Cannabidiol—from Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer
Abstract
1. Introduction
2. Pharmacology, Toxicology, and Route of Administration
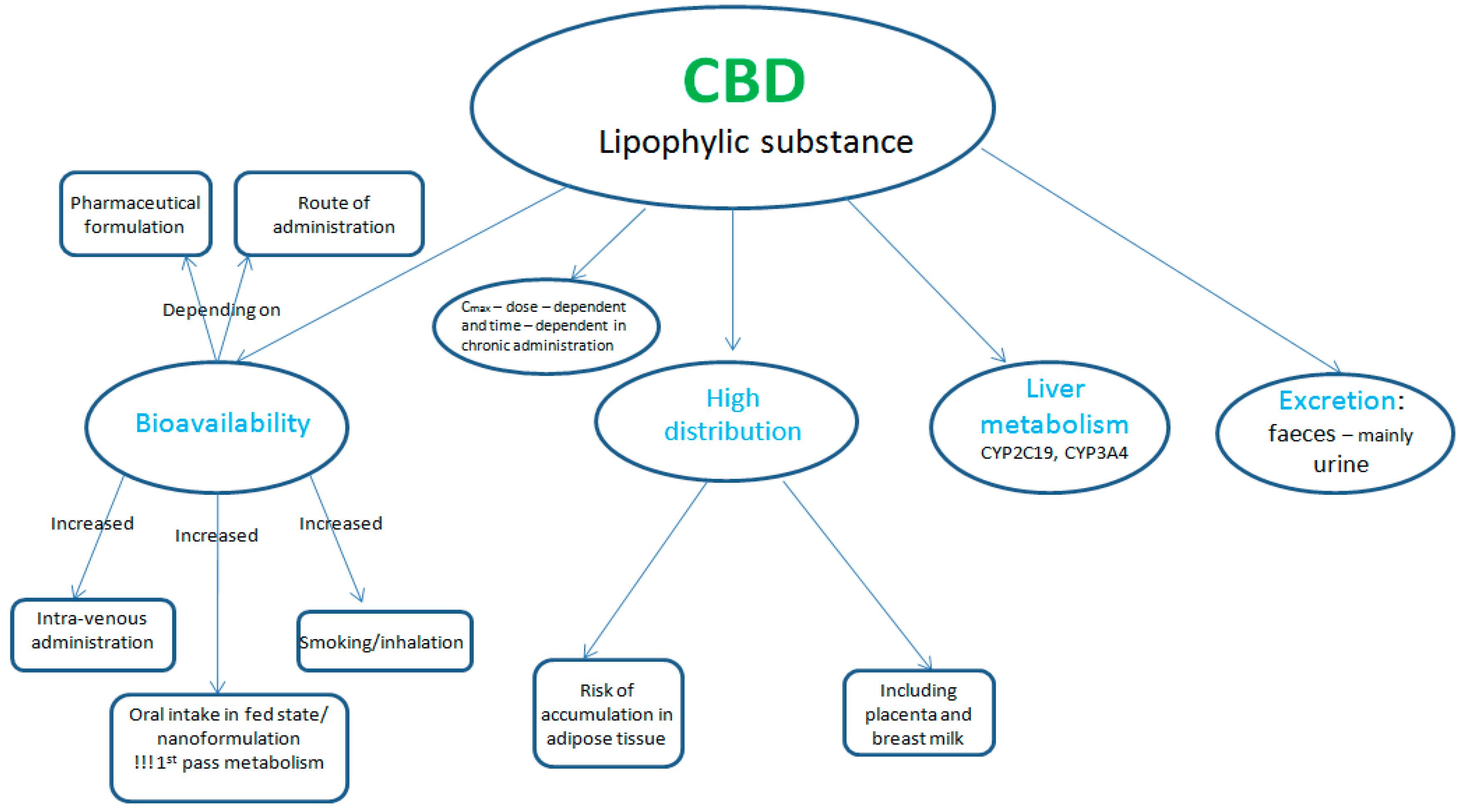
2.1. Pharmacokinetics
2.2. Pharmacodynamics
2.3. Pharmacological Actions and Indications
2.4. Toxicology
2.5. Route of Administration and Dosage
2.6. Cannabidiol and Hepatotoxicity: A Debate
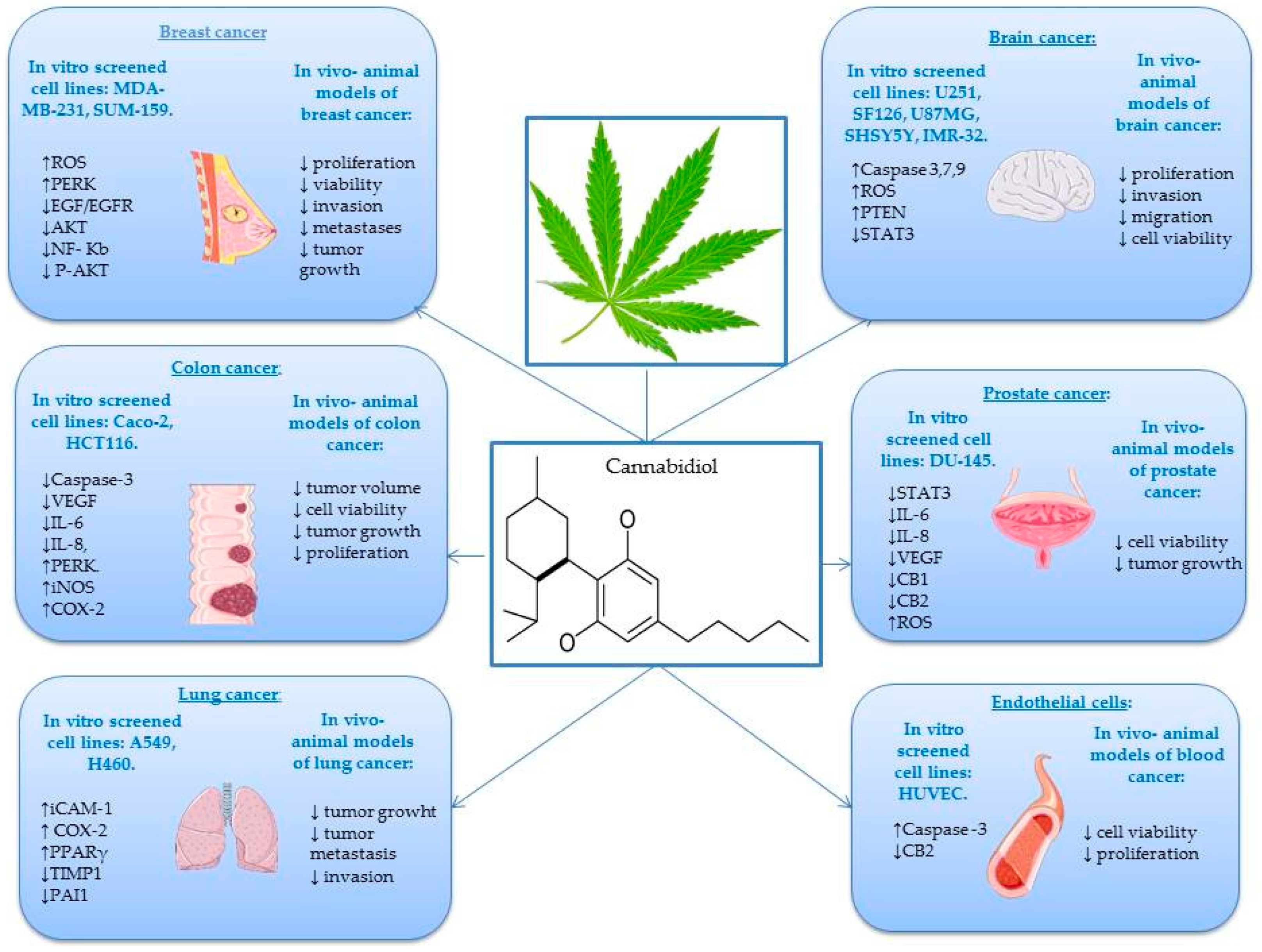
3. Anticancer Effects of CBD in In Vitro and In Vivo Studies
4. Immunomodulatory Effects of CBD
5. CBD in Inflammation-associated Carcinogenesis
6. Anti-angiogenic Effects of CBD
7. Clinical Evidence of the Anticancer Effects of CBD
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-HT1A | Serotonin receptor type 1A |
| ACF | Aberrant crypt foci |
| AD | Alzheimer’s disease |
| Aᵦ42 | Amiloid beta 42 |
| BCE | Before Common Era |
| BRCA-1 | Breast cancer gene 1 |
| BRCA-2 | Breast cancer gene 2 |
| cAMP | Cyclic adenosine monophosphate |
| CB1, CB2 | Cannabinoid receptor 1,2 |
| CBD | Cannabidiol |
| CBR | Cannabinoid receptor |
| CD-1; 4 | Cluster of differentiation 1; 4 |
| CGS | Glioma stem cells |
| CID 16020046 | Inverse agonist at the former orphan receptor GPR55 |
| COX | Cyclooxygenase |
| CT | Computed tomography scan |
| CXCL16 | Chemokine (C-X-C motif) ligand 16 |
| DNA | Deoxyribonucleic acid |
| EAE | Experimental autoimmune encephalomyelitis |
| EC50 | Half maximal effective concentration |
| EGF/EGFR | Epidermal growth factor/epidermal growth factor receptor |
| ELISA | Enzyme-linked immunosorbent assay |
| EMA | European Medicine Agency |
| EMV | Exosome and microvesicle |
| ENT-1 | Equilibrative nucleoside transporter-1 |
| ET-1 | Endothelin-1 |
| FDA | Food and Drug administration |
| GABA | Gamma-aminobutyric acid |
| GI | Gastro-intestinal |
| GPR55 | G-coupled protein receptor-55 |
| GSC | Glioma stem cell |
| GW9662 | Potent antagonist of Peroxisome proliferator-activated receptor gamma |
| GPX | Glutathione peroxidase |
| HUVEC | Human umbilical vein endothelial cells |
| HIF-1a | Regulatory subunit of the hypoxia- inducible transcription factor |
| i.p. | Intraperitoneal |
| IC50 | Half maximal inhibitory concentration |
| ICAM | Inter-intracellular adhesion molecule |
| ICR | Institute of Cancer Research |
| IFN-γ | Interferon Gamma |
| IGHM | Immunoglobulin heavy constant Mu |
| IL-2,6,8,9,12,IL-1A; 17A/F2 | Interleukin 2, 6,8,9,12, 1A, 17A/F2, |
| iNOS | Inducible nitric oxide synthase |
| Ki | Inhibitory constant binding affinity |
| KPC | KRAS, p53, Cre mice |
| MDA | Malonaldehyde |
| MIP-1α,1ᵦ,2 | Macrophage inflammatory protein 1α,1ᵦ, 2 |
| MMP2,9 | Matrix metalloproteinase-2, 9 |
| mRNA | Messenger RNA |
| Mtor | Mammalian target of rapamycin |
| NFκB | Nuclear factor κB |
| n.d. | Not determined |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PDGF-AA | Platelet-derived growth factor subunit A |
| PERK | Extracellular signal-regulated kinase phosphorylation |
| Phospho-Akt | Phosphorylated AKT |
| PPARγ | Nuclear peroxisome proliferation activated receptor |
| PSA | Prostate-specific antigen |
| PTEN | Phosphatase and tensin homolog |
| ROS | Reactive oxygen species |
| RT-PRC | Real-time polymerase chain reaction |
| s.c. | Sub cutaneous |
| S100A10B | S100 calcium-binding protein A10 |
| S1P | Sphingosine-1-phosphate |
| S1PR1 | Sphingosine-1-phosphate receptor 1 |
| Serpin E1 | Serpin Family E Member 1 |
| SGPL1 | Sphingosine-1 phosphate lyase |
| SiRNA | Small interfering RNA |
| SOD | Superoxide dismutase |
