Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options
Abstract
1. Introduction
2. Epidemiology
3. Pathophysiology and Risk Factors
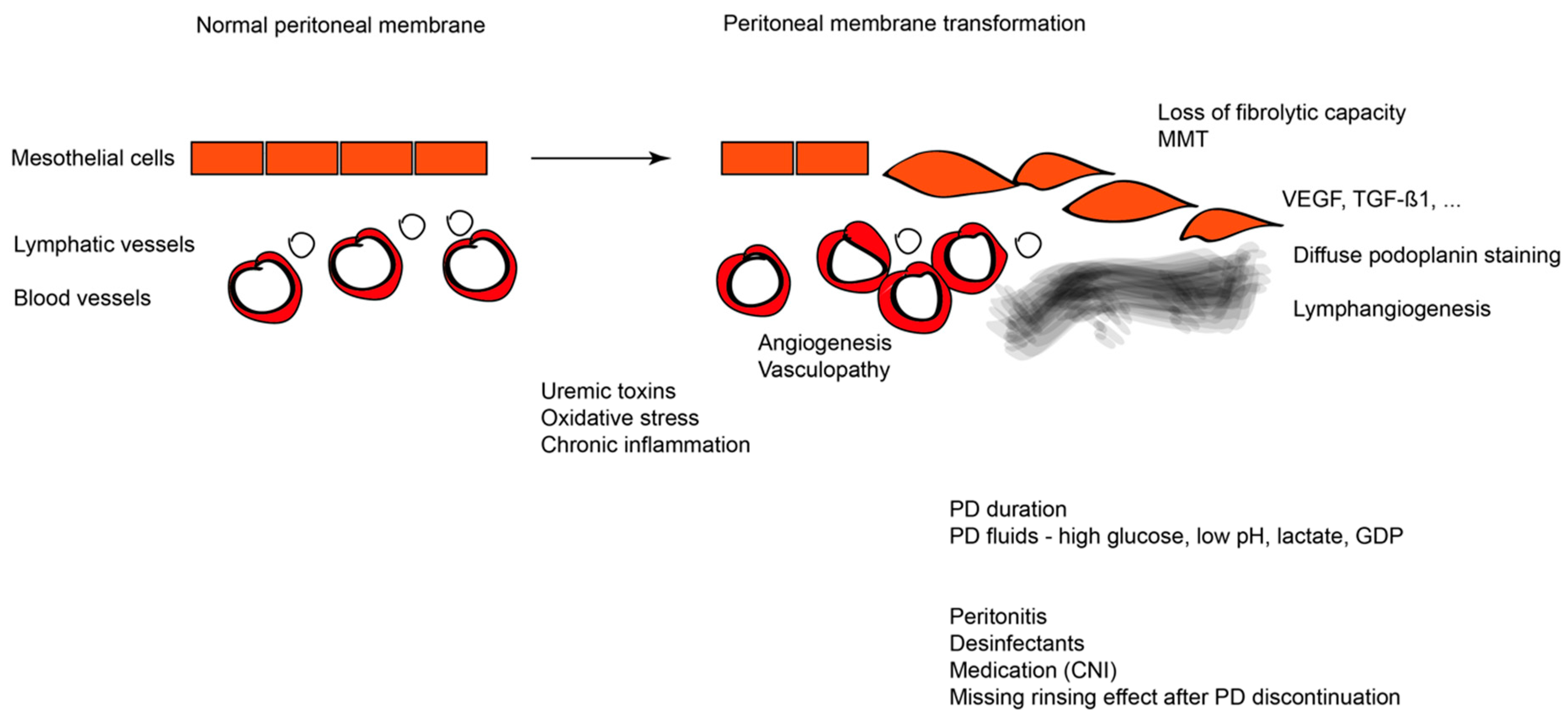
3.1. Pathophysiology
3.2. Risk Factors
4. Diagnosis of EPS
5. Treatment of EPS
5.1. Glucocorticosteroids (GC)
5.2. Non-Steroidal Immunosuppressants
5.2.1. mTOR Inhibitors
5.2.2. Mycophenolate Mofetil
5.2.3. Azathioprine
6. Other Therapies
6.1. Tamoxifen
6.2. Renin–Angiotensin–Aldosterone System (RAAS) Inhibition
6.3. Surgical Treatment
7. Experimental Approaches to EPS Treatment
7.1. N-Acetylcysteine
7.2. Colchicine
7.3. Pentoxifylline
7.4. Rosiglitazone
7.5. Pirfenidone
7.6. Thalidomide
7.7. Itraconazole
7.8. Dissolved Molecular Hydrogen (H2)
7.9. Peritoneal Stem Cell Treatment
8. Prognosis
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced Glycation End-products |
| a-SMA | a-Smooth Muscle Actin |
| AZA | Azathioprine |
| CNIs | Calcineurin Inhibitors |
| EMT | Epithelial-to-Mesenchymal Transition |
| eNOS | endothelial Nitric Oxide Synthase |
| EPS | Encapsulating Peritoneal Sclerosis |
| ESRD | End Stage Renal Disease |
| H2 | Dissolved Molecular Hydrogen |
| HD | Hemodialysis |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MCs | Mesothelial Cells |
| MMF | Mycophenolate Mofetil |
| MMP9 | Matrix MetalloProteinase-9 |
| MMT | Mesothelial-to-Mesenchymal Transition |
| mTOR | Mammalian Target of Rapamycin |
| NAC | N-Acetylcysteine |
| PD | Peritoneal Dialysis |
| PM | Peritoneal Membrane |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| PTX | Pentoxifylline |
| RAAS | Renin–Angiotensin–Aldosterone System |
| TGF-β1 | Transforming Growth Factor–β1 |
| UF | UltraFiltration |
| UFF | UltraFiltration Failure |
| VEGF | Vascular Endothelial Growth Factor |
References
- Aroeira, L.S.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; del Peso, G.; Jiménez-Heffernan, J.A.; Selgas, R.; López-Cabrera, M. Epithelial to Mesenchymal Transition and Peritoneal Membrane Failure in Peritoneal Dialysis Patients: Pathologic Significance and Potential Therapeutic Interventions. J. Am. Soc. Nephrol. 2007, 18, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Garosi, G.; Di Paolo, N. Morphological aspects of peritoneal sclerosis. J. Nephrol. 2001, 14 (Suppl. 4), S30–S38. [Google Scholar]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Vörös, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K.; et al. Neutral pH and low–glucose degradation product dialysis fluids induce major early alterations of the peritoneal membrane in children on peritoneal dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, V.C.; Humayun, H.M.; Ing, T.S.; Daugirdas, J.T.; Jablokow, V.R.; Iwatsuki, S.; Geis, W.P.; Hano, J.E. Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch. Intern. Med. 1980, 140, 1201–1203. [Google Scholar] [CrossRef] [PubMed]
- Rottembourg, J.; Gahl, G.M.; Poignet, J.L.; Mertani, E.; Strippoli, P.; Langlois, P.; Tranbaloc, P.; Legrain, M. Severe abdominal complications in patients undergoing continuous ambulatory peritoneal dialysis. Proc. Eur. Dial. Transplant. Assoc. 1983, 20, 236–242. [Google Scholar] [PubMed]
- Kawanishi, H.; Kawaguchi, Y.; Fukui, H.; Hara, S.; Imada, A.; Kubo, H.; Kin, M.; Nakamoto, M.; Ohira, S.; Shoji, T. Encapsulating peritoneal sclerosis in Japan: A prospective, controlled, multicenter study. Am. J. Kidney Dis. 2004, 44, 729–737. [Google Scholar] [CrossRef]
- Moinuddin, Z.; Summers, A.; van Dellen, D.; Augustine, T.; Herrick, S.E. Encapsulating peritoneal sclerosis—A rare but devastating peritoneal disease. Front. Physiol. 2015, 5, 470. [Google Scholar] [CrossRef]
- Habib, S.M.; Betjes, M.G.H.; Fieren, M.W.J.A.; Boeschoten, E.W.; Abrahams, A.C.; Boer, W.H.; Struijk, D.G.; Ruger, W.; Krikke, C.; Westerhuis, R.; et al. Management of encapsulating peritoneal sclerosis: A guideline on optimal and uniform treatment. Neth. J. Med. 2011, 69, 500–507. [Google Scholar]
- Kawaguchi, Y.; Kawanishi, H.; Mujais, S.; Topley, N.; Oreopoulos, D.G. Encapsulating peritoneal sclerosis: Definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit. Dial. Int. 2000, 20 (Suppl. 4), S43–S55. [Google Scholar]
- Rigby, R.J.; Hawley, C.M. Sclerosing peritonitis: The experience in Australia. Nephrol. Dial. Transplant 1998, 13, 154–159. [Google Scholar] [CrossRef]
- Johnson, D.W.; Cho, Y.; Livingston, B.E.R.; Hawley, C.M.; McDonald, S.P.; Brown, F.G.; Rosman, J.B.; Bannister, K.M.; Wiggins, K.J. Encapsulating peritoneal sclerosis: Incidence, predictors, and outcomes. Kidney Int. 2010, 77, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.; Preston, E.; Davenport, A. Risk factors for developing encapsulating peritoneal sclerosis in the icodextrin era of peritoneal dialysis prescription. Nephrol. Dial. Transplant 2010, 25, 1633–1638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, H.Y.; Kim, B.S.; Choi, H.Y.; Park, H.C.; Kang, S.W.; Choi, K.H.; Ha, S.K.; Han, D.S. Sclerosing encapsulating peritonitis as a complication of long-term continuous ambulatory peritoneal dialysis in Korea. Nephrology 2003, 8, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.A.; Van Biesen, W.; Finkelstein, F.O.; Hurst, H.; Johnson, D.W.; Kawanishi, H.; Pecoits-Filho, R.; Woodrow, G. ISPD Working Party Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: Position paper for ISPD. Perit. Dial. Int. 2009, 29, 595–600. [Google Scholar] [PubMed]
