A Role for Both V1a and V2 Receptors in Renal Heat Stress Injury Amplified by Rehydration with Fructose
Abstract
1. Introduction
2. Results
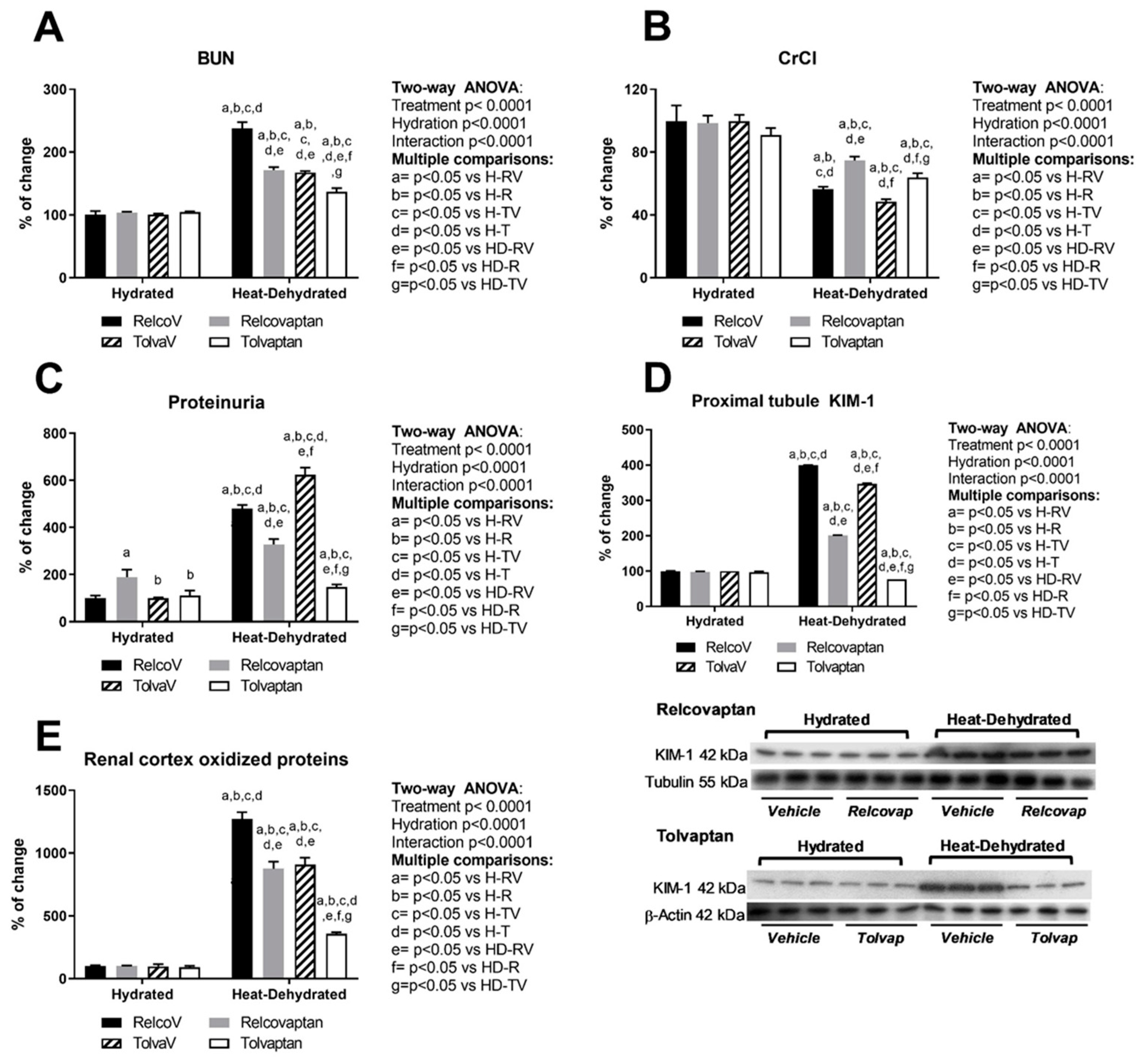
2.1. Relcovaptan and Tolvaptan Conferred Nephroprotection in HeatStress-Dehydrated Rats Rehydrated with a 10% Fructose Beverage
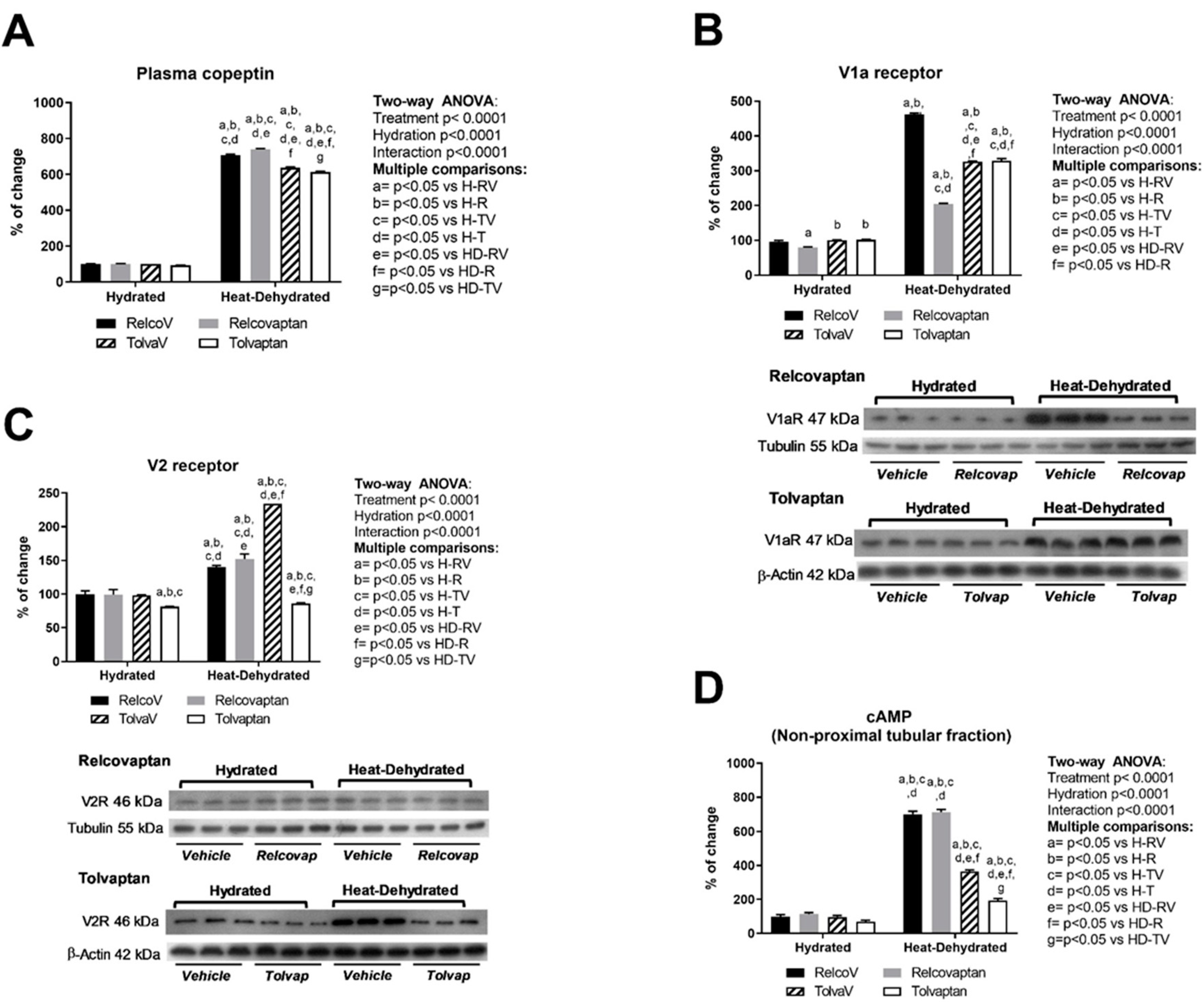
2.2. Plasma Copeptin and Vasopressin Receptors Expression in the Renal Cortex