| STAT-3,5 | Signal transducers and activators of transcription 3,5 |
| TGFBA | Transforming growth factor beta, alpha |
| THC | Tetrahydrocannabinol |
| THCA | Tetrahydrocannabinolic acid |
| TIMP1 | Tissue inhibitor of matrix metalloproteinases1 |
| TNF-α | Tumor necrosis factor α |
| Tp53 | Tumor protein p53 |
| TRP | Transient receptor potential |
| TRPA | Transient receptor potentialankyrin |
| TRPV1 | Transient receptor potential vanilloid type1 |
| TRPV2 | Transient receptor potential vanilloid type 2 |
| TRPM8 | Transient receptor potentialmelastatin 8 |
| uPA | Urokinase-type plasminogen activator |
| VEGF | Vascular endothelial growth factor |
| VR1, VR2 | Vanilloid receptor 1, 2 |
References
- Chandra, S.; Lata, H.; ElSohly, M.A.; Walker, L.A.; Potter, D. Cannabis cultivation: Methodological issues for obtaining medical-grade product. Epilepsy Behav. 2017, 70, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Guy, G.W. Models of Cannabis Taxonomy, Cultural Bias, and Conflicts between Scientific and Vernacular Names. Bot. Rev. 2017, 83, 327–381. [Google Scholar] [CrossRef]
- Grof, C.P.L. Cannabis, from plant to pill. Br. J.Clin. Pharmacol. 2018, 84, 2463–2467. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant. Sci. 2018, 9, 1969. [Google Scholar] [CrossRef]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and Medicinal Chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant. Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Thacker, D.L. A Brief Background on Cannabis: From Plant to Medical Indications. J. AOAC Int. 2019, 102, 412–420. [Google Scholar] [CrossRef]
- Russo, E.B. History of cannabis as medicine: Nineteenth century irish physicians and correlations of their observations to modern research. In Cannabis sativa L. Botany and Biotechnology; Suman, C., Ed.; Springer: Berlin, Germany, 2017; pp. 63–78. [Google Scholar]
- Pisanti, S.; Bifulco, M. Modern history of medical cannabis: From widespread use to prohibitionism and back. Trends Pharmacol. Sci. 2017, 38, 195–198. [Google Scholar] [CrossRef]
- Baron, E.P. Comprehensive review of medicinal marijuana, cannabinoids, and therapeutic implications in medicine and headache: What a long strange trip it’s been…. Headache 2015, 55, 885–916. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Handbook of Cannabis; Oxford University Press: Oxford, UK, 2014; pp. 768–781. [Google Scholar]
- Hammond, C.T.; Mahlberg, P.G. Morphogenesis of capitate glandular hairs of Cannabis sativa (Cannabaceae). Am. J. Bot. 1977, 64, 1023–1031. [Google Scholar] [CrossRef]
- Sirikantaramas, S.; Morimoto, S.; Shoyama, Y.; Ishikawa, Y.; Wada, Y.; Shoyama, Y.; Taura, F. The gene controlling marijuana psychoactivity–molecular cloning and heterologous expression of Delta(1)-tetrahydrocannabinolic acid synthase from Cannabis sativa L. J. Biol. Chem. 2004, 279, 39767–39774. [Google Scholar] [CrossRef]
- Marks, M.D.; Tian, L.; Wenger, J.P.; Omburo, S.N.; Soto-Fuentes, W.; He, J.; Gang, D.R.; Weiblen, G.D.; Dixon, R.A. Identification of candidate genes affecting Delta(9)-tetrahydrocannabinol biosynthesis in Cannabis sativa. J. Exp. Bot. 2009, 60, 3715–3726. [Google Scholar] [CrossRef]
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. PNAS 2012, 109, 2811–12816. [Google Scholar] [CrossRef]
- Carvalho, A.; Hansen, E.H.; Kayser, O.; Carlsen, S.; Stehle, F. Designing microorganisms for heterologous biosynthesis of cannabinoids. FEMS Yeast Res. 2017, 17. [Google Scholar] [CrossRef]