- Bansal, S.; Sheth, H.; Siddiqui, N.; Bender, F.H.; Johnston, J.R.; Piraino, B. Incidence of encapsulating peritoneal sclerosis at a single U.S. university center. Adv. Perit. Dial. 2010, 26, 75–81. [Google Scholar] [PubMed]
- Lambie, M.; Teece, L.; Johnson, D.W.; Petrie, M.; Mactier, R.; Solis-Trapala, I.; Belcher, J.; Bekker, H.L.; Wilkie, M.; Tupling, K.; et al. Estimating risk of encapsulating peritoneal sclerosis accounting for the competing risk of death. Nephrol. Dial. Transplant. 2019, 34, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, H. Long-Term Peritoneal Dialysis Study Group Encapsulating peritoneal sclerosis in Japan: Prospective multicenter controlled study. Perit. Dial. Int. 2001, 21 (Suppl. 3), S67–S71. [Google Scholar]
- Shroff, R.; Stefanidis, C.J.; Askiti, V.; Edefonti, A.; Testa, S.; Ekim, M.; Kavaz, A.; Ariceta, G.; Bakkaloglu, S.; Fischbach, M.; et al. Encapsulating peritoneal sclerosis in children on chronic PD: A survey from the European Paediatric Dialysis Working Group. Nephrol. Dial. Transplant. 2013, 28, 1908–1914. [Google Scholar] [CrossRef][Green Version]
- Nakayama, M.; Miyazaki, M.; Honda, K.; Kasai, K.; Tomo, T.; Nakamoto, H.; Kawanishi, H. Encapsulating peritoneal sclerosis in the era of a multi-disciplinary approach based on biocompatible solutions: The next-PD study. Perit. Dial. Int. 2014, 34, 766–774. [Google Scholar] [CrossRef]
- Alatab, S.; Najafi, I.; Pourmand, G.; Hosseini, M.; Shekarchian, S. Risk factors of severe peritoneal sclerosis in chronic peritoneal dialysis patients. Ren. Fail. 2017, 39, 32–39. [Google Scholar] [CrossRef]
- Morelle, J.; Sow, A.; Hautem, N.; Bouzin, C.; Crott, R.; Devuyst, O.; Goffin, E. Interstitial Fibrosis Restricts Osmotic Water Transport in Encapsulating Peritoneal Sclerosis. J. Am. Soc. Nephrol. 2015, 26, 2521–2533. [Google Scholar] [CrossRef] [PubMed]
- Petrie, M.C.; Traynor, J.P.; MacTier, R.A. Incidence and outcome of encapsulating peritoneal sclerosis. Clin. Kidney J. 2016, 9, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Phelan, P.J.; Walshe, J.J.; Al-Aradi, A.; Garvey, J.P.; Finnegan, K.; O’kelly, P.; Mcwilliams, J.; Ti, J.P.; Morrin, M.M.; Morgan, N.; et al. Encapsulating peritoneal sclerosis: Experience of a tertiary referral center Encapsulating peritoneal sclerosis. Ren. Fail. 2010, 32, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.; Edefonti, A.; Puteo, F.; Chimenz, R.; Gianoglio, B.; Lavoratti, G.; Leozappa, G.; Maringhini, S.; Mencarelli, F.; Pecoraro, C.; et al. Encapsulating peritoneal sclerosis in paediatric peritoneal dialysis patients: The experience of the Italian Registry of Pediatric Chronic Dialysis. Nephrol. Dial. Transplant. 2013, 28, 1603–1609. [Google Scholar] [CrossRef]
- Vizzardi, V.; Sandrini, M.; Zecchini, S.; Ravera, S.; Manili, L.; Cancarini, G. Encapsulating peritoneal sclerosis in an Italian center: Thirty year experience. J. Nephrol. 2016, 29, 259–267. [Google Scholar] [CrossRef]
- Slingeneyer, A. Preliminary report on a cooperative international study on sclerosing encapsulating peritonitis. Contrib. Nephrol. 1987, 57, 239–247. [Google Scholar]
- Hsu, H.J.; Yang, S.Y.; Wu, I.W.; Hsu, K.H.; Sun, C.Y.; Chen, C.Y.; Lee, C.C. Encapsulating Peritoneal Sclerosis in Long-Termed Peritoneal Dialysis Patients. BioMed Res. Int. 2018, 2018, 8250589. [Google Scholar] [CrossRef]
- Braun, N.; Alscher, M.D.; Kimmel, M.; Amann, K.; Büttner, M. Encapsulating peritoneal sclerosis—An overview. Nephrol. Ther. 2011, 7, 162–171. [Google Scholar] [CrossRef]
- Oulès, R.; Challah, S.; Brunner, F.P. Case-control study to determine the cause of sclerosing peritoneal disease. Nephrol. Dial. Transplant. 1988, 3, 66–69. [Google Scholar]
- Davies, S.J.; Phillips, L.; Griffiths, A.M.; Russell, L.H.; Naish, P.F.; Russell, G.I. What really happens to people on long-term peritoneal dialysis? Proc. Kidney Int. 1998, 54, 2207–2217. [Google Scholar] [CrossRef]
- Amoore, J.N.; Lemesre, Y.; Murray, I.C.; Vacher, E.; Mieke, S.; King, S.T.; Smith, F.E.; Murray, A. Validation of oscillometric noninvasive blood pressure measurement devices using simulators. Blood Press. Monit. 2007, 12, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic Changes in the Peritoneal Membrane of Patients with Renal Disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [PubMed]
- Dobbie, J.W. Serositis: Comparative analysis of histological findings and pathogenetic mechanisms in nonbacterial serosal inflammation. Perit. Dial. Int. 1993, 13, 256–269. [Google Scholar] [PubMed]
- Schwenger, V.; Morath, C.; Salava, A.; Amann, K.; Seregin, Y.; Deppisch, R.; Ritz, E.; Bierhaus, A.; Nawroth, P.P.; Zeier, M. Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. J. Am. Soc. Nephrol. 2006, 17, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Niwa, T. Advanced glycation end-products and peritoneal sclerosis. Proc. Semin. Nephrol. 2004, 24, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Spanos, G.; Harissis, H.V.; Dounousi, E.; Mitsis, M.; Pappas, H.; Georgiou, G.K.; Siamopoulos, K.C.; Fatouros, M. A case of encapsulating peritoneal sclerosis presented shortly after renal transplantation. CEN Case Rep. 2014, 3, 40–43. [Google Scholar] [CrossRef][Green Version]
