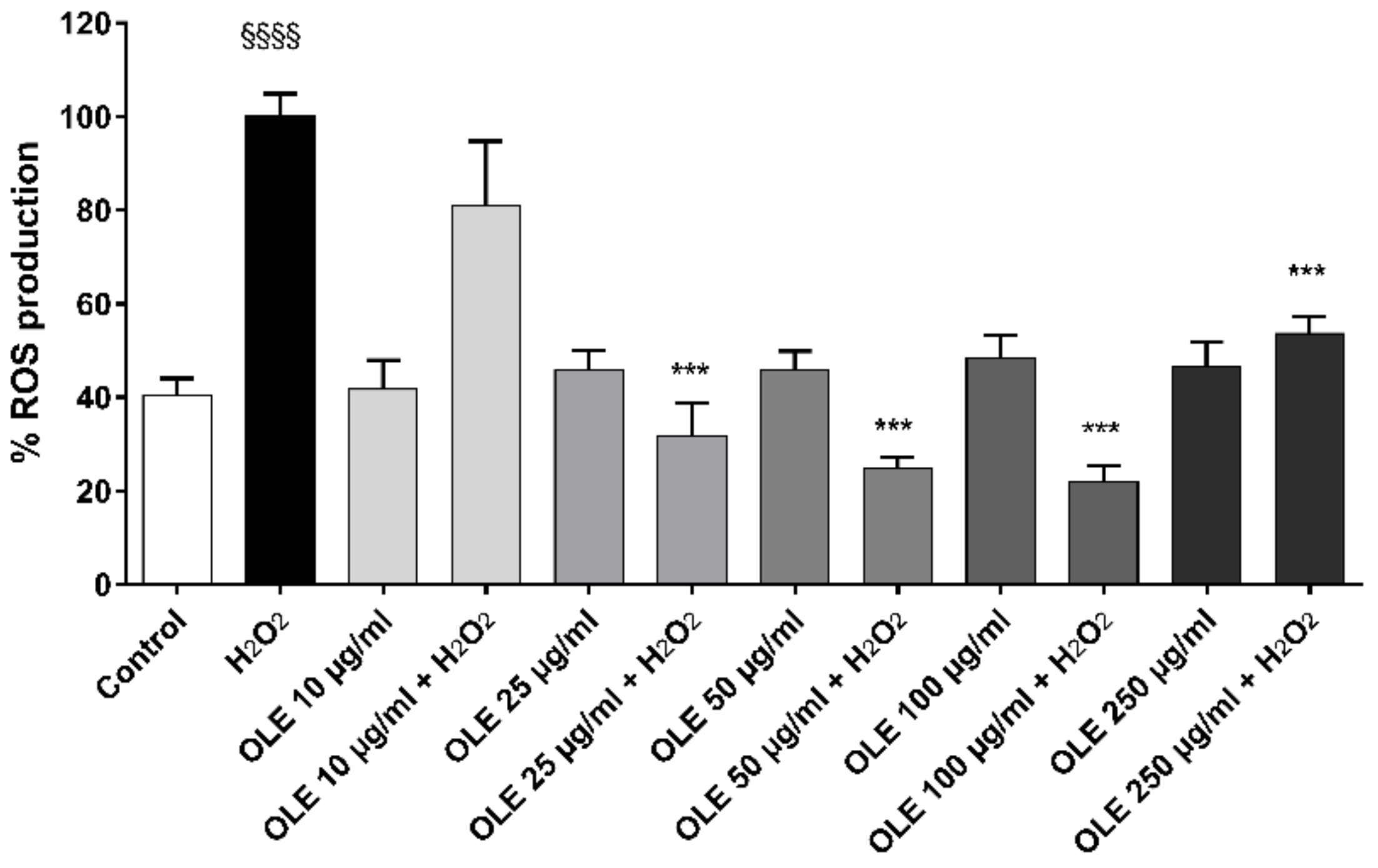
2.5. ROS Production in 2D Culture Model
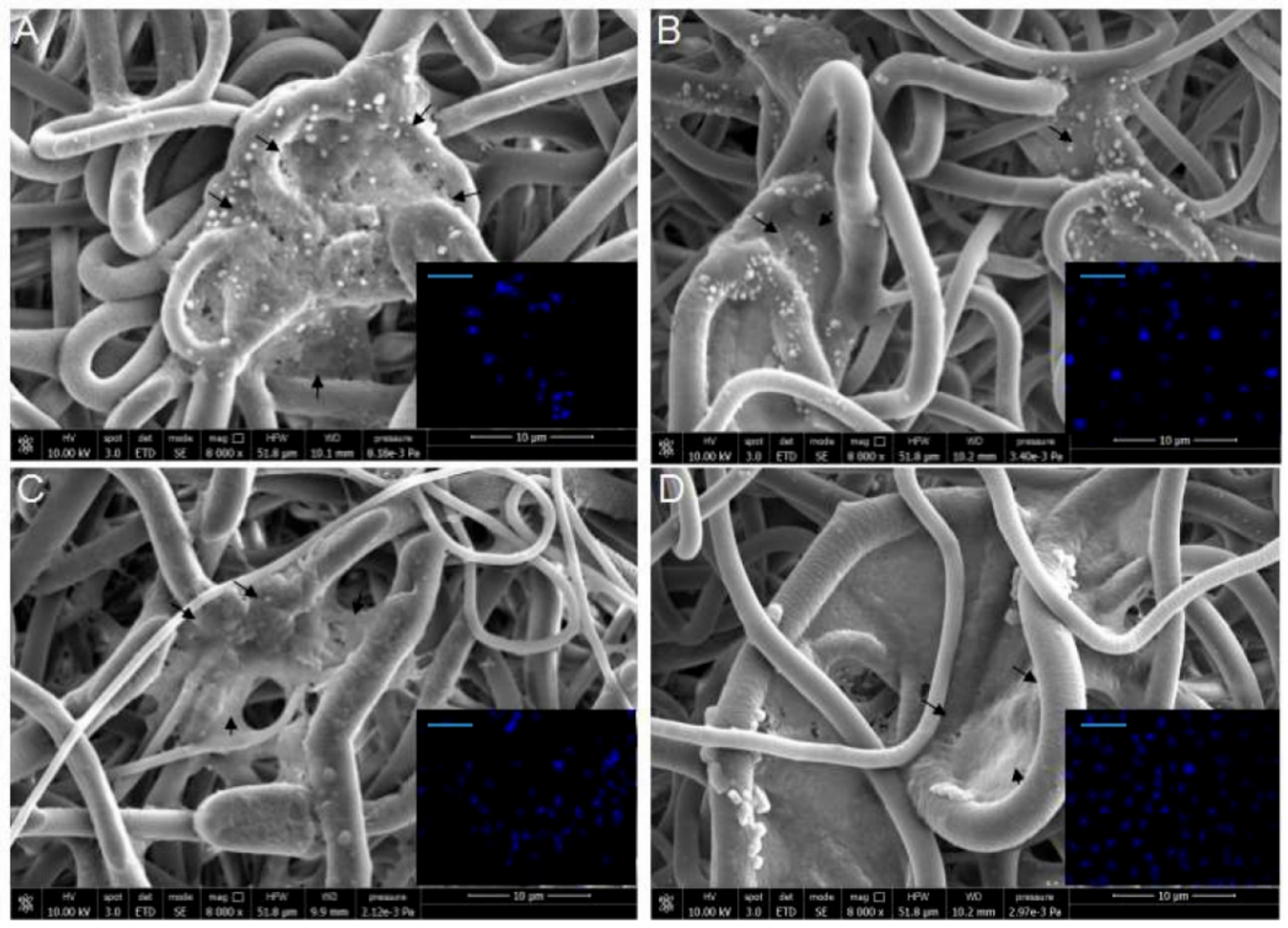
2.6. 3D Culture Model Characterization