- Taura, F.; Morimoto, S.; Shoyama, Y. Purification and characterization of cannabidiolic-acid synthase from CannabissativaL. Biochemical analysis of a novel enzyme that catalyzes the oxidocyclization of cannabigerolic acid to cannabidiolic acid. J. Biol. Chem. 1996, 271, 17411–17416. [Google Scholar] [CrossRef]
- Morimoto, S.; Komatsu, K.; Taura, F.; Shoyama, Y. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998, 49, 1525–1529. [Google Scholar] [CrossRef]
- Lange, B.M.; Turner, G.W. Terpenoid biosynthesis in trichomes—current status and future opportunities. Plant Biotechnol. J. 2013, 11, 2–22. [Google Scholar] [CrossRef]
- Happyana, N.; Agnolet, S.; Muntendam, R.; Van Dam, A.; Schneider, B.; Kayser, O. Analysis of cannabinoids in laser-microdissectedtrichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 2013, 87, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, F.; Stehle, F.; Kayser, O. The Biosynthesis of Cannabinoids. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 13–23. [Google Scholar]
- Grotenhermen, F.; Müller-Vahl, K. Medicinal Uses of Marijuana and Cannabinoids. CRC Crit. Rev. Plant. Sci. 2016, 35, 378–405. [Google Scholar] [CrossRef]
- Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol and Cancer —An Overview of the Preclinical Data. In Molecular Considerations and Evolving Surgical Management Issues in the Treatment of Patients with a Brain Tumor; Terry, L., Ed.; InTechOpen: London, UK, 2015; p. 13. [Google Scholar]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Agurell, S.; Carlsson, S.; Lindgren, J.E.; Ohlsson, A.; Gillespie, H.; Hollister, L. Interactions of delta 1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia 1981, 37, 1090–1092. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Ohlsson, A.; Lindgren, J.E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed. Environ. Mass. Spectrom. 1986, 13, 77–83. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Challapalli, P.V.; Stinchcomb, A.L. In vitro experiment optimization for measuring tetrahydrocannabinol skin permeation. Int. J. Pharm. 2002, 241, 329–339. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Martin, J.H.; Schneider, J.; Lucas, C.J.; Galettis, P. Exogenous Cannabinoid Efficacy: Merely a Pharmacokinetic Interaction? Clin. Pharmacokinet. 2018, 57, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Zendulka, O.; Dovrtělová, G.; Nosková, K.; Turjap, M.; Šulcová, A.; Hanuš, L.; Juřica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug. Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Consroe, P.; Laguna, J.; Allender, J.; Snider, S.; Stern, L.; Sandyk, R.; Kennedy, K.; Schram, K. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol. Biochem. Behav. 1991, 40, 701–708. [Google Scholar] [CrossRef]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef]
- Kogan, N.M.; Mechoulam, R. Cannabinoids in health and disease. Dialogues Clin. Neurosci. 2007, 9, 413–430. [Google Scholar]
- McCarberg, B.H.; Barkin, R.L. The future of cannabinoids as analgesic agents: A pharmacologic, pharmacokinetic, and pharmacodynamic overview. Am. J. Ther. 2007, 14, 475–483. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef]