2.3. Effect of Heat Stress and Rehydration with a Fructose-Containing Beverage on Systemic Inflammation and Plasma Cortisol Levels
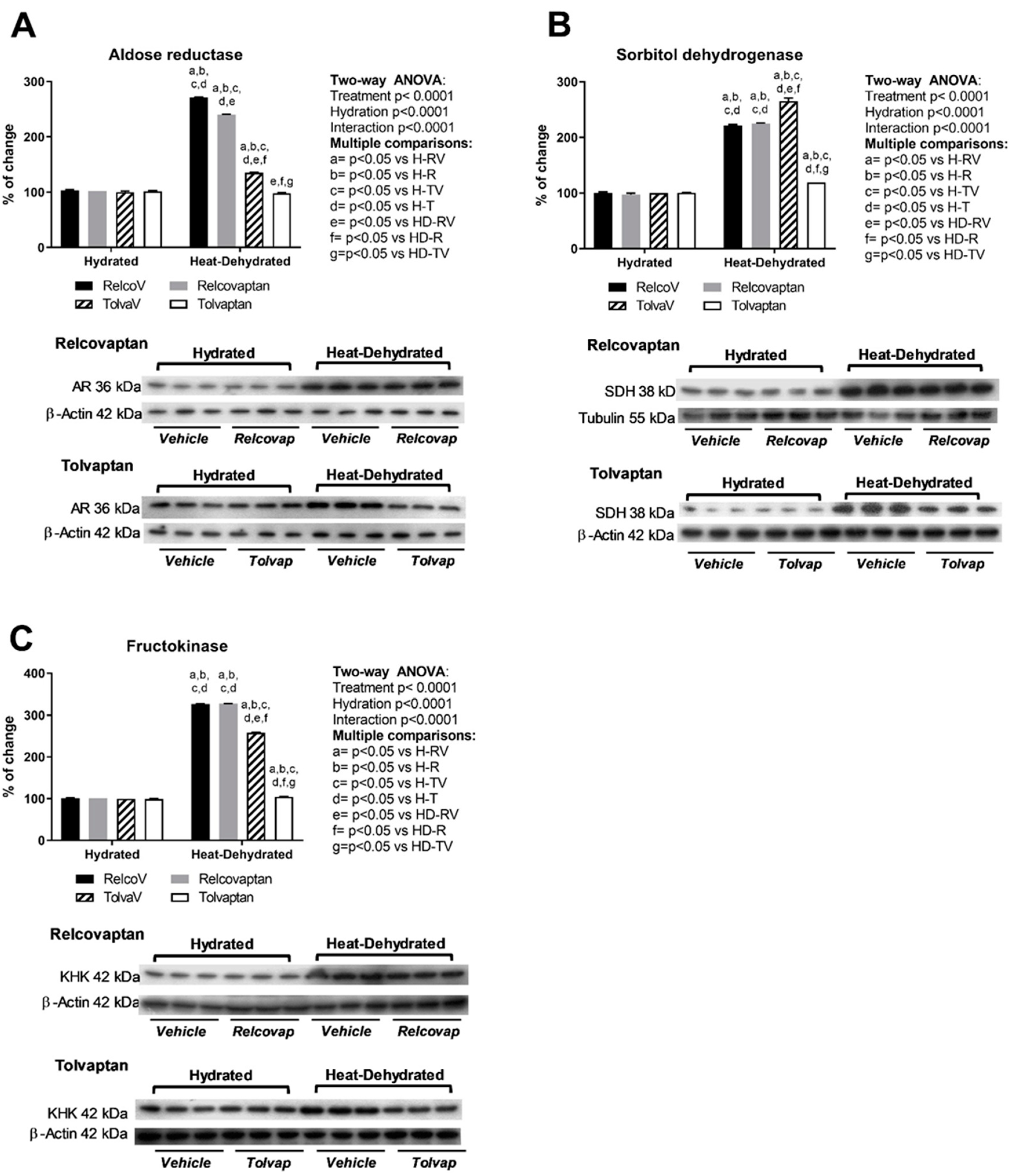
2.4. Tolvaptan Prevented the Overexpression of Polyol Pathway Enzymes (Aldose Reductase and Sorbitol Dehydrogenase) and Fructokinase in Heat Stress-Dehydrated Rats Rehydrated with a 10% Fructose Beverage