- Bartosova, M.; Schmitt, C.P. Biocompatible peritoneal dialysis: The target is still way off. Front. Physiol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Reimann, D.; Dachs, D.; Meye, C.; Gross, P. Amino acid-based peritoneal dialysis solution stimulates mesothelial nitric oxide production. Perit. Dial. Int. 2004, 24, 378–384. [Google Scholar]
- Yang, Y.M.; Yang, W.X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Honda, K.; Oda, H. Pathology of encapsulating peritoneal sclerosis. Perit. Dial. Int. 2005, 25 (Suppl. 4), S19–S29. [Google Scholar]
- Augustine, T.; Brown, P.W.; Davies, S.D.; Summers, A.M.; Wilkie, M.E. Encapsulating peritoneal sclerosis: Clinical significance and implications. Nephron Clin. Pract. 2009, 111, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, D.; Cetin, P.; Sipahi, S.; Hur, E.; Nar, H.; Ertilav, M.; Sezak, M.; Duman, S. The effects of renin-angiotensin system inhibition on regression of encapsulating peritoneal sclerosis. Perit. Dial. Int. 2008, 28 (Suppl. 5), S38–S42. [Google Scholar]
- Rougier, J.P.; Guia, S.; Hagège, J.; Nguyen, G.; Ronco, P.M. PAI-1 secretion and matrix deposition in human peritoneal mesothelial cell cultures: Transcriptional regulation by TGF-β1. Kidney Int. 1998, 54, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E.; Birnie, K.; Lansley, S.; Herrick, S.E.; Lim, C.B.; Prêle, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Afthentopoulos, I.E.; Passadakis, P.; Oreopoulos, D.G.; Bargman, J. Sclerosing peritonitis in continuous ambulatory peritoneal dialysis patients: One center’s experience and review of the literature. Adv. Ren. Replace. Ther. 1998, 5, 157–167. [Google Scholar] [CrossRef]
- Dobbie, J.W. Pathogenesis of peritoneal fibrosing syndromes (sclerosing peritonitis) in peritoneal dialysis. Perit. Dial. Int. 1992, 12, 14–27. [Google Scholar] [PubMed]
- Yáñez-Mó, M.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Sánchez-Madrid, F.; Lara-Pezzi, E.; Gamallo, C.; López-Cabrera, M.; Selgas, R.; Aguilera, A.; Sánchez-Tomero, J.A.; et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Wilson, R.B. Hypoxia, cytokines and stromal recruitment: Parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura Peritoneum 2018, 3. [Google Scholar] [CrossRef]
- Alscher, D.M.; Braun, N.; Biegger, D.; Fritz, P. Peritoneal Mast Cells in Peritoneal Dialysis Patients, Particularly in Encapsulating Peritoneal Sclerosis Patients. Am. J. Kidney Dis. 2007, 49, 452–461. [Google Scholar] [CrossRef]
- Braun, N.; Alscher, D.M.; Fritz, P.; Edenhofer, I.; Kimmel, M.; Gaspert, A.; Reimold, F.; Bode-Lesniewska, B.; Ziegler, U.; Biegger, D.; et al. Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 2011, 26, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.; Alscher, M.D.; Fritz, P.; Latus, J.; Edenhofer, I.; Reimold, F.; Alper, S.L.; Kimmel, M.; Biegger, D.; Lindenmeyer, M.; et al. The Spectrum of Podoplanin Expression in Encapsulating Peritoneal Sclerosis. PLoS ONE 2012, 7, e53382. [Google Scholar] [CrossRef] [PubMed]
- Latus, J.; Habib, S.M.; Kitterer, D.; Korte, M.R.; Ulmer, C.; Fritz, P.; Davies, S.; Lambie, M.; Alscher, M.D.; Betjes, M.G.H.; et al. Histological and clinical findings in patients with post-transplantation and classical encapsulating peritoneal sclerosis: A European multicenter study. PLoS ONE 2014, 9, e106511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abrahams, A.C.; Habib, S.M.; Dendooven, A.L.; Riser, B.L.; Van Der Veer, J.W.; Toorop, R.J.; Betjes, M.G.H.; Verhaar, M.C.; Watson, C.J.E.; Nguyen, T.Q.; et al. Patients with encapsulating peritoneal sclerosis have increased peritoneal expression of connective tissue growth factor (CCN2), transforming growth factor- β1, and vascular endothelial growth factor. PLoS ONE 2014, 9, e112050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakai, N.; Nakamura, M.; Lipson, K.E.; Miyake, T.; Kamikawa, Y.; Sagara, A.; Shinozaki, Y.; Kitajima, S.; Toyama, T.; Hara, A.; et al. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci. Rep. 2017, 7, 5392. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, Y.; Kawaguchi, Y.; Kubo, H.; Hirano, H.; Sakai, S.; Kurokawa, K. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: A report of the Japanese sclerosing encapsulating peritonitis study group. Am. J. Kidney Dis. 1996, 28, 420–427. [Google Scholar] [CrossRef]
- Lam, M.F.; Leung, J.C.K.; Lo, W.K.; Tam, S.; Chong, M.C.; Lui, S.L.; Tse, K.C.; Chan, T.M.; Lai, K.N. Hyperleptinaemia and chronic inflammation after peritonitis predicts poor nutritional status and mortality in patients on peritoneal dialysis. Nephrol. Dial. Transplant. 2007, 22, 1445–1450. [Google Scholar] [CrossRef]
- Sajwani, S.H.; Bargman, J.M. Novel ways to preserve the peritoneal membrane. Adv. Perit. Dial. 2012, 28, 37–41. [Google Scholar]
- Shaldon, S.; Koch, K.M.; Quellhorst, E.; Dinarello, C.A. Pathogenesis of sclerosing peritonitis in CAPD. ASAIO J. 1984, 30, 193–194. [Google Scholar]
- Flessner, M.F.; Credit, K.; Henderson, K.; Vanpelt, H.M.; Potter, R.; He, Z.; Henegar, J.; Robert, B. Peritoneal Changes after Exposure to Sterile Solutions by Catheter. J. Am. Soc. Nephrol. 2007, 18, 2294–2302. [Google Scholar] [CrossRef]