- Showalter, V.M.; Compton, D.R.; Martin, B.R.; Abood, M.E. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): Identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996, 278, 989–999. [Google Scholar]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Di Marzo, V. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Guida, F.; Moriello, A.S.; De Chiaro, M.; Piscitelli, F.; de Novellis, V.; Maione, S.; Di Marzo, V. N-palmitoyl-vanillamide (palvanil) is a non-pungent analogue of capsaicin with stronger desensitizing capability against the TRPV1 receptor and anti-hyperalgesic activity. Pharmacol. Res. 2011, 63, 294–299. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Orlando, P.; Moriello, A.S.; Aviello, G.; Stott, C.; Izzo, A.A.; Di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A cannabigerolquinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J. Neuroimmune. Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- Karst, M.; Salim, K.; Burstein, S.; Conrad, I.; Hoy, L.; Schneider, U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: A randomized controlled trial. JAMA 2003, 290, 1757–1762. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef]
- Rock, E.M.; Bolognini, D.; Limebeer, C.L.; Cascio, M.G.; Anavi-Goffer, S.; Fletcher, P.J.; Mechoulam, R.; Pertwee, R.G.; Parker, L.A. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendriticautoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef]
- van den Elsen, G.A.; Ahmed, A.I.; Lammers, M.; Kramers, C.; Verkes, R.J.; van der Marck, M.A.; Rikkert, M.G. Efficacy and safety of medical cannabinoids in older subjects: A systematic review. Ageing Res. Rev. 2014, 14, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, C.; Vermersch, P. A breakthrough for the treatment of spasticity in multiple sclerosis. Rev. Neurol. 2015, 171, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Russo, E.; Elia, M. Cannabidiol and epilepsy: Rationale and therapeutic potential. Pharmacol. Res. 2016, 107, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Sosnoff, J. Cannabidiol to Improve Mobility in People with Multiple Sclerosis. Front. Neurol. 2018, 9, 183. [Google Scholar] [CrossRef]
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician 2017, 20, E755–E796. [Google Scholar]
- Li, H.; Kong, W.; Chambers, C.R.; Yu, D.; Ganea, D.; Tuma, R.F.; Ward, S.J. The non-psychoactive phytocannabinoid cannabidiol (CBD) attenuates pro-inflammatory mediators, T cell infiltration, and thermal sensitivity following spinal cord injury in mice. Cell. Immunol. 2018, 329, 1–9. [Google Scholar] [CrossRef]
- Mannucci, C.; Navarra, M.; Calapai, F.; Spagnolo, E.V.; Busardò, F.P.; Cas, R.D.; Ippolito, F.M.; Calapai, G. Neurological Aspects of Medical Use of Cannabidiol. CNS Neurol. Disord. Drug Targets 2017, 16, 541–553. [Google Scholar] [CrossRef]
- Bitencourt, R.M.; Takahashi, R.N. Cannabidiol as a Therapeutic Alternative for Post-Traumatic Stress Disorder: From Bench Research to Confirmation in Human Trials. Front. Neurosci. 2018, 12, 502. [Google Scholar] [CrossRef]
- Osborne, A.L.; Solowij, N.; Weston-Green, K. A systematic review of the effect of cannabidiol on cognitive function: Relevance to schizophrenia. Neurosci. Biobehav. Rev. 2017, 72, 310–324. [Google Scholar] [CrossRef]
- Haustein, M.; Ramer, R.; Linnebacher, M.; Manda, K.; Hinz, B. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem. Pharmacol. 2014, 92, 312–325. [Google Scholar] [CrossRef]
- Morgan, C.J.; Das, R.K.; Joye, A.; Curran, H.V.; Kamboj, S.K. Cannabidiol reduces cigarette consumption in tobacco smokers: Preliminary findings. Addict. Behav. 2013, 38, 2433–2436. [Google Scholar] [CrossRef] [PubMed]
- World health organization-WHO. Available online: https://who.int/ (accessed on 20 May 2019).
- Medicines. Available online: https://www.medicines.org.uk/emc/product/602/smpc (accessed on 10 June 2019).
- Food and Drug administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf (accessed on 15 May 2019).
