MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness
Abstract
1. Introduction
2. Results
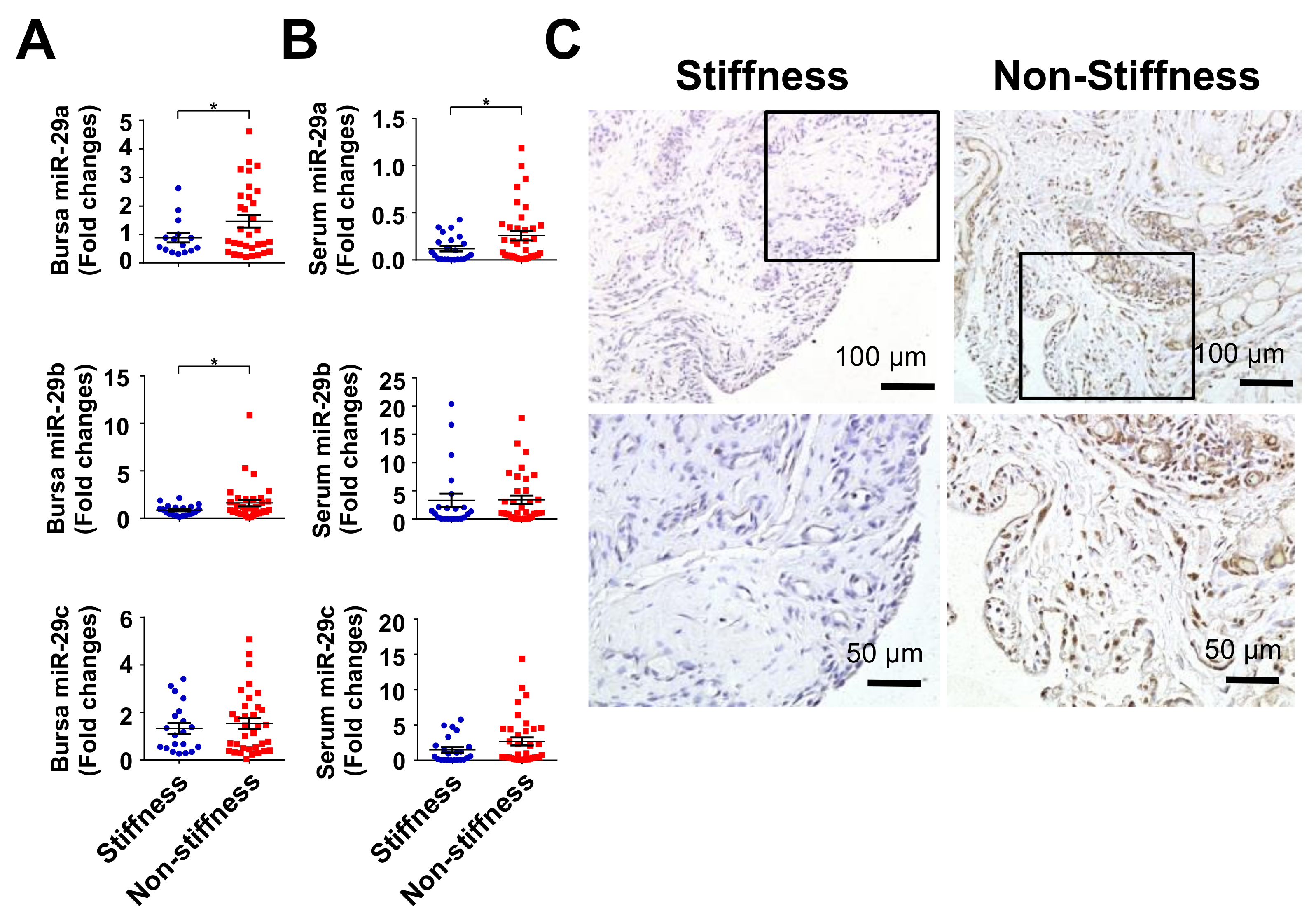
2.1. Subacromial Bursitis in Rotator Cuff Lesion with Shoulder Stiffness
2.2. Decreased miR-29a Expression in Rotator Cuff Lesion with Shoulder Stiffness
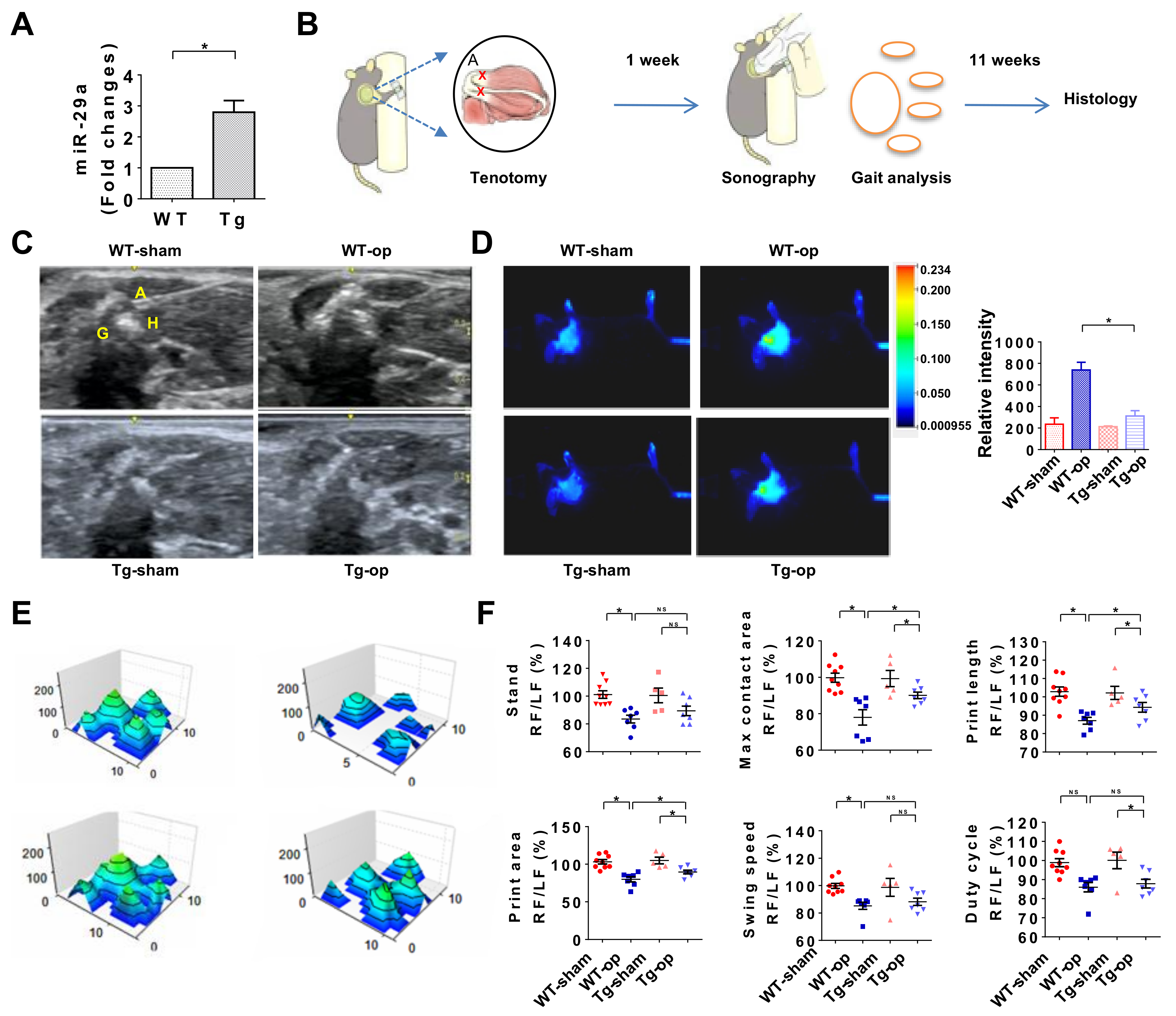
2.3. miR-29a Overexpression Attenuated Inflammation and Gait Irregularity
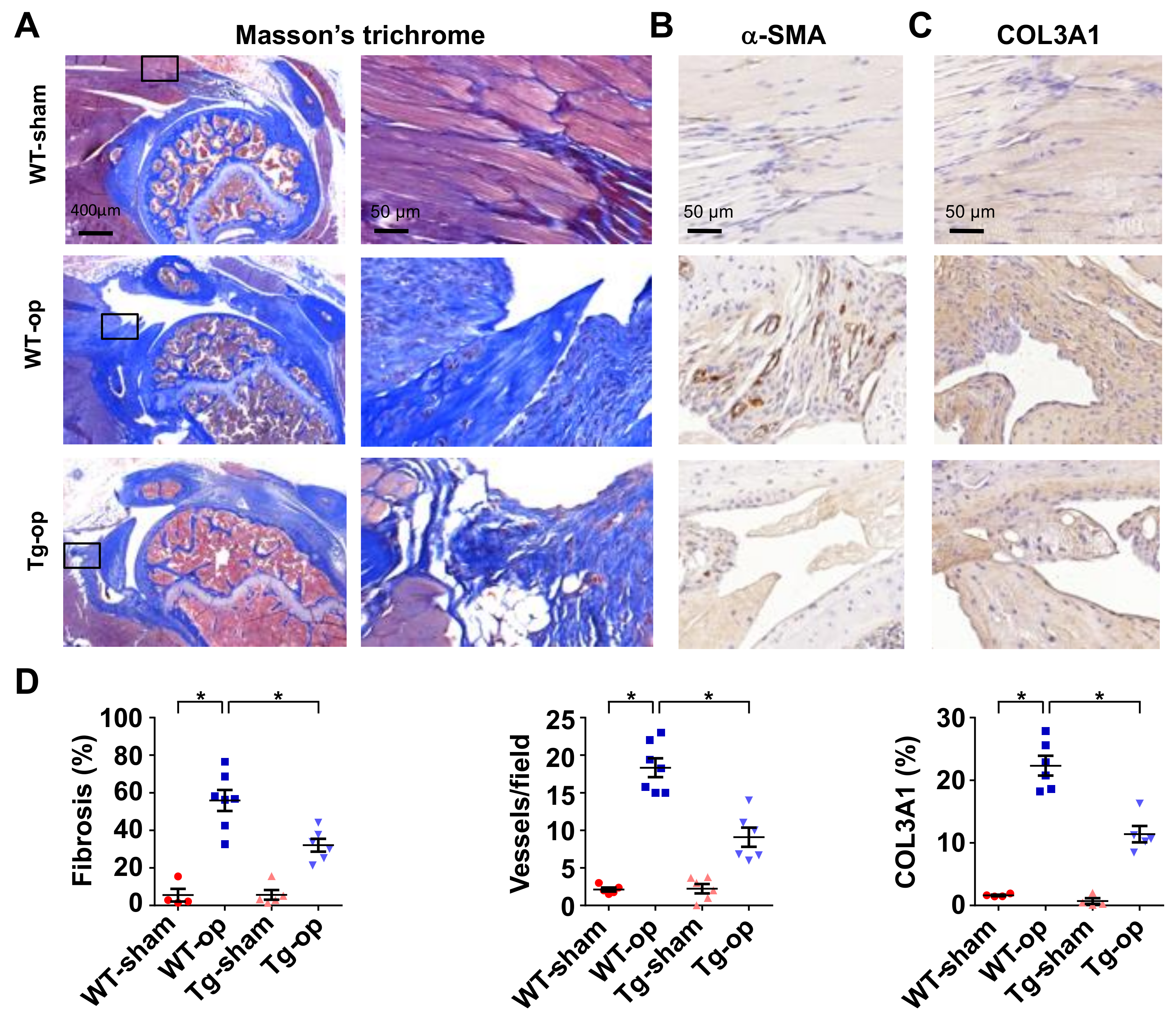
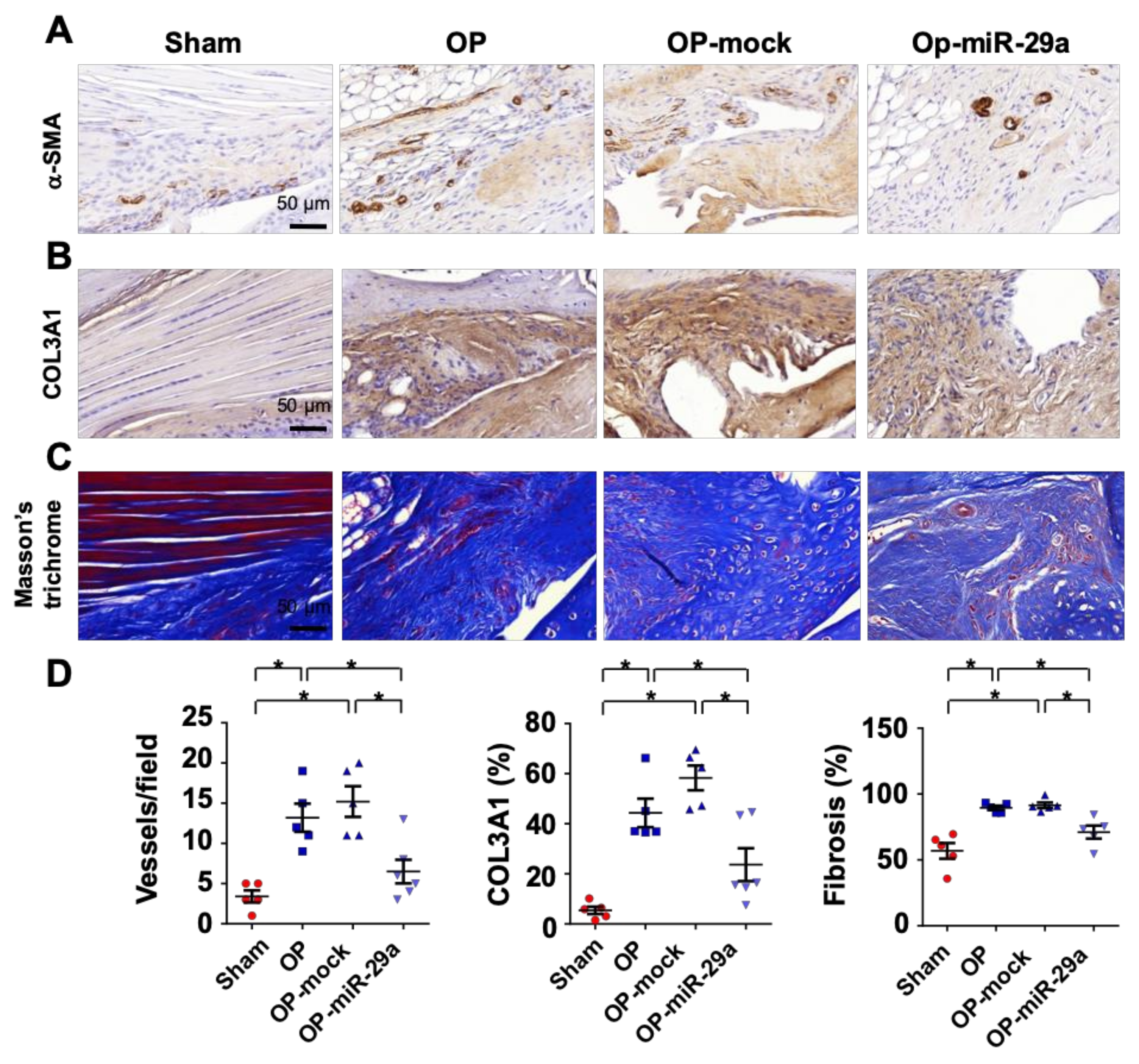
2.4. miR-29a Overexpression Downregulated Fibrotic Matrix Formation
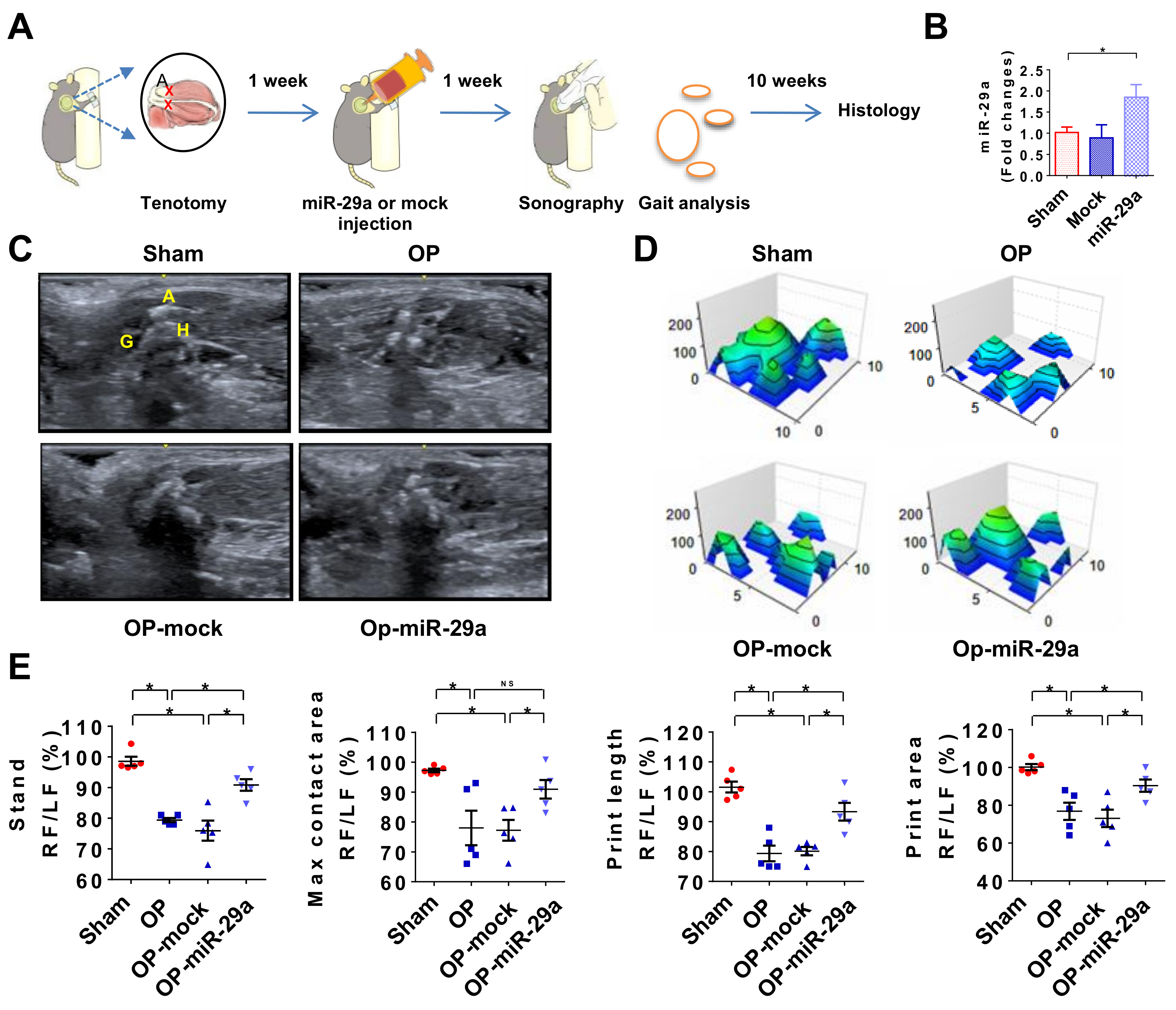
2.5. miR-29 Precursor Treatment Improved Tendon Fibrosis in Injured Shoulders
2.6. miR-29a Directly Targeted COL3A1 Transcription in Inflamed Tenocytes
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. Diagnosis of Shoulder Stiffness
4.3. Harvest of Clinical Specimens
4.4. Histomorphometry, In Situ Hybridization, and Immunohistochemistry
4.5. RT-Quantitative PCR
4.6. miR-29a Transgenic Mice
4.7. Shoulder Rotator Cuff Lesion in Mice
4.8. Desktop Digital Ultrasound Analysis
4.9. In Vivo Near-Infrared Fluorescence Assay
4.10. Gait Analysis
4.11. miR-29a Precursor Treatment
4.12. Transfection of Human Tenocyte Cultures
4.13. Luciferase Reporter Assessment of miR-29a Binding to 3′-UTR of COL3A1
4.14. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; de Sa, D.; Kanakamedala, A.; Sheean, A.J.; Vyas, D. Management of Concomitant Preoperative Rotator Cuff Pathology and Adhesive Capsulitis: A Systematic Review of Indications, Treatment Approaches, and Outcomes. Arthroscopy 2019, 35, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Blaine, T.A.; Cote, M.A.; Proto, A.; Mulcahey, M.; Lee, F.Y.; Bigliani, L.U. Interleukin-1beta stimulates stromal-derived factor-1alpha expression in human subacromial bursa. J. Orthop. Res. 2011, 29, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.K.; Flatow, E.L. Subacromial impingement syndrome. J. Am. Acad. Orthop. Surg. 2011, 19, 701–708. [Google Scholar] [CrossRef]