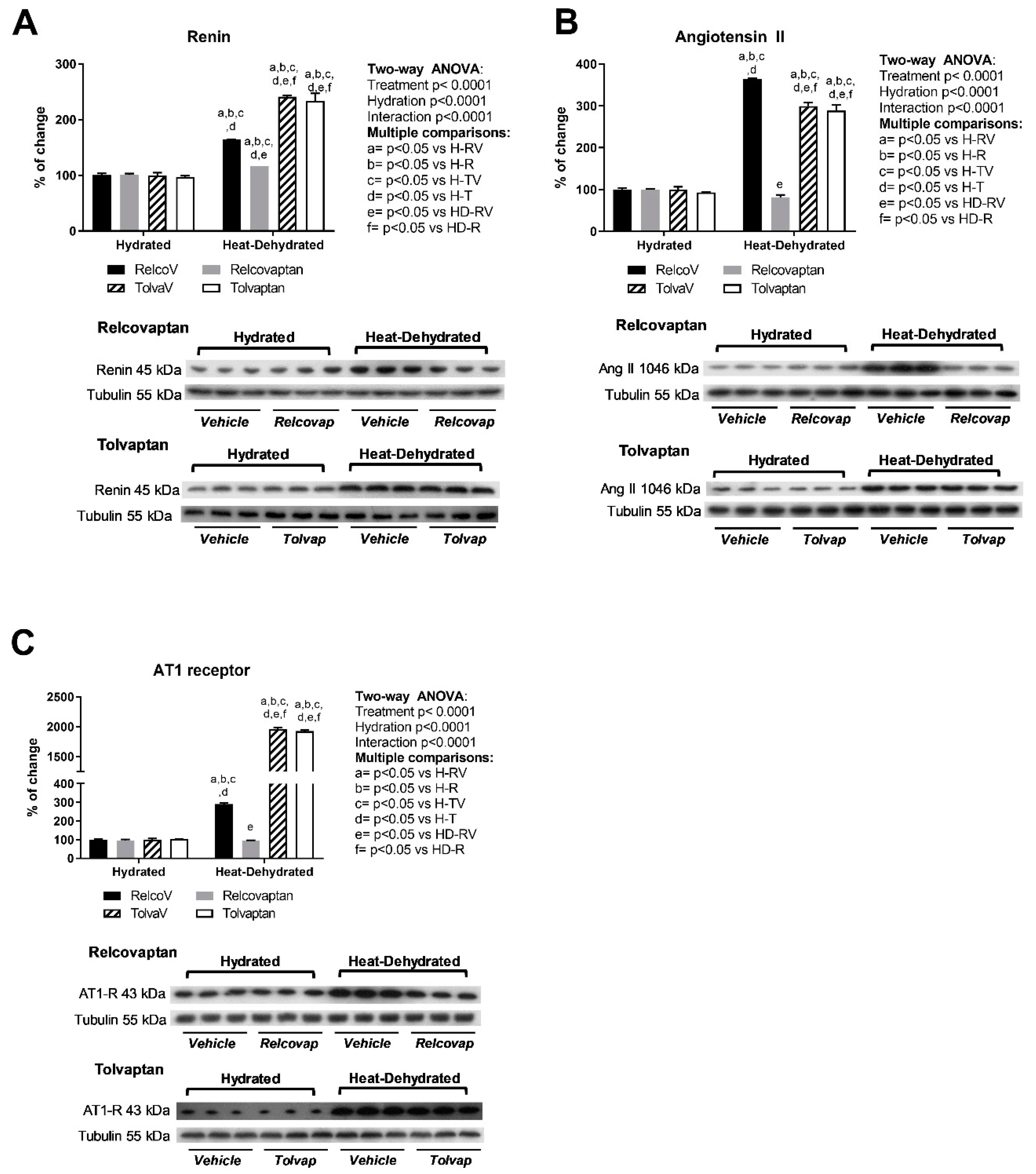
2.5. Relcovaptan Protected Against the Overactivation of the Renin–Angiotensin System in Heat Stress-Dehydrated Rats Rehydrated with a 10% Fructose Beverage
2.6. Relcovaptan Prevented the Overexpression of Glucocorticoid-Inducible Kinase 1 (SGK1) in Heat Stress-Dehydrated Rats Rehydrated with a 10% Fructose Beverage
3. Discussion
4. Materials and Methods
4.1. Animal Model
- HD-RV group, n = 5. Rats were treated with vehicle, followed by two hours of rehydration with fructose 10% after one hour of 37 °C thermal exposure and tap water the rest of the day.
- HD-R group, n = 5. Rats were treated with relcovaptan, followed by two hours of rehydration with fructose 10% after one hour of 37 °C thermal exposure and tap water the rest of the day.