- Stout, S.M.; Cimino, N.M. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug. Metab. Rev. 2014, 46, 86–95. [Google Scholar] [CrossRef]
- Mallat, A.; Teixeira-Clerc, F.; Deveaux, V.; Manin, S.; Lotersztajn, S. The endocannabinoid system as a key mediator during liver diseases: New insights and therapeutic openings. Br. J. Pharmacol. 2011, 163, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Mohanraj, R.; Pacher, P.; Horvath, B.; Batkai, S.; Park, O.; Tanashian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Rozenfeld, R.; Wu, D.; Devi, L.A.; Zhang, Z.; Cederbaum, A. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Rad. Biol. Med. 2014, 68, 260–267. [Google Scholar] [CrossRef]
- Ashino, T.; Hakukawa, K.; Itoh, Y.; Numazawa, S. Inhibitory effect of synthetic cannabinoids on CYP1A activity in mouse liver microsomes. J. Toxicol. Sci. 2014, 39, 815–820. [Google Scholar] [CrossRef]
- Avraham, Y.; Grigoriadis, N.; Poutahidis, T.; Vorobiev, L.; Magen, I.; Ilan, Y.; Mechoulam, R.; Berry, E. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br. J. Pharmacol. 2011, 162, 1650–1658. [Google Scholar] [CrossRef]
- Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules 2019, 24, 1694. [Google Scholar]
- Ramer, R.; Hinz, B. Cannabinoids as Anticancer Drugs. Cannabinoid Pharmacology. Adv. Pharmacol. 2017, 80, 397–436. [Google Scholar]
- Pertwee, R.G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: D9 –tetrahydrocannabinol, cannabidiol and D9 –tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Khan, M.I.; Soboci, A.A.; Czarnecka, A.M.; Król, M.; Botta, B. The Therapeutic Aspects of the Endocannabinoid System (ECS) for Cancer and their Development: From Nature to Laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef] [PubMed]
- Dariš, B.; Tancer Verboten, M.; Knez, Ž.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Carchman, R.A. Antineoplastic Activity of Cannabinoids. J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef]
- Cridge, B.J.; Rosengeren, R.J. Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag. Res. 2013, 5, 301–313. [Google Scholar]
- Velasco, G.; Hernandez-Tiedra, S.; Davila, D.; Lorente, M. The use of cannabinoids as anticancer agents. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 259–266. [Google Scholar] [CrossRef]
- Śledziński, P.; Zeyland, J.; Słomski, R.; Nowak, A. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med. 2018, 7, 765–775. [Google Scholar] [CrossRef]
- Azar, F.E.; Azami-aghdash, S.; Pournaghi-Azar, F.; Mazdaki, A.; Rezapour, A.; Ebrahimi, P.; Yousefzadeh, N. Cost-effectiveness of lung cancer screening and treatment methods: A systematic review of systematic review. BMC Health Serv. Res. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health; Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014.
- Ramer, R.; Rohde, A.; Merkord, J.; Rohde, H.; Hinz, B. Decrease of Plasminogen Activator Inhibitor-1 May Contribute to the Anti-Invasive Action of Cannabidiol on Human Lung Cancer Cells. Pharm. Res. 2010, 27, 2162–2174. [Google Scholar] [CrossRef]
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966. [Google Scholar] [CrossRef]
- McMahon, G.A.; Petitclerc, E.; Stefansson, S.; Smith, E.; Westrick, R.J.; Ginsburg, D.; Brooks, P.C.; Lawrence, D.A. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J. Biol. Chem. 2001, 276, 33964–33968. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-g Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Canc. Ther. 2012, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Spatz, A.; Grob, J.J.; Malvehy, L.; Newton-Bishop, J.; Stratigos, A.; et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline – Update 2012. Eur. J. Cancer 2012, 48, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Simmerman, E.; Qin, X.; Yu, J.C.; Baban, B. Cannabinoids as a Potential New and Novel Treatment for Melanoma: A Pilot Study in a Murine Model. J. Surg. Res. 2018, 235, 210–215. [Google Scholar] [CrossRef]
- Blázquez, C.; Carracedo, A.; Barrado, L.; Real, P.J.; Fernández-Luna, J.L.; Velasco, G.; Malumbres, M.; Guzmán, M. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006, 20, 2633–2635. [Google Scholar] [CrossRef]
- Armstrong, J.L.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Anagnostou, M.E.; Babatunde, F.; Corazzari, M.; Redfern, C.P.F.; et al. Exploiting Cannabinoid-Induced Cytotoxic Autophagy to Drive Melanoma Cell Death. J. Invest. Dermatol. 2015, 135, 1629–1637. [Google Scholar] [CrossRef]
- Sullivan, R.; Peppercorn, J.; Sikora, K.; Zalcberg, J.; Meropol, N.J.; Amir, E.; Khayat, D.; Boyle, P.; Autier, P.; Tannock, I.F.; et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011, 12, 933–980. [Google Scholar] [CrossRef]
- Barnard, M.E.; Boeke, C.E.; Tamimi, R.M. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim. Biophys. Acta 2015, 1856, 73–85. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Sultan, A.S.; Marie, M.A.; Sheweita, S.A. Novel mechanism of cannabidiol-induced apoptosis in breast cancer cell lines. Breast 2018, 41, 34–41. [Google Scholar] [CrossRef]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.E., 3rd; et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef]
- McAllister, S.D.; Murase, R.; Rigel, T.C.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C.; et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Br. Can. Res. Treat. 2010, 129, 37–47. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer Treatment and Survivorship Statistics. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar]
- Bhandari, A.; Woodhouse, M.; Gupta, S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: A SEER-based analysis with comparison to other young-onset cancers. J. Investig. Med. 2017, 65, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Andersen, L.; Hasenöhrl, C.; Feuersinger, D.; Stan, A.; Fauland, A.; Magnes, C.; El-Heliebi, A.; Lax, S.; Uranitsch, S.; et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. Br. J. Pharmacol. 2016, 173, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef]
- Honarmand, M.; Namazi, F.; Mohammadi, A.; Nazifi, S. Can cannabidiol inhibit angiogenesis in colon cancer? Comp. Clin. Path. 2019, 28, 165–172. [Google Scholar] [CrossRef]
- Rebbeck, T.R. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin. Radiat. Oncol. 2016, 27, 3–10. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Nunn, A.V.; Guy, G.W.; Thomas, E.L.; Inal, J.M.; Bell, J.D.; Lange, S. Cannabidiol (CBD) is a Novel Inhibitor for Exosome and Microvesicle (EMV) Release in Cancer. Front. Pharmacol. 2018, 9, 889. [Google Scholar] [CrossRef]
- Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Iappelli, M.; Verde, R.; Stott, C.G.; Cristino, L.; Orlando, P.; Di Marzo, V. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 2012, 168, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Hudson, J.B.; Adomat, H.; Guns, E.; Cox, M.E. In Vitro Anticancer Activity of Plant-Derived Cannabidiol on Prostate Cancer Cell Lines. Pharmacol. Pharm. 2014, 5, 806–820. [Google Scholar] [CrossRef]
- Sreevalsan, S.; Joseph, S.; Jutooru, I.; Chadalapaka, G.; Safe, S.H. Induction of Apoptosis by Cannabinoids in Prostate and Colon Cancer Cells Is Phosphatase Dependent. Anticancer Res. 2012, 31, 3799–3807. [Google Scholar]
- Shah, V.; Kochar, P. Brain Cancer: Implication to Disease, Therapeutic Strategies and Tumor Targeted Drug Delivery Approaches. Recent Pat. Anticancer Drug Discov. 2018, 13, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Vaccani, A.; Ceruti, S.; Colombo, A.; Abbracchio, M.P. Antitumor Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, on Human Glioma Cell Lines. J. Pharmacol. Exp. Ther. 2004, 308, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Judkins, J.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; Mcallister, S.; Soroceanu, L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015, 6, e1601–e1611. [Google Scholar] [CrossRef] [PubMed]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol enhances the inhibitory effects of Δ9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2011, 9, 180–189. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cantelmo, A.R.; Cattaneo, M.G.; Cammarota, R.; Bartolini, D.; Cinquina, V.; Valenti, M.; Vicentini, L.M.; Noonan, D.M.; et al. Cannabidiol inhibits angiogenesis by multiple mechanism. Br. J. Pharmacol. 2012, 167, 1218–1231. [Google Scholar] [CrossRef]
- Perez de la Ossa, D.H.; Lorente, M.; Gil-Alegre, M.E.; Torres, S.; Garcia-Taboada, E.; Aberturas, M.R.; Molpeceres, J.; Velasco, G.; Torres-Suarez, A.I. Local Delivery of Cannabinoid-Loaded Microparticles Inhibits Tumor Growth in a Murine Xenograft Model of Glioblastoma Multiforme. Plos ONE 2013, 8, e54795. [Google Scholar] [CrossRef]
- Alharris, E.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M.; Alharris, E.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Role of miRNA in the regulation of cannabidiol-mediated apoptosis in neuroblastoma cells. Oncotarget 2019, 10, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Kiyotani, K.; Chan, H.T.; Nakamura, Y. Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens. Cancer Sci. 2018, 109, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tsung, K. Tumor reductive therapies and antitumor immunity. Oncotarget 2017, 8, 55736–55749. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.M.; Korbut, R.; Kania, P.W.; Buchmann, K. Cannabidiol effects on behaviour and immune gene expression in zebrafish (Danio rerio). Plos ONE 2018, 13, e0200016. [Google Scholar] [CrossRef] [PubMed]
- Tanasescu, R.; Constantinescu, C.S. Cannabinoids and the immune system: An overview. Immunobiology 2010, 215, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Hu, C.M.; Huang, C.H.; Wey, S.P.; Jan, T.R. Cannabidiol attenuates delayed-type hypersensitivity reactions via suppressing T-cell and macrophage reactivity. Acta Pharmacol. Sin. 2010, 31, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Croxford, J.L.; Yamamura, T. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J. Neuroimmunol. 2005, 166, 3–18. [Google Scholar] [CrossRef]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef]
- Watzl, B.; Scuderi, P.; Watson, R.R. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int. J. Immunopharmacol. 1991, 13, 1091–1097. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control. Antioxidants 2019, 8, 103. [Google Scholar] [CrossRef]
- Sacerdote, P.; Martucci, C.; Vaccani, A.; Bariselli, F.; Panerai, A.E.; Colombo, A.; Parolaro, D.; Massi, P. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J. Neuroimmunol. 2005, 159, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Adenosine as an endogenous immunoregulator in cancer pathogenesis: Where to go? Purinergic Signal. 2013, 9, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.L.F.; Springs, A.E.B.; Kaminski, N.E. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem. Pharmacol. 2008, 76, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. [Google Scholar] [CrossRef]
- Jan, T.R.; Su, S.T.; Wu, H.Y.; Liao, M.H. Suppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c mice. Int. Immunopharmacol. 2007, 7, 773–780. [Google Scholar] [CrossRef]
- Schwiebs, A.; Herrero San Juan, M.; Schmidt, K.G.; Wiercinska, E.; Anlauf, M.; Ottenlinger, F.; Thomas, D.; Elwakeel, E.; Weigert, A.; Farin, H.F.; et al. Cancer-induced inflammation and inflammation-induced cancer in colon: A role for S1P lyase. Oncogene 2019, 38, 4788–4803. [Google Scholar] [CrossRef]
- Rayburn, E.R.; Ezell, S.J.; Zhang, R. Anti-Inflammatory Agents for Cancer Therapy. Mol. Cell. Pharmacol. 2009, 1, 29–43. [Google Scholar] [CrossRef]
- Esposito, G.; De Filippis, D.; Maiuri, M.C.; De Stefano, D.; Carnuccio, R.; Iuvone, T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in β-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-κB involvement. Neurosci. Lett. 2006, 399, 91–95. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a Non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells through a Multitarget Effect. Plos ONE 2013, 8, e76918. [Google Scholar] [CrossRef]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc. Res. 2015, 107, 568–578. [Google Scholar] [CrossRef]
- Kisková, T.; Mungenast, F.; Suváková, M.; Jäger, W.; Thalhammer, T. Future Aspects for Cannabinoids in Breast Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 1673. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, A. The Trouble with CBD Oil. Med. Cannabis Cannabinoids 2018, 1, 65–72. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Marzo, V.D.; Pertwee, R.G. Are cannabidiol and Δ9 -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L.O. Cannabidiol – Recent Advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Amin, M.R.; Ali, D.W. Pharmacology of Medical Cannabis. Recent Advances in Cannabinoid Physiology and Pathology; Springer: Berlin, Germany, 2019; pp. 151–165. [Google Scholar]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03607643 (accessed on 4 May 2019).