- Wong, Y.Y.; Wong, P.N.; Mak, S.K.; Chan, S.F.; Cheuk, Y.Y.; Ho, L.Y.; Lo, K.Y.; Lo, M.W.; Lo, K.C.; Tong, G.M.W.; et al. Persistent sterile peritoneal inflammation after catheter removal for refractory bacterial peritonitis predicts full-blown encapsulating peritoneal sclerosis. Perit. Dial. Int. 2013, 33, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Moriishi, M.; Kawanishi, H. Icodextrin and intraperitoneal inflammation. Perit. Dial. Int. 2008, 28 (Suppl. 3), S96–S100. [Google Scholar]
- Moriishi, M.; Kawanishi, H.; Tsuchiya, S. Impact on peritoneal membrane of use of icodextrin-based dialysis solution in peritoneal dialysis patients. Adv. Perit. Dial. 2006, 22, 24. [Google Scholar] [PubMed]
- Pollock, C.A. Diagnosis and management of encapsulating peritoneal sclerosis. Perit. Dial. Int. 2001, 21 (Suppl. 3), S61–S66. [Google Scholar]
- Summers, A.M.; Brenchley, P.E.C. An international encapsulating peritoneal sclerosis registry and DNA bank: Why we need one now. Perit. Dial. Int. 2006, 26, 559–563. [Google Scholar] [PubMed]
- Margetts, P.J.; Hoff, C.; Liu, L.; Korstanje, R.; Walkin, L.; Summers, A.; Herrick, S.; Brenchley, P. Transforming growth factor β-induced peritoneal fibrosis is mouse strain dependent. Nephrol. Dial. Transplant. 2013, 28, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Pecoits-Filho, R.; Carvalho, M.J.; Stenvinkel, P.; Lindholm, B.; Heimbürger, O. Systemic and intraperitoneal interleukin-6 system during the first year of peritoneal dialysis. Perit. Dial. Int. 2006, 26, 53–63. [Google Scholar]
- Wong, T.Y.H.; Szeto, C.C.; Szeto, C.Y.K.; Lai, K.B.; Chow, K.M.; Li, P.K.T. Association of ENOS polymorphism with basal peritoneal membrane function in uremic patients. Am. J. Kidney Dis. 2003, 42, 781–786. [Google Scholar] [CrossRef]
- Gillerot, G.; Goffin, E.; Michel, C.; Evenepoel, P.; Van Biesen, W.; Tintillier, M.; Stenvinkel, P.; Heimbürger, O.; Lindholm, B.; Nordfors, L.; et al. Genetic and clinical factors influence the baseline permeability of the peritoneal membrane. Kidney Int. 2005, 67, 2477–2487. [Google Scholar] [CrossRef]
- Lo, W.K.; Chan, K.T.; Leung, A.C.; Pang, S.W.; Tse, C.Y. Sclerosing peritonitis complicating prolonged use of chlorhexidine in alcohol in the connection procedure for continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 1991, 11, 166–172. [Google Scholar]
- Keating, J.P.; Neill, M.; Hill, G.L. Sclerosing encapsulating peritonitis after intraperitoneal use of Povidone Iodine. Aust. N. Z. J. Surg. 1997, 67, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Marigold, J.H.; Pounder, R.E.; Pemberton, J.; Thompson, R.P. Propranolol, oxprenolol, and sclerosing peritonitis. Br. Med. J. 1982, 284, 870. [Google Scholar] [CrossRef] [PubMed]
- Liappas, G.; González-Mateo, G.; Aguirre, A.R.; Abensur, H.; Albar-Vizcaino, P.; Parra, E.G.; Sandoval, P.; Ramírez, L.G.; Del Peso, G.; Acedo, J.M.; et al. Nebivolol, a β1-adrenergic blocker, protects from peritoneal membrane damage induced during peritoneal dialysis. Oncotarget 2016, 7, 30133–30146. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Plummer, M.; Bromberek, C.; Bresnahan, B.; Hariharan, S. Expression of TGF-β and fibrogenic genes in transplant recipients with tacrolimus and cyclosporine nephrotoxicity. Kidney Int. 2002, 62, 2257–2263. [Google Scholar] [CrossRef]
- Van Westrhenen, R.; Aten, J.; Hajji, N.; De Boer, O.J.; Kunne, C.; De Waart, D.R.; Krediet, R.T. Cyclosporin A induces peritoneal fibrosis and angiogenesis during chronic peritoneal exposure to a glucose-based, lactate-buffered dialysis solution in the rat. Blood Purif. 2008, 25, 466–472. [Google Scholar] [CrossRef]
- Fieren, M.W.J.A.; Betjes, M.G.H.; Korte, M.R.; Boer, W.H. Posttransplant encapsulating peritoneal sclerosis: A worrying new trend? Perit. Dial. Int. 2007, 27, 619–624. [Google Scholar]
- Balasubramaniam, G.; Brown, E.A.; Davenport, A.; Cairns, H.; Cooper, B.; Fan, S.L.S.; Farrington, K.; Gallagher, H.; Harnett, P.; Krausze, S.; et al. The Pan-Thames EPS study: Treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 2009, 24, 3209–3215. [Google Scholar] [CrossRef]
- Tarzi, R.M.; Lim, A.; Moser, S.; Ahmad, S.; George, A.; Balasubramaniam, G.; Clutterbuck, E.J.; Gedroyc, W.; Brown, E.A. Assessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosis. Clin. J. Am. Soc. Nephrol. 2008, 3, 1702–1710. [Google Scholar] [CrossRef]
- Nakamoto, H. Encapsulating peritoneal sclerosis—A clinician’s approach to diagnosis and medical treatment. Perit. Dial. Int. 2005, 25 (Suppl. 4), S30–S38. [Google Scholar]
- Brown, E.A.; Bargman, J.; Van Biesen, W.; Chang, M.Y.; Finkelstein, F.O.; Hurst, H.; Johnson, D.W.; Kawanishi, H.; Lambie, M.; De Moraes, T.P.; et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis—Position paper for ISPD: 2017 update. Perit. Dial. Int. 2017, 37, 362–374. [Google Scholar] [CrossRef]
- Vlijm, A.; Van Schuppen, J.; Lamers, A.B.G.N.; Struijk, D.G.; Krediet, R.T. Imaging in encapsulating peritoneal sclerosis. Nephrol. Dial.Transplant. Plus 2011, 4, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Yoshida, H.; Matsuo, N.; Maruyama, Y.; Kawamura, Y.; Yamamoto, R.; Hanaoka, K.; Ikeda, M.; Yamamoto, H.; Nakayama, M.; et al. Serum beta2 microglobulin (beta2MG) level is a potential predictor for encapsulating peritoneal sclerosis (EPS) in peritoneal dialysis patients. Clin. Nephrol. 2008, 69, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Tawada, M.; Yuasa, H.; Ryuzaki, M. New Japanese Society of Dialysis Therapy Guidelines for Peritoneal Dialysis. Proc. Contrib. Nephrol. 2019, 198, 52–61. [Google Scholar]