- Carr, A.J.; Murphy, R.; Dakin, S.G.; Rombach, I.; Wheway, K.; Watkins, B.; Franklin, S.L. Platelet-Rich Plasma Injection with Arthroscopic Acromioplasty for Chronic Rotator Cuff Tendinopathy: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 2891–2897. [Google Scholar] [CrossRef]
- Siu, K.K.; Zheng, L.B.; Ko, J.Y.; Wang, F.S.; Wang, C.J.; Wong, T.; Chou, W.Y. Increased interleukin 1beta levels in the subacromial fluid in diabetic patients with rotator cuff lesions compared with nondiabetic patients. J. Shoulder Elb. Surg. 2013, 22, 1547–1551. [Google Scholar] [CrossRef]
- Ko, J.Y.; Wang, F.S.; Huang, H.Y.; Wang, C.J.; Tseng, S.L.; Hsu, C. Increased IL-1beta expression and myofibroblast recruitment in subacromial bursa is associated with rotator cuff lesions with shoulder stiffness. J. Orthop. Res. 2008, 26, 1090–1097. [Google Scholar] [CrossRef]
- Deacon, D.C.; Nevis, K.R.; Cashman, T.J.; Zhou, Y.; Zhao, L.; Washko, D.; Guner-Ataman, B.; Burns, C.G.; Burns, C.E. The miR-143-adducin3 pathway is essential for cardiac chamber morphogenesis. Development 2010, 137, 1887–1896. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Georgantas, R.W.; Streicher, K.; Greenberg, S.A.; Greenlees, L.M.; Zhu, W.; Brohawn, P.Z.; Higgs, B.W.; Czapiga, M.; Morehouse, C.A.; Amato, A.; et al. Inhibition of myogenic microRNAs 1133, and 206 by inflammatory cytokines links inflammation and muscle degeneration in adult inflammatory myopathies. Arthritis Rheumatol. 2014, 66, 1022–1033. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, H.; Li, M.; Frid, M.G.; Flockton, A.R.; McKeon, B.A.; Yeager, M.E.; Fini, M.A.; Morrell, N.W.; Pullamsetti, S.S.; et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ. Res. 2014, 114, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Oglesby, I.K.; Chotirmall, S.H.; McElvaney, N.G.; Greene, C.M. Regulation of cystic fibrosis transmembrane conductance regulator by microRNA-145, -223, and -494 is altered in DeltaF508 cystic fibrosis airway epithelium. J. Immunol. 2013, 190, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Denby, L.; Ramdas, V.; Lu, R.; Conway, B.R.; Grant, J.S.; Dickinson, B.; Aurora, A.B.; McClure, J.D.; Kipgen, D.; Delles, C.; et al. MicroRNA-214 antagonism protects against renal fibrosis. J. Am. Soc. Nephrol. 2014, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, C.; Lin, X.; Li, J. MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie 2013, 95, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, V.; McBride, M.; Denby, L.; Baker, A.H. Canonical transforming growth factor-beta signaling regulates disintegrin metalloprotease expression in experimental renal fibrosis via miR-29. Am. J. Pathol. 2013, 183, 1885–1896. [Google Scholar] [CrossRef]
- Kwiecinski, M.; Elfimova, N.; Noetel, A.; Tox, U.; Steffen, H.M.; Hacker, U.; Nischt, R.; Dienes, H.P.; Odenthal, M. Expression of platelet-derived growth factor-C and insulin-like growth factor I in hepatic stellate cells is inhibited by miR-29. Lab. Invest. 2012, 92, 978–987. [Google Scholar] [CrossRef]
- Xiao, J.; Meng, X.M.; Huang, X.R.; Chung, A.C.; Feng, Y.L.; Hui, D.S.; Yu, C.M.; Sung, J.J.; Lan, H.Y. miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol. Ther. 2012, 20, 1251–1260. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Wang, Y.; Zhang, M.; Li, L.; Zhu, D.; Li, X.; Gu, H.; Zhang, C.Y.; Zen, K. Protective role of estrogen-induced miRNA-29 expression in carbon tetrachloride-induced mouse liver injury. J. Biol. Chem. 2012, 287, 14851–14862. [Google Scholar] [CrossRef]