- Sulé-Suso, J.; Watson, N.A.; Pittius, D.G. Striking lung cancer response to self- administration of cannabidiol: A case report and literature review. SAGE Open Med. Case Rep. 2019, 7. [Google Scholar] [CrossRef]
- Kenyon, J.; Liu, W.A.I.; Dalgleiersh, A. Report of Objective Clinical Responses of Cancer Patients to Pharmaceutical-grade Synthetic Cannabidiol. Anticancer Res. 2018, 38, 5831–5835. [Google Scholar] [CrossRef]
- Dall’Stella, P.B.; Docema, M.F.L.; Maldaun, M.V.C.; Feher, O.; Lancellotti, C.L.P.; Ware, M. Case Report: Clinical Outcome and Image Response of Two Patients With Secondary High-Grade Glioma Treated With Chemoradiation, PCV, and Cannabidiol. Front. Oncol. 2019, 8, 1–7. [Google Scholar] [CrossRef]
- Barrie, A.M.; Gushue, A.C.; Eskander, R.N. Gynecologic Oncology Reports Dramatic response to Laetrile and cannabidiol (CBD) oil in a patient with metastatic low grade serous ovarian carcinoma. Gynecol. Oncol. Rep. 2019, 29, 10–12. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef] [PubMed]
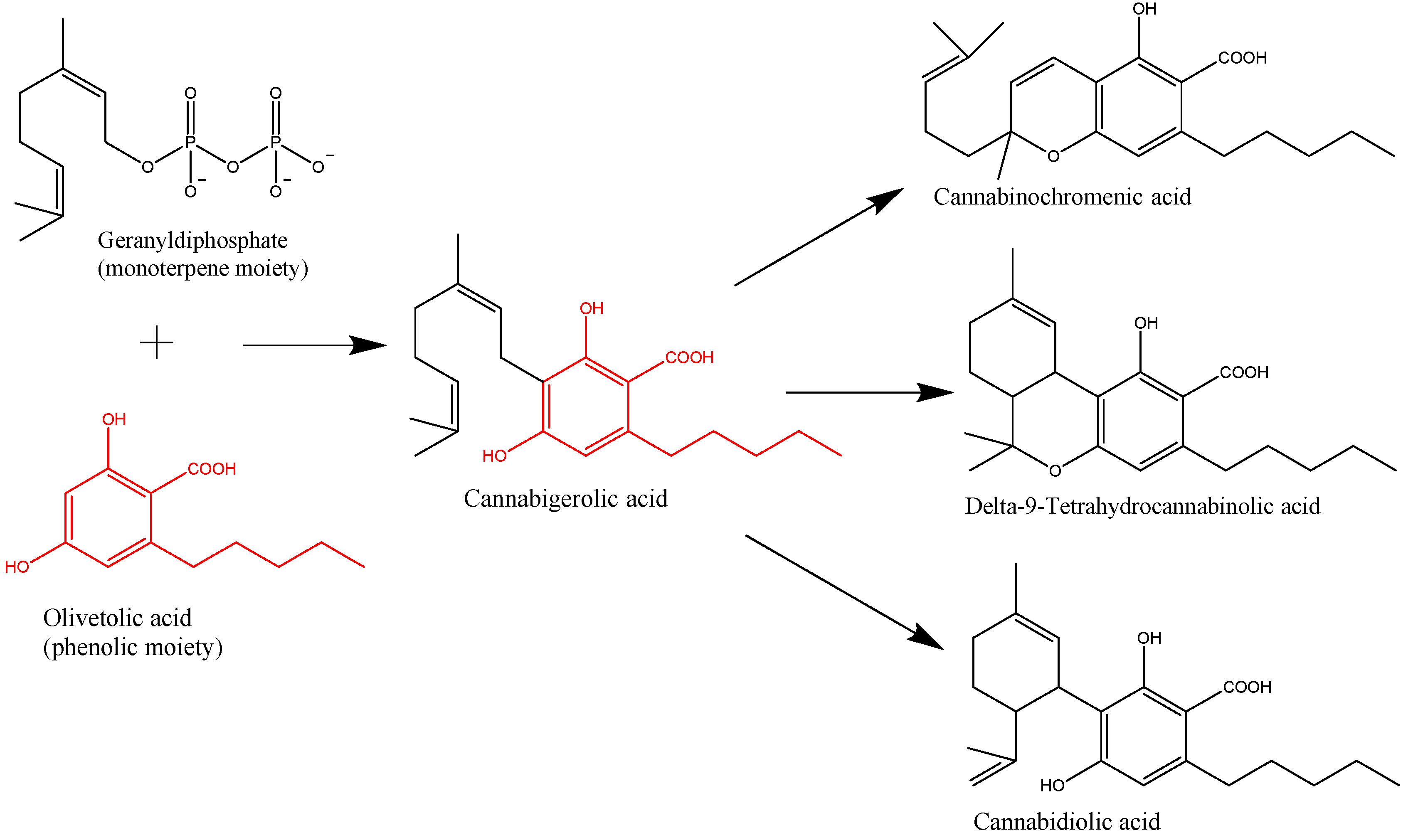
| Receptor | Effect | Ki; EC50; IC50 | References |
|---|---|---|---|
| CB1 | Antagonist | Ki = 4350–4900 nM | [43,44] |
| CB2 | Inverse agonist | Ki = 2860–4200 nM | [43,45] |
| GPR55 | Antagonist | IC50 = 445 nM | [45] |
| TRPM8 | Antagonist | IC50 = 80–140 nM | [46,47] |
| TRPV1 | Agonist | EC50 = 1000 nM | [46,48] |
| TRPV2 | Agonist | EC50 = 1250 nM | [46,48] |
| TRPV3 | Agonist | EC50 = 3700 nM | [46,49] |
| TRPA1 | Agonist | EC50 = 110 nM | [46,48] |
| PPARϒ | Agonist | EC50 = 20,100 nM | [50] |
| CYP 450 – Isoenzymes | Substrates | Inducers | Inhibitors |
|---|---|---|---|
| CYP3A4 | CBD Alprazolam Diazepam Amlodipine Simvastatine Atorvastatine Apixaban Rivaroxaban | Carbamazepine Fenitoine Phenobarbital | Eritromicine Claritromicine Verapamil Diltiazem Fluconazol Itraconazol |
| CYP2C19 | CBD Clopidogrel Fenitoine Diazepam | Carbamazepine Rifampicine | Fluoxetine Fluvoxamine Ketoconazole Omeprazole |
| CYP1A2 | Theophylline Clozapine Naproxen | CBD Tobacco smoke | Ciprofloxacine Ofloxacine Levofloxacine Amiodarone |
| P-Glycoproteine (intestinal absorption) | Loperamide Morphine Dabigatran Atorvastatine Simvastatine Paclitaxel Antraciclines | Carbamazepine Rifampicine Phenobarbital | CBD Ketoconazol Itraconazol Eritromicine Clarytromicine Propafenone Amiodarone |
| Type/Cancer Cell Line | Cell Line | In vitro | In vivo | Conc. | Conclusion | Ref. |
|---|---|---|---|---|---|---|
| Colorectal cancer | HCT116 | √ | 0–8 µM 100 mg·kg−1 | CBD induces apoptosis by regulating many pro- and anti-apoptotic proteins, and decreases tumor volume | [105] | |
| Colorectal cancer | DLD-1 | √ | 0–8 µM 100 mg·kg−1 | |||
| Colon cancer | CaCo-2 HCT116 | √ IC50 = 0.67 µM | √ | 5 mg/kg | Reduced aberrant crypt foci (ACF) number of polyps and tumors | [103] |
| Colon cancer | CT26 | √ | 5 mg/kg | CBD induces apoptosis, showed anti-angiogenesis and anti-metastatic effect | [106] | |
| Colon cancer | HCT116 | √ | 5 mg·kg−1 | CBD reduces colon cancer cells | [104] | |
| Prostate cancer | PC3 | √ | 1–5 µM | CBD reduces exosome release. | [108] | |
| Prostate cancer | LNCaP | √ | 1; 10; 100 mg/kg | CBD decreased cell viability and tumor growth | [109] | |