- Kawaguchi, Y.; Saito, A.; Kawanishi, H.; Nakayama, M.; Miyazaki, M.; Nakamoto, H.; Tranaeus, A. Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: Diagnosis, predictive markers, treatment, and preventive measures. Perit. Dial. Int. 2005, 25 (Suppl. 4), S83–S95. [Google Scholar]
- Bozkurt, D.; Sipahi, S.; Cetin, P.; Hur, E.; Ozdemir, O.; Ertilav, M.; Sen, S.; Duman, S. Does immunosuppressive treatment ameliorate morphology changes in encapsulating peritoneal sclerosis? Perit. Dial. Int. 2009, 29 (Suppl. 2), S206–S210. [Google Scholar]
- Mori, Y.; Matsuo, S.; Sutoh, H.; Toriyama, T.; Kawahara, H.; Hotta, N. A case of a dialysis patient with sclerosing peritonitis successfully treated with corticosteroid therapy alone. Am. J. Kidney Dis. 1997, 30, 275–278. [Google Scholar] [CrossRef]
- Jung, J.Y.; Cho, J.T. A case of fulminant sclerosing peritonitis presented like acute culture-negative peritonitis and successfully treated with corticosteroid therapy. J. Korean Med. Sci. 2013, 28, 620–623. [Google Scholar] [CrossRef]
- Martins, L.S.; Rodrigues, A.S.; Cabrita, A.N.; Guimaraes, S. Sclerosing encapsulating peritonitis: A case successfully treated with immunosuppression. Perit. Dial. Int. 1999, 19, 478–481. [Google Scholar]
- Dejagere, T.; Evenepoel, P.; Claes, K.; Kuypers, D.; Maes, B.; Vanrenterghem, Y. Acute-onset, steroid-sensitive, encapsulating peritoneal sclerosis in a renal transplant recipient. Am. J. Kidney Dis. 2005, 45, e33–e37. [Google Scholar] [CrossRef]
- Kuriyama, S.; Tomonari, H. Corticosteroid therapy in encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 2001, 16, 1304–1305. [Google Scholar] [CrossRef][Green Version]
- Maruyama, Y.; Nakayama, M. Encapsulating peritoneal sclerosis in Japan. Perit. Dial. Int. 2008, 28 (Suppl. 3), S201–S204. [Google Scholar]
- Bhandari, S.; Wilkinson, A.; Sellars, L. Sclerosing peritonitis: Value of immunosuppression prior to surgery. Nephrol. Dial. Transplant. 1994, 9, 436–437. [Google Scholar] [PubMed]
- Kawanishi, H.; Harada, Y.; Noriyuki, T.; Kawai, T.; Takahashi, S.; Moriishi, M.; Tsuchiya, S. Treatment options for encapsulating peritoneal sclerosis based on progressive stage. Adv. Perit. Dial. 2001, 21 (Suppl. 3), S67–S71. [Google Scholar]
- Rajani, R. Differential Effect of sirolimus vs. prednisolone in the treatment of sclerosing encapsulating peritonitis. Nephrol. Dial. Transplant. 2002, 17, 2278–2280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, C.F.; Beshir, S.; Khalil, A.; Pai, P.; Ahmad, R. Successful treatment of encapsulating peritoneal sclerosis with azathioprine and prednisolone. Perit. Dial. Int. 2005, 25, 285–287. [Google Scholar] [PubMed]
- Lafrance, J.P.; Létourneau, I.; Ouimet, D.; Bonnardeaux, A.; Leblanc, M.; Mathieu, N.; Pichette, V. Successful Treatment of Encapsulating Peritoneal Sclerosis with Immunosuppressive Therapy. Am. J. Kidney Dis. 2008, 51, e7–e10. [Google Scholar] [CrossRef] [PubMed]
- Junor, B.J.; McMillan, M.A. Immunosuppression in sclerosing peritonitis. Adv. Perit. Dial. 1993, 9, 187–189. [Google Scholar]
- Fagugli, R. Immunosuppressive treatment for sclerosing peritonitis. Nephrol. Dial. Transplant. 1999, 14, 1343–1345. [Google Scholar] [CrossRef][Green Version]
- Cho, R.; Ghag, D.; Karim, M.A.; Lo, C. Encapsulating peritoneal sclerosis: Surgery, sustained drug therapy and treatment of recurrence at 1 year. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef]
- Minetto Brabo, A.; Soares Do Carmo Reis, N.; Barretti, P.; Ponce, D. A combination of corticosteroid, sirolimus, and intradialytic parenteral nutrition in encapsulating peritoneal sclerosis: Case report and literature review. Hemodial. Int. 2017, 21, 307–311. [Google Scholar] [CrossRef]
- Messina, M.; Ariaudo, C.; Mella, A.; Cantaluppi, V.; Segoloni, G.P.; Biancone, L. mTOR inhibitors for medical treatment of post-transplantation encapsulating peritoneal sclerosis: A favourable single center experience. J. Nephrol. 2015, 28, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Duman, S.; Bozkurt, D.; Sipahi, S.; Sezak, M.; Ozkan, S.; Ertilav, M.; Sen, S.; Ok, E. Effects of everolimus as an antiproliferative agent on regression of encapsulating peritoneal sclerosis in a rat model. Adv. Perit. Dial. 2008, 24, 104–110. [Google Scholar] [PubMed]
- Sekiguchi, Y.; Zhang, J.; Patterson, S.; Liu, L.; Hamada, C.; Tomino, Y.; Margetts, P.J. Rapamycin inhibits transforming growth factor β-induced peritoneal angiogenesis by blocking the secondary hypoxic response. J. Cell. Mol. Med. 2012, 16, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Aroeira, L.S.; Ramirez-Huesca, M.; Perez-Lozano, M.L.; Cirugeda, A.; Bajo, M.A.; Del Peso, G.; Valenzuela-Fernandez, A.; Sanchez-Tomero, J.A.; Lopez-Cabrera, M.; et al. Effects of rapamycin on the epithelial-to-mesenchymal transition of human peritoneal mesothelial cells. Int. J. Artif. Organs 2005, 28, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Huddam, B.; Azak, A.; Koçak, G.; Başaran, M.; Voyvoda, N.; Duranay, M. Additive effectiveness of everolimus plus tamoxifen therapy in treatment of encapsulating peritoneal sclerosis. Ren. Fail. 2012, 34, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Sud, R.; Garry, L.; Spicer, S.T.; Allen, R.D.M.; Eris, J.M.; Wyburn, K.; Verran, D.; Cooper, C.L.; Chadban, S. A role for everolimus in post-transplant encapsulating peritoneal sclerosis: First case report. Nephrology 2014, 19, 27–30. [Google Scholar] [CrossRef]