- 3.
- HD-TV group, n = 5. Rats were treated with vehicle, followed by two hours of rehydration with fructose 10% after one hour of 37 °C thermal exposure and tap water the rest of the day.
- 4.
- HD-T group, n = 7. Rats were treated with tolvaptan, followed by two hours of rehydration with fructose 10% after one hour of 37 °C thermal exposure and tap water the rest of the day.
4.2. Blood Testing
4.3. Plasma Testing
4.4. Urine testing
4.5. Isolation of Tubular Fractions
4.6. Renal Cortex Homogenates
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radhakrishnan, J.; Remuzzi, G.; Saran, R.; Williams, D.E.; Rios-Burrows, N.; Powe, N.; Team, C.-C.S.; Bruck, K.; Wanner, C.; Stel, V.S.; et al. Taming the chronic kidney disease epidemic: A global view of surveillance efforts. Kidney Int. 2014, 86, 246–250. [Google Scholar] [CrossRef]
- Garcia-Garcia, G.; Jha, V.; Committee, W. Environmental and occupational factors in CKD. Occup. Environ. Med. 2015, 72, 238. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cleary, C.; Ortiz, A. CKD hotspots around the world: Where, why and what the lessons are. A CKJ review series. Clin. kidney J. 2014, 7, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Lemery, J.; Rajagopalan, B.; Diaz, H.F.; Garcia-Trabanino, R.; Taduri, G.; Madero, M.; Amarasinghe, M.; Abraham, G.; Anutrakulchai, S.; et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: The case for heat stress nephropathy. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Roncal Jimenez, C.A.; Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Nakagawa, T.; Ejaz, A.A.; Cicerchi, C.; Inaba, S.; Le, M.; Miyazaki, M.; et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int. 2014, 86, 294–302. [Google Scholar] [CrossRef]
- Garcia-Arroyo, F.E.; Cristobal, M.; Arellano-Buendia, A.S.; Osorio, H.; Tapia, E.; Soto, V.; Madero, M.; Lanaspa, M.A.; Roncal-Jimenez, C.; Bankir, L.; et al. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R57–R65. [Google Scholar] [CrossRef]
- García-Arroyo, F.E.; Tapia, E.; Blas-Marron, M.G.; Gonzaga, G.; Silverio, O.; Cristóbal, M.; Osorio, H.; Arellano-Buendía, A.S.; Zazueta, C.; Aparicio-Trejo, O.E.; et al. Vasopressin mediates the renal damage induced by limited fructose rehydration in recurrently dehydrated rats. Int. J. Biol. Sci. 2017, 13, 976–984. [Google Scholar]
- Enhörning, S.; Christensson, A.; Melander, O. Plasma copeptin as a predictor of kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Renal Assoc. 2019, 34, 74–82. [Google Scholar] [CrossRef]
- Enhorning, S.; Wang, T.J.; Nilsson, P.M.; Almgren, P.; Hedblad, B.; Berglund, G.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Lindholm, E.; et al. Plasma copeptin and the risk of diabetes mellitus. Circulation 2010, 121, 2102–2108. [Google Scholar] [CrossRef]
- Boustany, R.; Tasevska, I.; Meijer, E.; Kieneker, L.M.; Enhörning, S.; Lefèvre, G.; Mohammedi, K.; Marre, M.; Fumeron, F.; Balkau, B.; et al. Plasma copeptin and chronic kidney disease risk in 3 European cohorts from the general population. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Villela-Torres, M.; Higareda-Mendoza, A.; Gómez-García, A.; Alvarez-Paredes, A.; García-López, E.; Stenvikel, P.; Gu, H.F.; Rashid-Qureshi, A.; Lindholm, B.; Alvarez-Aguilar, C. Copeptin plasma levels are associated with decline of renal function in patients with type 2 diabetes mellitus. Arch. Med Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tasevska, I.; Enhörning, S.; Christensson, A.; Persson, M.; Nilsson, P.M.; Melander, O. Increased levels of copeptin, a surrogate marker of arginine vasopressin, are associated with an increased risk of chronic kidney disease in a general population. Am. J. Nephrol. 2016, 44, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Bouby, N.; Hassler, C.; Bankir, L. Contribution of vasopressin to progression of chronic renal failure: Study in Brattleboro rats. Life Sci. 1999, 65, 991–1004. [Google Scholar] [CrossRef]