- Ko, J.Y.; Lee, M.S.; Lian, W.S.; Weng, W.T.; Sun, Y.C.; Chen, Y.S.; Wang, F.S. MicroRNA-29a counteracts synovitis in knee osteoarthritis pathogenesis by targeting VEGF. Sci. Rep. 2017, 7, 3584. [Google Scholar] [CrossRef]
- Tiao, M.M.; Wang, F.S.; Huang, L.T.; Chuang, J.H.; Kuo, H.C.; Yang, Y.L.; Huang, Y.H. MicroRNA-29a protects against acute liver injury in a mouse model of obstructive jaundice via inhibition of the extrinsic apoptosis pathway. Apoptosis 2014, 19, 30–41. [Google Scholar] [CrossRef]
- Lin, C.L.; Lee, P.H.; Hsu, Y.C.; Lei, C.C.; Ko, J.Y.; Chuang, P.C.; Huang, Y.T.; Wang, S.Y.; Wu, S.L.; Chen, Y.S.; et al. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J. Am. Soc. Nephrol. 2014, 25, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Gilchrist, D.S.; Akbar, M.; Reilly, J.H.; Kerr, S.C.; Campbell, A.L.; Murrell, G.A.C.; Liew, F.Y.; Kurowska-Stolarska, M.; McInnes, I.B. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat. Commun. 2015, 6, 6774. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.J.; Killian, M.L.; Choi, A.J.; Lin, E.; Esparza, M.C.; Galatz, L.M.; Thomopoulos, S.; Ward, S.R. Skeletal muscle fibrosis and stiffness increase after rotator cuff tendon injury and neuromuscular compromise in a rat model. J. Orthop. Res. 2014, 32, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Celeng, C.; de Keizer, B.; Merkely, B.; de Jong, P.; Leiner, T.; Takx, R.A.P. PET molecular targets and near-infrared fluorescence imaging of artherosclerosis. Curr. Cardiol. Rep. 2018, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.Y.; Sun, Y.C.; Li, W.C.; Wang, F.S. Chaperonin 60 regulation of SOX9 ubiquitination mitigates the development of knee osteoarthritis. J. Mol. Med. 2016, 94, 755–769. [Google Scholar] [CrossRef] [PubMed]