- Temple, S.; Zaltzman, J.; Perl, J. Development of encapsulating peritoneal sclerosis in a renal transplant recipient on sirolimus immunotherapy. Perit. Dial. Int. 2010, 30, 475–477. [Google Scholar] [CrossRef]
- Romagnoli, J.; Pedroso, J.A.; Paola Salerno, M.; Favi, E.; Spagnoletti, G.; Citterio, F. Posttransplant encapsulating peritoneal sclerosis, long-term success with everolimus and low-dose CNI: A case report. Transplant. Proc. 2014, 46, 2368–2370. [Google Scholar] [CrossRef]
- Klawitter, J.; Nashan, B.; Christians, U. Everolimus and sirolimus in transplantation-related but different. Expert Opin. Drug Saf. 2015, 14, 1055–1070. [Google Scholar] [CrossRef]
- Waller, J.R.; Brook, N.R.; Bicknell, G.R.; Murphy, G.J.; Nicholson, M.L. Mycophenolate mofetil inhibits intimal hyperplasia and attenuates the expression of genes favouring smooth muscle cell proliferation and migration. Transplant. Proc. 2005, 37, 164–166. [Google Scholar] [CrossRef]
- Huddam, B.; Başaran, M.; Koçak, G.; Azak, A.; Yalçın, F.; Reyhan, N.H.; Duranay, M. The use of mycophenolate mofetil in experimental encapsulating peritoneal sclerosis. Int. Urol. Nephrol. 2015, 47, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.; Bozkurt, D.; Timur, O.; Bicak, S.; Sarsik, B.; Akcicek, F.; Duman, S. The effects of mycophenolate mofetil on encapsulated peritoneal sclerosis model in rats. Clin. Nephrol. 2012, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.; Aguilera, A.; Selgas, R.; Sandoval, P.; Albar-Vizcaíno, P.; Pérez-Lozano, M.L.; Ruiz-Carpio, V.; Majano, P.L.; Lamas, S.; Rodríguez-Pascual, F.; et al. Blocking TGF-β1 Protects the Peritoneal Membrane from Dialysate-Induced Damage. J. Am. Soc. Nephrol. 2011, 22, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Moustafellos, P.; Hadjianastassiou, V.; Roy, D.; Velzeboer, N.E.; Maniakyn, N.; Vaidya, A.; Friend, P.J. Tamoxifen Therapy in Encapsulating Sclerosing Peritonitis in Patients After Kidney Transplantation. Transplant. Proc. 2006, 38, 2913–2914. [Google Scholar] [CrossRef]
- Margetts, P.J.; Bonniaud, P.; Liu, L.; Hoff, C.M.; Holmes, C.J.; West-Mays, J.A.; Kelly, M.M. Transient overexpression of TGF-β1 induces epithelial mesenchymal transition in the rodent peritoneum. J. Am. Soc. Nephrol. 2005, 16, 425–436. [Google Scholar] [CrossRef]
- Loureiro, J.; Sandoval, P.; del Peso, G.; Gónzalez-Mateo, G.; Fernández-Millara, V.; Santamaria, B.; Bajo, M.A.; Sánchez-Tomero, J.A.; Guerra-Azcona, G.; Selgas, R.; et al. Tamoxifen Ameliorates Peritoneal Membrane Damage by Blocking Mesothelial to Mesenchymal Transition in Peritoneal Dialysis. PLoS ONE 2013, 8, e61165. [Google Scholar] [CrossRef]
- Huang, J.W.; Yen, C.J.; Wu, H.Y.; Chiang, C.K.; Cheng, H.T.; Lien, Y.C.; Hung, K.Y.; Tsai, T.J. Tamoxifen downregulates connective tissue growth factor to ameliorate peritoneal fibrosis. Blood Purif. 2011, 31, 252–258. [Google Scholar] [CrossRef]
- Turner, M.W.; Holleman, J.H. Successful Therapy of Sclerosing Peritonitis. Semin. Dial. 1992, 5, 316. [Google Scholar] [CrossRef]
- De Sousa-Amorim, E.; Del Peso, G.; Bajo, M.A.; Alvarez, L.; Ossorio, M.; Gi, F.; Bellon, T.; Selgas, R. Can EPS development be avoided with early interventions? The potential role of tamoxifen—A single-center study. Perit. Dial. Int. 2014, 34, 582–593. [Google Scholar] [CrossRef]
- Summers, A.M.; Clancy, M.J.; Syed, F.; Harwood, N.; Brenchley, P.E.C.; Augustine, T.; Riad, H.; Hutchison, A.J.; Taylor, P.; Pearson, R.; et al. Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int. 2005, 68, 2381–2388. [Google Scholar] [CrossRef]
- Del Peso, G.; Bajo, M.A.; Gil, F.; Aguilera, A.; Ros, S.; Costero, O.; Castro, M.J.; Selgas, R. Clinical experience with tamoxifen in peritoneal fibrosing syndromes. Adv. Perit. Dial. 2003, 19, 32–35. [Google Scholar] [PubMed]
- Guest, S. Tamoxifen therapy for encapsulating peritoneal sclerosis: Mechanism of action and update on clinical experiences. Perit. Dial. Int. 2009, 29, 252–255. [Google Scholar] [PubMed]
- Eltoum, M.A.; Wright, S.; Atchley, J.; Mason, J.C. Four consecutive cases of peritoneal dialysis-related encapsulating peritoneal sclerosis treated successfully with tamoxifen. Perit. Dial. Int. 2006, 26, 203–206. [Google Scholar]
- Wong, C.F. Clinical experience with tamoxifen in encapsulating peritoneal sclerosis. Perit. Dial. Int. 2006, 26, 183–184. [Google Scholar] [PubMed]
- Allaria, P.M.; Giangrande, A.; Gandini, E.; Pisoni, I.B. Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: Tamoxifen as a new therapeutic agent? J. Nephrol. 1999, 12, 395–397. [Google Scholar] [PubMed]