- Morooka, H.; Iwanaga, Y.; Tamaki, Y.; Takase, T.; Akahoshi, Y.; Nakano, Y.; Fujiki, H.; Miyazaki, S. Chronic administration of oral vasopressin type 2 receptor antagonist tolvaptan exerts both myocardial and renal protective effects in rats with hypertensive heart failure. Circ. Heart Fail. 2012, 5, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Iwanaga, Y.; Watanabe, H.; Morooka, H.; Akahoshi, Y.; Fujiki, H.; Miyazaki, S. Effects of Long-term blockade of vasopressin receptor types 1a and 2 on cardiac and renal damage in a Rat model of hypertensive heart failure. J. Cardiovasc. Pharm. 2015, 66, 487–496. [Google Scholar] [CrossRef]
- Perico, N.; Zoja, C.; Corna, D.; Rottoli, D.; Gaspari, F.; Haskell, L.; Remuzzi, G. V1/V2 Vasopressin receptor antagonism potentiates the renoprotection of renin-angiotensin system inhibition in rats with renal mass reduction. Kidney Int. 2009, 76, 960–967. [Google Scholar] [CrossRef]
- Kuwabara, M.; Hisatome, I.; Roncal-Jimenez, C.A.; Niwa, K.; Andres-Hernando, A.; Jensen, T.; Bjornstad, P.; Milagres, T.; Cicerchi, C.; Song, Z.; et al. Increased serum sodium and serum osmolarity are independent risk factors for developing chronic kidney disease; 5 year cohort study. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Wolf, J.P.; Nguyen, N.U.; Dumoulin, G.; Berthelay, S. Influence of hypertonic monosaccharide infusions on the release of plasma arginine vasopressin in normal humans. Horm. Metab. Res. Hormon.und. Stoffwechs. Horm. Metab. 1992, 24, 379–383. [Google Scholar] [CrossRef]
- Song, Z.; Roncal-Jimenez, C.A.; Lanaspa-Garcia, M.A.; Oppelt, S.A.; Kuwabara, M.; Jensen, T.; Milagres, T.; Andres-Hernando, A.; Ishimoto, T.; Garcia, G.E.; et al. Role of fructose and fructokinase in acute dehydration-induced vasopressin gene expression and secretion in mice. J. Neurophysiol. 2017, 117, 646–654. [Google Scholar] [CrossRef]
- Chapman, C.L.; Johnson, B.D.; Sackett, J.R.; Parker, M.D.; Schlader, Z.J. soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019. [Google Scholar] [CrossRef]
- Ko, B.C.; Ruepp, B.; Bohren, K.M.; Gabbay, K.H.; Chung, S.S. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J. Biol. Chem. 1997, 272, 16431–16437. [Google Scholar] [CrossRef] [PubMed]
- Herman, B.A.; Ferguson, K.M.; Fernandez, J.V.B.; Kauffman, S.; Spicher, J.T.; King, R.J.; Halterman, J.A. NFAT5 is differentially expressed in Sprague-Dawley rat tissues in response to high salt and high fructose diets. Genet. Mol. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Guelinckx, I.; Lemetais, G.; Melander, O. Two liters a day keep the doctor away? considerations on the pathophysiology of suboptimal fluid intake in the common population. Kidney Blood Press. Res. 2017, 42, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, J.D.; Persaud, P.; Williams, C.K.; Chen, Y.; Burg, M.B. cAMP-independent role of PKA in tonicity-induced transactivation of tonicity-responsive enhancer/ osmotic response element-binding protein. Proc. Natl. Acad. Sci. USA 2002, 99, 16800–16805. [Google Scholar] [CrossRef]