- de Oliveria, C.; Patel, K.; Mishra, V.; Trivedi, R.N.; Noel, P.; Singh, A.; Yaron, J.R.; Singh, V.P. Characterization and predictive value of near infrared 2-deoxyglucose optical imaging in sever acute pancreatitis. PLoS ONE 2016, 11, e0149073. [Google Scholar]
- Huegel, J.; Williams, A.A.; Soslowsky, L.J. Rotator cuff biology and biomechanics: A review of normal and pathological conditions. Curr. Rheumatol. Rep. 2015, 17, 476. [Google Scholar] [CrossRef]
- Lai, J.; Gagnier, J.J. The effect of lipid disorders on the risk of rotator cuff disease: A systematic review and meta-analysis. JBJS Open Access 2018, 3, e0018. [Google Scholar] [CrossRef]
- Bishop, J.Y.; Santiago-Torres, J.E.; Rimmke, N.; Flanigan, D.C. Smoking predisposes to rotator cuff pathology and shoulder dysfunction: A systematic review. Arthroscopy 2015, 31, 1598–1605. [Google Scholar] [CrossRef]
- Sabzevari, S.; Kachooei, A.R.; Giugale, J.; Lin, A. One-stage surgical treatment for concomitant rotator cuff tears with shoulder stiffness has comparable results with isolated rotator cuff tears: A systematic review. J. Shoulder Elbow. Surg. 2017, 26, e252–e258. [Google Scholar] [CrossRef]
- Franceschi, F.; Papalia, R.; Franceschetti, E.; Palumbo, A.; Del Buono, A.; Paciotti, M.; Maffulli, N.; Denaro, V. Double-row repair lowers the retear risk after accelerated rehabilitation. Am. J. Sports Med. 2016, 44, 948–956. [Google Scholar] [CrossRef]
- Skedros, J.G.; Adondakis, M.G.; Knight, A.N.; Pilkington, M.B. Frequency of shoulder corticosteroid injections for pain and stiffness after shoulder surgery and their potential to enhance outcomes with physiotherapy: A retrospective study. Pain Ther. 2017, 6, 45–60. [Google Scholar] [CrossRef]