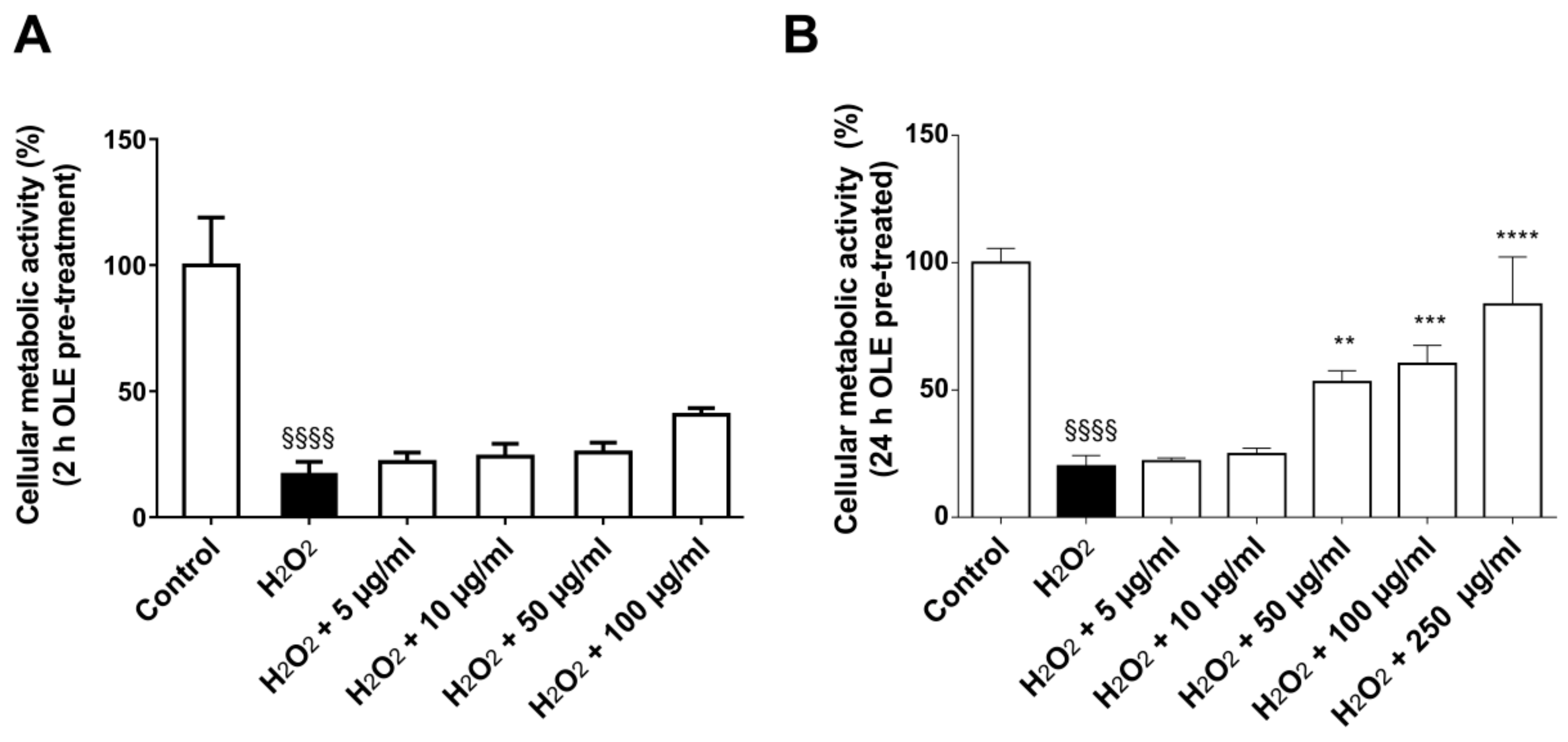
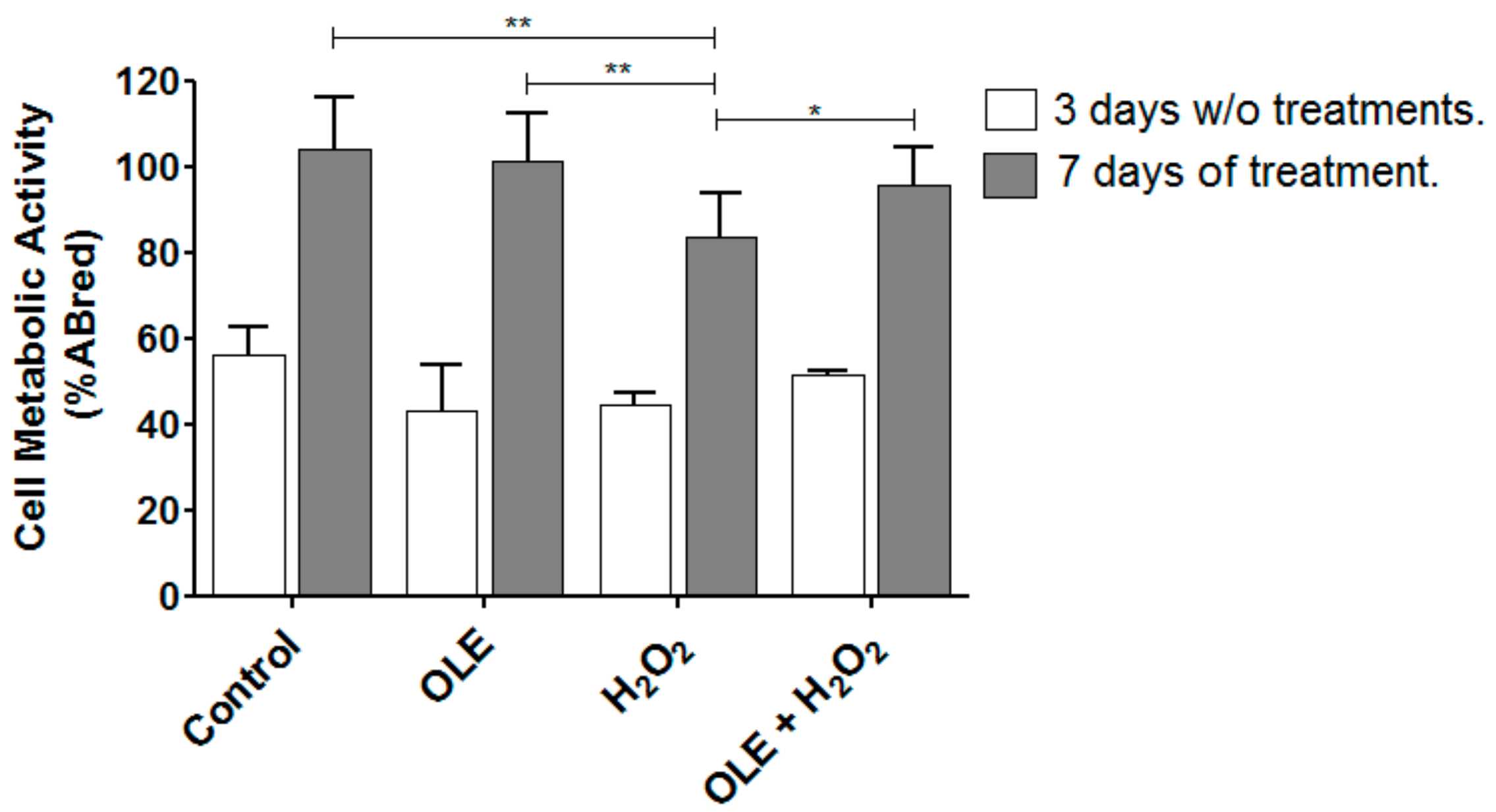
2.7. OLE Effect on Metabolic Activity in 3D Culture Model
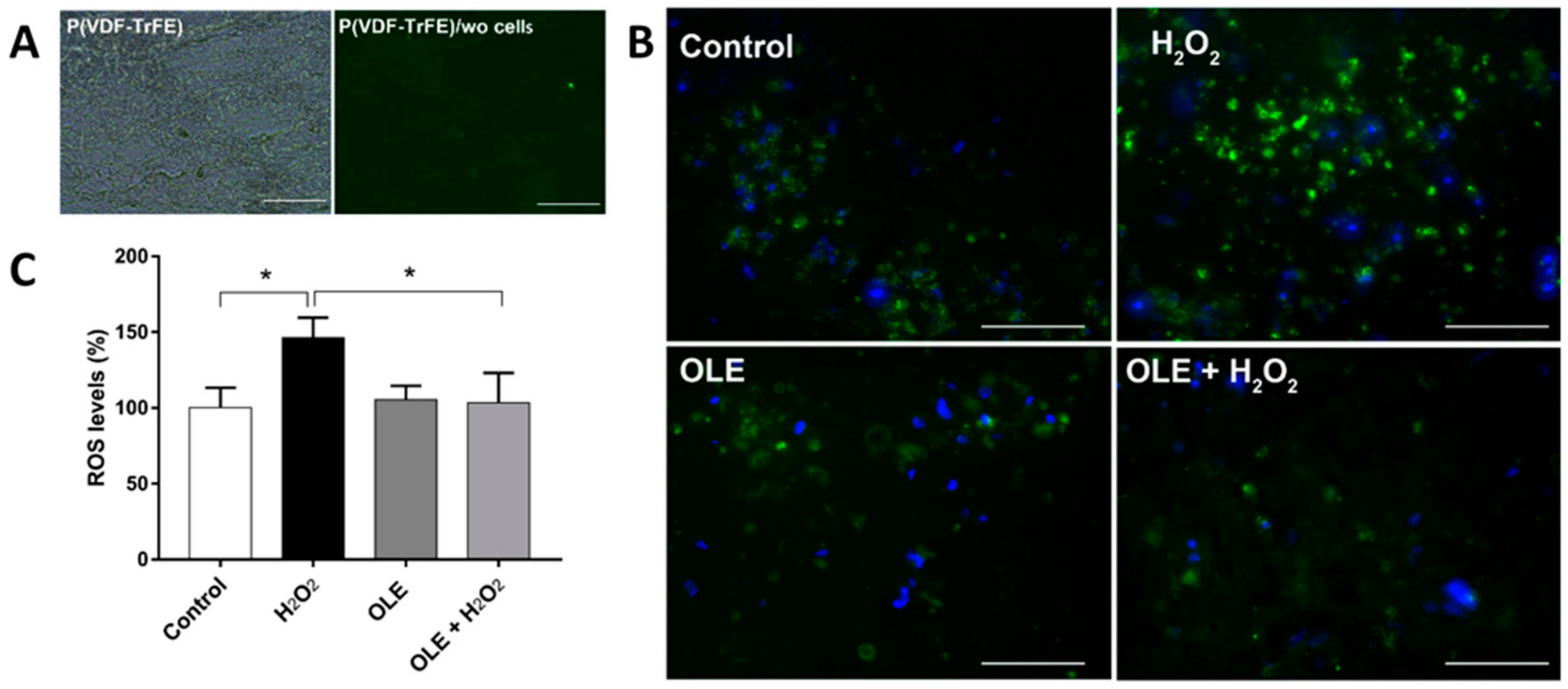
2.8. OLE Effect on ROS in 3D Culture Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. OLE Polyphenol Characterization
4.4. HPLC Characterization
4.5. Endothelial Cell Isolation and Culture
4.6. Scaffold Fabrication and Characterization
4.7. Investigation of OLE Effects
4.7.1. 2D HUVEC Model
4.7.2. 3D HUVEC Model
- Cell/scaffold construct (control);
- Cell/scaffold construct + OLE (100 µg/mL GAE) for 24 h;
- Cell/scaffold construct + H2O2 (100 µM) for 1 h;
- Cell/scaffold construct + OLE (100 µg/mL GAE for 24 h) + H2O2 (100 µM for 1 h).