- Garcia-Arroyo, F.E.; Gonzaga, G.; Munoz-Jimenez, I.; Osorio-Alonso, H.; Iroz, A.; Vecchio, M.; Tapia, E.; Roncal-Jimenez, C.A.; Johnson, R.J.; Sanchez-Lozada, L.G. Antioxidant supplements as a novel mean for blocking recurrent heat stress-induced kidney damage following rehydration with fructose-containing beverages. Free Radic. Biol. Med. 2019, 141, 182–191. [Google Scholar] [CrossRef]
- Aoyagi, T.; Izumi, Y.; Hiroyama, M.; Matsuzaki, T.; Yasuoka, Y.; Sanbe, A.; Miyazaki, H.; Fujiwara, Y.; Nakayama, Y.; Kohda, Y.; et al. Vasopressin regulates the renin-angiotensin-aldosterone system via V1a receptors in macula densa cells. Am. J. Physiol. Renal Physiol. 2008, 295, F100–F107. [Google Scholar] [CrossRef]
- Hussain, A.; Wyatt, A.W.; Wang, K.; Bhandaru, M.; Biswas, R.; Avram, D.; Foller, M.; Rexhepaj, R.; Friedrich, B.; Ullrich, S.; et al. SGK1-dependent upregulation of connective tissue growth factor by angiotensin II. Kidney blood Press. Res. 2008, 31, 80–86. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Annunziata, G.; Vecchiarini, M.; Orio, F.; Di Somma, C.; Colao, A.; Savastano, S. Water intake keeps type 2 diabetes away? Focus copeptin. Endocr. 2018, 62, 292–298. [Google Scholar] [CrossRef]
- Yamamura, Y.; Nakamura, S.; Itoh, S.; Hirano, T.; Onogawa, T.; Yamashita, T.; Yamada, Y.; Tsujimae, K.; Aoyama, M.; Kotosai, K.; et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: Pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J. Pharmacol. Exp. Ther. 1998, 287, 860–867. [Google Scholar]
- Serradeil-Le Gal, C.; Wagnon, J.; Garcia, C.; Lacour, C.; Guiraudou, P.; Christophe, B.; Villanova, G.; Nisato, D.; Maffrand, J.P.; Le Fur, G.; et al. Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. J. Clin. Invest. 1993, 92, 224–231. [Google Scholar] [CrossRef]
- Ferreira-Pego, C.; Guelinckx, I.; Moreno, L.A.; Kavouras, S.A.; Gandy, J.; Martinez, H.; Bardosono, S.; Abdollahi, M.; Nasseri, E.; Jarosz, A.; et al. Total fluid intake and its determinants: Cross-sectional surveys among adults in 13 countries worldwide. Eur. J. Nutr. 2015, 54 (Suppl. 2), 35–43. [Google Scholar] [CrossRef]
- Stookey, J.D.; Brass, B.; Holliday, A.; Arieff, A. What is the cell hydration status of healthy children in the USA? Preliminary data on urine osmolality and water intake. Public health Nutr. 2012, 15, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, I.; Santaliestra-Pasias, A.M.; Bel-Serrat, S.; Sadalla-Collese, T.; Miguel-Berges, M.L.; Moreno, L.A. Fluid consumption, total water intake and first morning urine osmolality in Spanish adolescents from Zaragoza: Data from the HELENA study. Eur. J. Clin. Nutr. 2016, 70, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Sichert-Hellert, W.; Kersting, M.; Manz, F. Fifteen year trends in water intake in German children and adolescents: Results of the DONALD Study. dortmund nutritional and anthropometric longitudinally designed study. Acta Paediatr. 2001, 90, 732–737. [Google Scholar] [PubMed]
- Bonnet, F.; Lepicard, E.M.; Cathrin, L.; Letellier, C.; Constant, F.; Hawili, N.; Friedlander, G. French children start their school day with a hydration deficit. Ann. Nutr. Metab. 2012, 60, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.K.; Mennella, J.A. Innate and learned preferences for sweet taste during childhood. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 379–384. [Google Scholar] [CrossRef]