- Gupta, S.; Woodrow, G. Successful treatment of fulminant encapsulating peritoneal sclerosis following fungal peritonitis with tamoxifen. Clin. Nephrol. 2007, 68, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukarasu, T.; Saxena, R.; Anijeet, H.; Pai, P.; Wong, C.F. Encapsulating peritoneal sclerosis presenting with recurrent ascites and tamoxifen: Case reports and review of the literature. Ren. Fail. 2007, 29, 775–776. [Google Scholar] [CrossRef]
- Mesquita, M.; Guillaume, M.P.; Dratwa, M. First use of tamoxifen in an HIV patient with encapsulating peritoneal sclerosis. Clin. Drug Investig. 2007, 27, 727–729. [Google Scholar] [CrossRef]
- Mohamed, A.O.; Kamar, N.; Nogier, M.-B.; Esposito, L.; Duffas, J.P.; Rostaing, L. Tamoxifen therapy in kidney-transplant patients presenting with severe encapsulating peritoneal sclerosis after treatment for acute humoral rejection. Exp. Clin. Transplant. 2009, 7, 164–167. [Google Scholar]
- Korte, M.R.; Fieren, M.W.; Sampimon, D.E.; Lingsma, H.F.; Weimar, W.; Betjes, M.G.H. Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: Results of the Dutch Multicentre EPS Study. Nephrol. Dial. Transplant. 2011, 26, 691–697. [Google Scholar] [CrossRef]
- Cornelis, T.; Oreopoulos, D.G. Update on potential medical treatments for encapsulating peritoneal sclerosis; Human and experimental data. Int. Urol. Nephrol. 2011, 43, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.L.; Wu, M.J.; Chen, C.H.; Tsai, S.F. Case Report of a Patient Undergoing Peritoneal Dialysis with Encapsulating Peritoneal Sclerosis Superimposed with Calciphylaxis. Iran. Red Crescent Med. J. 2016, 18, e30913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korzets, A.; Ori, Y.; Zevin, D.; Chagnac, A.; Herman, M.; Rozen-Zvi, B.; Gafter, U. A worrying thought—Could there be a connection between encapsulating peritoneal sclerosis, tamoxifen and calciphylaxis? Nephrol. Dial. Transplant. 2006, 21, 2975–2978. [Google Scholar] [CrossRef] [PubMed]
- Kyuden, Y.; Ito, T.; Masaki, T.; Yorioka, N.; Kohno, N. Tgf-beta1 induced by high glucose is controlled by angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker on cultured human peritoneal mesothelial cells. Perit. Dial. Int. 2005, 25, 483–491. [Google Scholar]
- Kolesnyk, I.; Dekker, F.W.; Noordzij, M.; le Cessie, S.; Struijk, D.G.; Krediet, R.T. Impact of ACE inhibitors and AII receptor blockers on peritoneal membrane transport characteristics in long-term peritoneal dialysis patients. Perit. Dial. Int. 2007, 27, 446–453. [Google Scholar]
- Duman, S.; Sen, S.; Duman, C.; Oreopoulos, D.G. Effect of valsartan versus lisinopril on peritoneal sclerosis in rats. Int. J. Artif. Organs 2005, 28, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, H.; Imai, H.; Fukushima, R.; Ishida, Y.; Yamanouchi, Y.; Suzuki, H. Role of the renin-angiotensin system in the pathogenesis of peritoneal fibrosis. Perit. Dial. Int. 2008, 28 (Suppl. 3), S83–S87. [Google Scholar]
- Sampimon, D.E.; Kolesnyk, I.; Korte, M.R.; Fieren, M.W.J.A.; Struijk, D.G.; Krediet, R.T. Use of angiotensin II inhibitors in patients that develop encapsulating peritoneal sclerosis. Perit. Dial. Int. 2010, 30, 656–659. [Google Scholar] [CrossRef]
- Kawanishi, H.; Banshodani, M.; Yamashita, M.; Shintaku, S.; Dohi, K. Surgical Treatment for Encapsulating Peritoneal Sclerosis: 24 Years’ Experience. Perit. Dial. Int. 2019, 39, 169–174. [Google Scholar] [CrossRef]
- Kawanishi, H.; Shintaku, S.; Moriishi, M.; Dohi, K.; Tsuchiya, S. Seventeen years’ experience of surgical options for encapsulating peritoneal sclerosis. Adv. Perit. Dial. 2011, 27, 53–58. [Google Scholar]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Bozkurt, D.; Bicak, S.; Sipahi, S.; Taskin, H.; Hur, E.; Ertilav, M.; Sen, S.; Duman, S. The effects of colchicine on the progression and regression of encapsulating peritoneal sclerosis. Perit. Dial. Int. 2008, 28 (Suppl. 5), S53–S57. [Google Scholar]
- Yang, Y.L.; Lee, M.T.G.; Lee, C.C.; Su, P.I.; Chi, C.Y.; Liu, C.H.; Wu, M.C.; Yen, Z.S.; Chen, S.C. Pentoxifylline decreases post-operative intra-abdominal adhesion formation in an animal model. PeerJ 2018, 6, e5434. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.Y.; Huang, J.W.; Chiang, C.K.; Tsai, T.J. Preservation of peritoneal morphology and function by pentoxifylline in a rat model of peritoneal dialysis: Molecular studies. Nephrol. Dial. Transplant. 2008, 23, 3831–3840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Yang, X.; Zhang, Y.J.; Sun, Y.L.; Zou, X.L.; Kong, Q.Y.; Dong, X.Q.; Ye, X.Q.; Yu, X.Q. Peroxisome proliferator-activated receptor-gamma is expressed by rat peritoneal mesothelial cells: Its potential role in peritoneal cavity local defense. Am. J. Nephrol. 2006, 26, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, D.; Taskin, H.; Sezak, M.; Biçak, S.; Sen, S.; Ok, E.; Duman, S. Rosiglitazone, a peroxisome proliferator-activated receptor agonist, improves peritoneal alterations resulting from an encapsulated peritoneal sclerosis model. Adv. Perit. Dial. 2008, 24, 32–38. [Google Scholar] [PubMed]
- Bayhan, Z.; Zeren, S.; Kocak, F.E.; Kocak, C.; Akcilar, R.; Kargi, E.; Tiryaki, C.I.; Yaylak, F.; Akcilar, A.I. Antiadhesive and anti-inflammatory effects of pirfenidone in postoperative intra-abdominal adhesion in an experimental rat model. J. Surg. Res. 2016, 201, 348–355. [Google Scholar] [CrossRef]