- Honda, H.; Gotoh, M.; Kanazawa, T.; Ohzono, H.; Nakamura, H.; Ohta, K.; Nakamura, K.I.; Fukuda, K.; Teramura, T.; Hashimoto, T.; et al. Hyaluronic acid accelerates tendon-to-bone healing after rotator cuff Repair. Am. J. Sports Med. 2017, 45, 3322–3330. [Google Scholar] [CrossRef]
- Depres-Tremblay, G.; Chevrier, A.; Snow, M.; Rodeo, S.; Buschmann, M.D. Freeze-dried chitosan-platelet-rich plasma implants improve supraspinatus tendon attachment in a transosseous rotator cuff repair model in the rabbit. J. Biomater. Appl. 2019, 33, 792–807. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Qiao, Y.; Ge, Y.; Li, H.; Chen, J.; Hua, Y.; Li, Y. A Long preoperative duration of symptoms is associated with worse functional outcomes after 1-stage arthroscopic treatment of rotator cuff tears with shoulder stiffness. Am. J. Sports Med. 2017, 45, 2336–2344. [Google Scholar] [CrossRef]
- Zhuo, H.; Li, J. Comparison of one-stage versus two-stage procedure for the management of patients with rotator cuff tear and concomitant shoulder stiffness. J. Orthop. Surg. Res. 2019, 14, 40. [Google Scholar] [CrossRef]
- Cho, C.H.; Jang, H.K.; Bae, K.C.; Lee, S.W.; Lee, Y.K.; Shin, H.K.; Hwang, I. Clinical outcomes of rotator cuff repair with arthroscopic capsular release and manipulation for rotator cuff tear with stiffness: A matched-pair comparative study between patients with and without stiffness. Arthroscopy 2015, 31, 482–487. [Google Scholar] [CrossRef]
- Yoo, H.J.; Choi, J.Y.; Hong, S.H.; Kang, Y.; Park, J.; Kim, S.H.; Kang, H.S. Assessment of the postoperative appearance of the rotator cuff tendon using serial sonography after arthroscopic repair of a rotator cuff tear. J. Ultrasound Med. 2015, 34, 1183–1190. [Google Scholar] [CrossRef]