- Yngve, A.; Haapala, I.; Hodge, A.; McNeill, G.; Tseng, M. Making soft drinks the dietary version of the cigarette. Public Health Nutr. 2012, 15, 1329–1330. [Google Scholar] [CrossRef]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.; Lim, S.; Andrews, K.G.; Engell, R.E.; Ezzati, M.; Mozaffarian, D.; Global Burden of Diseases Nutrition; et al. Global, Regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: A systematic assessment of beverage intake in 187 countries. PLoS ONE 2015, 10, e0124845. [Google Scholar] [CrossRef]
- Miranda, C.A.; Lee, J.W.; Chou, C.L.; Knepper, M.A. Tolvaptan as a tool in renal physiology. Am. J. Physiol. Renal Physiol. 2014, 306, F359–F366. [Google Scholar] [CrossRef]
- Vinay, P.; Gougoux, A.; Lemieux, G. Isolation of a pure suspension of rat proximal tubules. Am. J. Physiol. 1981, 241, F403–F411. [Google Scholar] [CrossRef]

| Parameter | Relcovaptan | Tolvaptan | ||||||
|---|---|---|---|---|---|---|---|---|
| Hydrated | Heat-dehydrated | Hydrated | Heat-dehydrated | |||||
| H-RV | H-R | HD-RV | HD-R | H-TV | H-T | HD-TV | HD-T | |
| 24 h fluid intake (mL/24 h) | 32 ± 1 | 33 ± 1 | 35 ± 3 | 34 ± 5 | 36 ± 2 | 39 ± 2 | 35 ± 3 | 40 ± 5 |
| Rehydration fluid intake (mL/2 h) | 8 ± 1 | 7 ± 2 | 7 ± 1 | 7 ± 1 | 7 ± 2 | 8 ± 2 | 8 ± 3 | 9 ± 1 |
| BW loss after heat stress (% BW *) | 2.3 ± 0.1 | 2.2 ± 1 | 2.8 ± 1 | 2.8 ± 1 | ||||
| Parameter | Tolvaptan | Relcovaptan | ||||||
|---|---|---|---|---|---|---|---|---|
| Hydrated | Heat-dehydrated | Hydrated | Heat-dehydrated | |||||
| H-RV | H-R | HD-RV | HD-R | H-TV | H-T | HD-TV | HD-T | |
| Plasma Cortisol * pg/mL | 25 ± 1 | 25 ± 2 | 383 ± 3 a,b,c,d | 11 ± 4 a,b,c,d,e | 30 ± 5 | 24 ± 2 | 298 ± 8 a,b,c,d,e,f | 110 ± 4 a,b,c,d,e,f,g |
| Plasma IL−6 * pg/mL | 38 ± 2 | 37 ± 1 | 82 ± 3 a,b,c,d | 67 ± 2 a,b,c,d,e | 38 ± 2 | 36 ± 2 | 101 ± 1 a,b,c,d,e,f | 75 ± 3 a,b,c,d,e,f,g |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Arroyo, F.E.; Muñoz-Jiménez, I.; Gonzaga, G.; Tapia, E.; Osorio-Alonso, H.; Roncal-Jiménez, C.A.; Iroz, A.; Vecchio, M.; Reyes-García, J.G.; Johnson, R.J.; et al. A Role for Both V1a and V2 Receptors in Renal Heat Stress Injury Amplified by Rehydration with Fructose. Int. J. Mol. Sci. 2019, 20, 5764. https://doi.org/10.3390/ijms20225764
García-Arroyo FE, Muñoz-Jiménez I, Gonzaga G, Tapia E, Osorio-Alonso H, Roncal-Jiménez CA, Iroz A, Vecchio M, Reyes-García JG, Johnson RJ, et al. A Role for Both V1a and V2 Receptors in Renal Heat Stress Injury Amplified by Rehydration with Fructose. International Journal of Molecular Sciences. 2019; 20(22):5764. https://doi.org/10.3390/ijms20225764
Chicago/Turabian StyleGarcía-Arroyo, Fernando E., Itzel Muñoz-Jiménez, Guillermo Gonzaga, Edilia Tapia, Horacio Osorio-Alonso, Carlos A Roncal-Jiménez, Alison Iroz, Mariacristina Vecchio, Juan G. Reyes-García, Richard J Johnson, and et al. 2019. "A Role for Both V1a and V2 Receptors in Renal Heat Stress Injury Amplified by Rehydration with Fructose" International Journal of Molecular Sciences 20, no. 22: 5764. https://doi.org/10.3390/ijms20225764
APA StyleGarcía-Arroyo, F. E., Muñoz-Jiménez, I., Gonzaga, G., Tapia, E., Osorio-Alonso, H., Roncal-Jiménez, C. A., Iroz, A., Vecchio, M., Reyes-García, J. G., Johnson, R. J., & Sánchez-Lozada, L. G. (2019). A Role for Both V1a and V2 Receptors in Renal Heat Stress Injury Amplified by Rehydration with Fructose. International Journal of Molecular Sciences, 20(22), 5764. https://doi.org/10.3390/ijms20225764