| Prostate cancer | DU-145 | √ | 20–80 µg/mL | CBD is a potent inhibitor of cancer cell growth and has lowest potency in non-cancer cells | [110] | |
| Prostate cancer | LNCaP | √ | 20–80 µg/mL | |||
| Prostate cancer | PC3 LNCaP | √ | 5–15 µM | CBD induces apoptosis | [111] | |
| Lung cancer | A549 | √ | 5 mg/kg | CBD decreased tumor growth | [87] | |
| Lung cancer | H460 | √ | 3µM | CBD decreased tumor metastasis | [88] | |
| Lung cancer | A549 | √ | 3 µM | ICAM-1 present an essential objective for CBD in executing its antitumorigenic function. | [64] | |
| Lung cancer | A549 H460 | √ | √ | 3 µmol/L 5–10 mg/kg | CBD induces cancer cell apoptosis | [90] |
| Brain tumor | U87 U373 | √ | 5–10 µM | CBD induces apoptosis through activation of serotonin and vanilloid receptors | [113] | |
| Brain tumor | GSC | √ IC50 = 3.5 µM | √ | 15 mg/kg | CBD induces apoptosis through the production of ROS | [114] |
| Brain tumor | U251 SF126 | √ IC50 = 1.1–1.3 µM | 0.4 µM | CBD induces apoptosis and reduces cell viability and invasion | [115] | |
| Brain tumor | U87MG | √ | 10 µM | CBD activates TRPV2 receptors to promote cancer cell death. | [116] | |
| Brain tumor | U87MG | √ | 6.7 mg | CBD enhances apoptosis and decreases cell proliferation. | [118] | |
| Brain tumor | SH SY5Y IMR-32 | √ | 10 µM | CBD induces apoptosis and reduces cancer cell migration and invasion | [119] | |
| Skin cancer | Murine B16F10 melanoma tumors | √ | 5 mg/kg | CBD reduces tumor size | [92] | |
| Breast cancer | MDA-MB-231 | √ IC50 = 6–10.6 µM | √ | 10 mg/kg | Decreased tumor growth | [51] |
| Breast cancer | T47D MDA-MB-231 | √ | 10 mg/kg | Decreased tumor metastasis | [98] | |
| Breast cancer | MDA-MB-231 | √ | 5 mg/kg | CBD induces cancer cells apoptosis | [97] | |
| Breast cancer | SUM-159 | √ | √ | 3–18 µM | CBD induces both apoptosis and autophagy-induced death in cancer cells | [99] |
| Endothelial cells | HUVEC | √ | √ | 1–19 µM | CBD inhibited cell proliferations and exhibited potent antiangiogenic properties inhibiting cell invasion and migration | [117] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol—from Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. https://doi.org/10.3390/ijms20235905
Kis B, Ifrim FC, Buda V, Avram S, Pavel IZ, Antal D, Paunescu V, Dehelean CA, Ardelean F, Diaconeasa Z, et al. Cannabidiol—from Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. International Journal of Molecular Sciences. 2019; 20(23):5905. https://doi.org/10.3390/ijms20235905
Chicago/Turabian StyleKis, Brigitta, Feng Chen Ifrim, Valentina Buda, Stefana Avram, Ioana Zinuca Pavel, Diana Antal, Virgil Paunescu, Cristina Adriana Dehelean, Florina Ardelean, Zorita Diaconeasa, and et al. 2019. "Cannabidiol—from Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer" International Journal of Molecular Sciences 20, no. 23: 5905. https://doi.org/10.3390/ijms20235905
APA StyleKis, B., Ifrim, F. C., Buda, V., Avram, S., Pavel, I. Z., Antal, D., Paunescu, V., Dehelean, C. A., Ardelean, F., Diaconeasa, Z., Soica, C., & Danciu, C. (2019). Cannabidiol—from Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. International Journal of Molecular Sciences, 20(23), 5905. https://doi.org/10.3390/ijms20235905