4.8. DAPI Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AcOH | Acetic Acid | |
| ANOVA | Analysis of variance | |
| CM-H2DCFDA | 5-(and-6)-chloromethyl-2’,7’-dichloro-di-hydro-fluorescein diacetate, acetyl | |
| DAPI | 4’,6-Diamidino-2-phenylindole dihydrochloride | |
| ECM | Extracellular matrix | |
| EGM-2 | Endothelial Cell Growth Medium | |
| FBS | Fetal Bovine Serum | |
| GAE | Gallic Acid Equivalent | |
| H2O2 | Hydrogen Peroxide | |
| HEPES | (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) | |
| HPLC | High Performance Liquid Chromatography | |
| HUVEC | Human Umbilical Vein Endothelial Cells | |
| M199 | Medium 199 | |
| MeOH | Methanol | |
| MEK | Methyl Ethyl Ketone | |
| Na2CO3 | Sodium Carbonate | |
| OLE | Olive Leaf Extract | |
| PVDF | Poly(vinylidene fluoride) | |
| ROS | Reactive Oxygen Species | |
| SD | Standard deviation | |
| SEM | Scanning Electronic Microscopy | |
| P(VDF-TrFE) | Poly(vinylidenefluoride-co-trifluoroethylene) | |
| TE | Tissue Engineering | |
| TP | Total Polyphenol | |
| UV | Ultraviolet | |
| WST-1 | 4-[3-(4-iodophenyl)-2-(4nitrophenyl)-2H-5-tetrazolium]-1,3-benzenedisulfonate ester | |
References
- Selin, S.; Mehmet, B. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Moltancini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219–228. [Google Scholar] [CrossRef]
- Romero, M.; Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016, 7, 584–593. [Google Scholar] [CrossRef]
- Koca, U.; Suntar, I.; Kupeli, E.; Yilmazer, D.; Alper, M. Wound repair potential of Olea europaea L. leaf extracts revealed by in vivo experimental models and comparative evaluation of the extracts’ antioxidant activity. J. Med. Food 2011, 14, 140–146. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 57–68. [Google Scholar] [CrossRef]
- Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wholett, S.; Hunt, J.A. Reactive oxygen species (ROS)-A family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur. Cell. Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Geir Maller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Susalit, N.; Agus, I.; Effendi, R.R.; Tjandrawinata, D.; Nofiarny, T.; Perrinjaquet-Moccetti Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef]
- Muriana, F.J.G.; Montserrat-de la Paz, S.; Lucas, R.; Bermudez, B.; Jaramillo, S.; Morales, J.C.; Abiaa, R.; Lopez, S. Tyrosol and its metabolites as antioxidative and antiinflammatory molecules in human endothelial cells. Food Funct. 2017, 8. [Google Scholar] [CrossRef]
- Shaik, M.M.; Dapkekar, A.; Rajwade, J.M.; Jadhav, S.H.; Kowshik, M. Antioxidant-antibacterial containing bi-layer scaffolds as potential candidates for management of oxidative stress and infections in wound healing. J. Mater. Sci. Mater. Med. 2019, 30, 13. [Google Scholar] [CrossRef]
- Zineh, B.R.; Shabgard, M.R.; Roshangar, L. An experimental study on the mechanical and biological properties of bio-printed alginate/halloysite nanotube/methylcellulose/Russian olive-based scaffolds. Adv. Pharm. Bull. 2018, 8, 643–655. [Google Scholar] [CrossRef]
- Ingber, D.E.; Mow, V.C.; Butler, D.; Niklason, L.; Huard, J.; Mao, J.; Yannas, I.; Kaplan, D.; Vunjak-Novakovic, G. Tissue engineering and developmental biology: Going biomimetic. Tissue Eng. 2006, 12, 3265–3283. [Google Scholar] [CrossRef]