- Arai, H.; Furusu, A.; Nishino, T.; Obata, Y.; Nakazawa, Y.; Nakazawa, M.; Hirose, M.; Abe, K.; Koji, T.; Kohno, S. Thalidomide Prevents the Progression of Peritoneal Fibrosis in Mice. Acta Histochem. Cytochem. 2011, 44, 51–60. [Google Scholar] [CrossRef][Green Version]
- Millrine, D.; Kishimoto, T. A Brighter Side to Thalidomide: Its Potential Use in Immunological Disorders. Trends Mol. Med. 2017, 23, 348–361. [Google Scholar] [CrossRef]
- Mondello, S.; Mazzon, E.; Di Paola, R.; Crisafulli, C.; Mondello, P.; Buemi, M.; Aloisi, C.; Cuzzocrea, S. Thalidomide suppresses sclerosing encapsulating peritonitis in a rat experimental model. Shock 2009, 32, 332–339. [Google Scholar] [CrossRef]
- Kim, J.S.; Cho, K.S.; Park, S.H.; Lee, S.H.; Lee, J.H.; Jeong, K.H.; Lee, T.W. Itraconazole attenuates peritoneal fibrosis through its effect on the sonic hedgehog signaling pathway in Mice. Am. J. Nephrol. 2018, 48, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Zhu, W.J.; Watanabe, K.; Gibo, A.; Sherif, A.M.; Kabayama, S.; Ito, S. Dissolved molecular hydrogen (H2) in Peritoneal Dialysis (PD) solutions preserves mesothelial cells and peritoneal membrane integrity. BMC Nephrol. 2017, 18, 327. [Google Scholar] [CrossRef] [PubMed]
- Terawaki, H.; Nakano, H.; Zhu, W.J.; Nakayama, M. Successful treatment of encapsulating peritoneal sclerosis by hemodialysis and peritoneal lavage using dialysate containing dissolved hydrogen. Perit. Dial. Int. 2015, 35, 107–112. [Google Scholar] [CrossRef]
- Alatab, S.; Najafi, I.; Atlasi, R.; Pourmand, G.; Tabatabaei-Malazy, O.; Ahmadbeigi, N. A systematic review of preclinical studies on therapeutic potential of stem cells or stem cells products in peritoneal fibrosis. Minerva Urol. Nefrol. 2018, 70, 162–178. [Google Scholar]
- Alatab, S.; Shekarchian, S.; Najafi, I.; Moghadasali, R.; Ahmadbeigi, N.; Pourmand, M.R.; Bolurieh, T.; Jaroughi, N.; Pourmand, G.; Aghdami, N. Systemic infusion of autologous adipose tissue-derived mesenchymal stem cells in peritoneal dialysis patients: Feasibility and safety. Cell J. 2019, 20, 483–495. [Google Scholar] [CrossRef]
- Brown, M.C.; Simpson, K.; Kerssens, J.J.; Mactier, R.A. Encapsulating peritoneal sclerosis in the new millennium: A national cohort study. Clin. J. Am. Soc. Nephrol. 2009, 29, 595–600. [Google Scholar] [CrossRef]
- Bartosova, M.; Schaefer, B.; Vondrak, K.; Sallay, P.; Taylan, C.; Cerkauskiene, R.; Dzierzega, M.; Milosevski-Lomic, G.; Büscher, R.; Zaloszyc, A.; et al. Peritoneal Dialysis Vintage and Glucose Exposure but Not Peritonitis Episodes Drive Peritoneal Membrane Transformation During the First Years of PD. Front. Physiol. 2019, 10, 356. [Google Scholar] [CrossRef]
| Class | Drug Name | Mode of Action |
|---|---|---|
| Glucocorticosteroids | Prednisone | Immunosuppressant, inhibits monocyte chemoattractant protein 1 (MCP-1) synthesis, regulates extracellular matrix (ECM) protein synthesis, ECM protein maturation |
| Prednisolone | ||
| Immuno-suppressants | Azathioprene | Inhibits DNA/RNA synthesis |
| Rapamycin/Sirolimus | Inhibits T-cell/B-cell activation | |
| Mycophenolate mofetil | De-novo purine synthesis blockade | |
| Cyclosporine | Lowered T-cell activity | |
| Hormonal antagonist | Tamoxifen | Blocks transforming growth factor-β1 (TGF-β1) signaling |
| Angiotensin converting enzyme inhibitor (ACEi)/Angiotensin II receptor blocker (ARB) | Blocks TGF-β1 signaling | |
| Perindopril | Blocks TGF-β1 signaling, lowered cell proliferation | |
| Candesartan | ||
| Mucolytic | Ν-acetylcysteine (NAC) | Reactive oxygen species scavenger |
| alkaloid | Colchicine | Blocks TGF-β1 mRNA expression |
| Xanthine derivative | Pentoxifylline | Fibrinoltyic, suppressed collagen synthesis, angiogenesis |
| Anti-diabetic | Rosiglitazone | Peroxisome Proliferator-Activated Receptor (PPAR)-agonist, suppressed inflammation, neovasculature |
| Anti-fibrotic, anti-inflammatory | Pirfenidone | Reduces tissue inhibitor of metalloproteinases-1 (TIMP-1), tumor necrosis factor-α (TNF-α), and TGF-β1 expression, |
| Immuno-modulator | Thalidomide | Anti-angiogenic, anti-proliferative, antifibrotic |
| Anti-fungal | Itraconazole | Decreased TGF-β1 expression |
| Autologous stem cell therapy | Mesothelial/submesothelial cellular layer repair |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagirdar, R.M.; Bozikas, A.; Zarogiannis, S.G.; Bartosova, M.; Schmitt, C.P.; Liakopoulos, V. Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options. Int. J. Mol. Sci. 2019, 20, 5765. https://doi.org/10.3390/ijms20225765
Jagirdar RM, Bozikas A, Zarogiannis SG, Bartosova M, Schmitt CP, Liakopoulos V. Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options. International Journal of Molecular Sciences. 2019; 20(22):5765. https://doi.org/10.3390/ijms20225765
Chicago/Turabian StyleJagirdar, Rajesh M., Andreas Bozikas, Sotirios G. Zarogiannis, Maria Bartosova, Claus Peter Schmitt, and Vassilios Liakopoulos. 2019. "Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options" International Journal of Molecular Sciences 20, no. 22: 5765. https://doi.org/10.3390/ijms20225765
APA StyleJagirdar, R. M., Bozikas, A., Zarogiannis, S. G., Bartosova, M., Schmitt, C. P., & Liakopoulos, V. (2019). Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options. International Journal of Molecular Sciences, 20(22), 5765. https://doi.org/10.3390/ijms20225765