- Jensen, G.; Morrill, C.; Huang, Y. 3D tissue engineering, an emerging technique for pharmaceutical research. Acta. Pharm. Sin. B 2018, 8, 756–766. [Google Scholar] [CrossRef]
- Mota, C.; Labardi, M.; Trombi, L.; Astolfi, L.; D’Acunto, M.; Puppi, D.; Gallone, G.; Chiellini, F.; Berrettini, S.; Bruschini, L.; et al. Design, fabrication and characterization of composite piezoelectric ultrafine fibers for cochlear stimulation. Mater. Des. 2017, 122, 206–219. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for Tissue Engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Stocco, T.; Bassous, N.J.; Zhao, S.; Granato, A.E.C.; Webster, T.J.; Lobo, A.O. Nanofibrous scaffolds for biomedical applications. Nanoscale 2018, 10, 12228–12255. [Google Scholar] [CrossRef]
- Woywodt, A.; Kirsch, T.; Haubitz, M. Circulating endothelial cells in renal disease: Markers and mediators of vascular damage. Nephrol. Dial. Transplant. 2008, 23, 7–10. [Google Scholar] [CrossRef][Green Version]
- Kouka, P.; Priftis, A.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xinos, N.; Skaltsounis, A.; Mamoulakis, C.; Tsatsakis, A.M.; Spandidos, D.A.; et al. Assessment of the antioxidant activity of an olive oil total polyphenolic fraction and hydroxytyrosol from a Greek Olea europea variety in endothelial cells and myoblasts. Int. J. Mol. Med. 2017, 40, 703–712. [Google Scholar] [CrossRef]
- Maurya, D.K.; Devasagayam, T.P. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef]
- Micucci, M.; Malagueti, M.; Gallini-Toschi, T.; Di Lecce, G.; Aldini, R.; Angeletti, A.; Chiarini, A.; Budriesi, R.; Hrelia, S. Cardiac and vascular synergic protective effect of Olea europea L. leaves and Hibiscus sabdariffa L. flower extracts. Oxid. Med. Cell. Longev. 2015, 2015, 318125. [Google Scholar] [CrossRef]
- Salomone, R.; Loppolo, G. Environmental impacts of olive oil production: A Life Cycle Assessment case study in the province of Messina (Sicily). J. Clean. Prod. 2012, 28, 88–100. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefining 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Lafka, T.-I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic extracts from wild olive leaves and their potential as edible oils antioxidants. Foods 2013, 2, 18–31. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Zheng, Q.; Shi, B.; Li, Z.; Wang, Z.L. Recent progress on piezoelectric and triboelectric energy harvesters in biomedical systems. Adv. Sci. 2017, 4, 1700029. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; Trombi, L.; D’Alessandro, D.; Coltelli, M.B.; Serino, L.P.; Pini, R.; Lazzeri, A.; Petrini, M.; Danti, S. Pore size distribution and blend composition affect in vitro prevascularized bone matrix formation on poly(vinyl alcohol)/gelatin sponges. Macromol. Mater. Eng. 2017, 302, 1700300. [Google Scholar] [CrossRef]
- Azimi, B.; Milazzo, M.; Lazzeri, L.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.; Danti, S. Electrospinning piezoelectric fibers for biocompatible devices. Adv. Healthc. Mat. 2019, 1901287. [Google Scholar] [CrossRef]
- Fukada, E.; Hara, K. Piezoelectric effect in blood vessel walls. J. Phys. Soc. Jpn. 1969, 26, 777–780. [Google Scholar] [CrossRef]
- Lenz, T.; Hummel, R.; Katsouras, I.; Groen, W.A.; Nijemeisland, M.; Ruemmler, R.; Schäfer, M.K.E.; de Leeuw, D.M. Ferroelectricity and piezoelectricity in soft biological tissue: Porcine aortic walls revisited. Appl. Phys. Lett. 2017, 111, 133701. [Google Scholar] [CrossRef]
- Jung, K.A.; Kwak, M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Río, J.A. Antioxidant activity of phenolics extracted from Olea europea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Benam, KH.; Dauth, S.; Hassell, B.; Herland, A.; Jain, A.; Jang, K.J.; Karalis, K.; Kim, H.J.; MacQueen, L.; Mahmoodian, R.; et al. Engineered in vitro disease models. Annu. Rev. Pathol. 2015, 10, 195–262. [Google Scholar] [CrossRef]
- Tabandeh, M.R.; Oryan, A.; Mohammadalipour, A. Polysaccharides of Aloe vera induce MMP-3 and TIMP-2 gene expression during the skin wound repair of rat. Int. J. Biol. Macromol. 2014, 65, 424–430. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, E.; Tsatsakis, A.; Hayes, W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef]
- Zaden, K.; Luyt, A.; Zarif, L.; Augustine, R.; Hasan, A.; Messori, M.; Hasssan, M.; Yalcin, H. Electrospun polylactic acid/date palm polyphenol extract nanofibres for tissue engineering applications. Emerg. Mater. 2019, 2, 141–151. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Prot. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Palla, M.; Digiacomo, M.; Cristani, C.; Bertini, S.; Giovannetti, M.; Macchia, M.; Manera, C.; Agnolucci, M. Composition of health-promoting phenolic compounds in two extra virgin olive oils and diversity of associated yeasts. J. Food Compost. Anal. 2018, 74, 27–33. [Google Scholar] [CrossRef]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef] [PubMed]


| Sampling Period (Month-Year) | TP (mg/g) |
|---|---|
| 02–2018 | 27.83 |
| 05–2018 | 23.06 |
| 10–2018 | 14.99 |
| 03–2019 | 23.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Ossa, J.G.; Felice, F.; Azimi, B.; Esposito Salsano, J.; Digiacomo, M.; Macchia, M.; Danti, S.; Di Stefano, R. Waste Autochthonous Tuscan Olive Leaves (Olea europaea var. Olivastra seggianese) as Antioxidant Source for Biomedicine. Int. J. Mol. Sci. 2019, 20, 5918. https://doi.org/10.3390/ijms20235918
De la Ossa JG, Felice F, Azimi B, Esposito Salsano J, Digiacomo M, Macchia M, Danti S, Di Stefano R. Waste Autochthonous Tuscan Olive Leaves (Olea europaea var. Olivastra seggianese) as Antioxidant Source for Biomedicine. International Journal of Molecular Sciences. 2019; 20(23):5918. https://doi.org/10.3390/ijms20235918
Chicago/Turabian StyleDe la Ossa, Jose Gustavo, Francesca Felice, Bahareh Azimi, Jasmine Esposito Salsano, Maria Digiacomo, Marco Macchia, Serena Danti, and Rossella Di Stefano. 2019. "Waste Autochthonous Tuscan Olive Leaves (Olea europaea var. Olivastra seggianese) as Antioxidant Source for Biomedicine" International Journal of Molecular Sciences 20, no. 23: 5918. https://doi.org/10.3390/ijms20235918
APA StyleDe la Ossa, J. G., Felice, F., Azimi, B., Esposito Salsano, J., Digiacomo, M., Macchia, M., Danti, S., & Di Stefano, R. (2019). Waste Autochthonous Tuscan Olive Leaves (Olea europaea var. Olivastra seggianese) as Antioxidant Source for Biomedicine. International Journal of Molecular Sciences, 20(23), 5918. https://doi.org/10.3390/ijms20235918