- White, E.A.; Schweitzer, M.E.; Haims, A.H. Range of normal and abnormal subacromial/subdeltoid bursa fluid. J. Comput. Assist. Tomogr. 2006, 30, 316–320. [Google Scholar] [CrossRef]
- Gibbons, M.C.; Singh, A.; Engler, A.J.; Ward, S.R. The role of mechanobiology in progression of rotator cuff muscle atrophy and degeneration. J. Orthop. Res. 2018, 36, 546–556. [Google Scholar] [CrossRef]
- Morita, W.; Snelling, S.J.; Dakin, S.G.; Carr, A.J. Profibrotic mediators in tendon disease: A systematic review. Arthritis Res. Ther. 2016, 18, 269. [Google Scholar] [CrossRef] [PubMed]
- Dubin, J.A.; Greenberg, D.R.; Iglinski-Benjamin, K.C.; Abrams, G.D. Effect of micro-RNA on tenocytes and tendon-related gene expression: A systematic review. J. Orthop. Res. 2018, 36, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Roos, T.R.; Roos, A.K.; Kleimeyer, J.P.; Ahmed, M.A.; Goodlin, G.T.; Fredericson, M.; Ioannidis, J.P.; Avins, A.L.; Dragoo, J.L. Genome-wide association screens for Achilles tendon and ACL tears and tendinopathy. PLoS ONE 2017, 12, e0170422. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.E.; Millar, N.L.; Platt, J.; Kitson, S.M.; Akbar, M.; Rech, R.; Griffin, J.; Pool, R.; Hughes, T.; McInnes, I.B.; et al. MicroRNA29a treatment improves early tendon injury. Mol. Ther. 2017, 25, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Levian, B.; Shah, P.; Mosich, G.M.; Husman, R.; Arinello, A.; Gatto, J.D.; Hu, V.J.; McClintick, D.J.; Jensen, A.R.; et al. Aged mice demonstrate greater muscle degeneration of chronically injured rotator cuff. J. Orthop. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Flood, M.D.; Bedi, A.; Kramer, H.F.; Russell, A.J.; Mendias, C.L. Inhibition of proly 4-hydroxylase decreases muscle fibrosis following chronic rotator cuff tear. Bone Jt. Res. 2017, 6, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Yoishida, R.; Alaee, F.; Dyrna, F.; Kronenbergy, M.S.; Maye, P.; Kalajzic, I.; Rowe, D.W.; Maszzocca, A.D.; Dyment, N.A. Murine supraspinatus tendon injury model to identify the cellular origins of rotator cuff healing. Connect. Tissue Res. 2016, 57, 507–515. [Google Scholar] [CrossRef]
- Eliasberg, C.D.; Dar, A.; Jensen, A.R.; Murray, I.R.; Hardy, W.R.; Kowalski, T.J.; Garagozlo, C.A.; Natsuhara, K.M.; Khan, A.Z.; McBride, O.J.; et al. Perivascular stem cells diminish muscle atrophy following massive rotator cuff tears in a small animal model. J. Bone Joint. Am. 2017, 99, 331–341. [Google Scholar] [CrossRef]


| Stiffness | Non-Stiffness | p Value | |
|---|---|---|---|
| Participants | 22 | 35 | |
| Gender | |||
| Female | 19 (86.4%) | 24 (68.6%) | 0.8 |
| Male | 3 (13.6%) | 11 (31.4%) | - |
| Involved shoulder | |||
| Right | 13 (59%) | 24 (68.6%) | 0.78 |
| Left | 9 (41%) | 11 (31.4%) | - |
| Age (years) | 62.1 ± 2 | 64.8 ± 1.2 | 0.12 |
| (48–77) | (52–80) | ||
| BMI | 25.1 ± 1 | 25.4 ± 0.5 | 0.42 |
| (16.6–32.2) | (20.8–33.4) | ||
| Functional score of Constant and Murley | 39.2 ± 2.2 | 63.5 ± 1.5 | <0.001 |
| (21–67) | (49–85) | ||
| Sum of passive range of motion (SROM) | 196.6 ± 10.1 | 365 ± 9.7 | <0.001 |
| (115–270) | (275–520) | ||
| Flexion | 83.4 ± 5 | 147.1 ± 3.5 | <0.001 |
| (35–130) | (100–180) | ||
| Abduction | 72.5 ± 4.8 | 119.7 ± 3.5 | <0.001 |
| (40–110) | (75–165) | ||
| External rotation | 15.2 ± 2.6 | 41.9 ± 3.1 | <0.001 |
| (0–40) | (10–75) | ||
| Internal rotation | 26 ± 6 | 54.6 ± 4.6 | <0.001 |
| (0–80) | (0–90) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.-Y.; Lian, W.-S.; Tsai, T.-C.; Chen, Y.-S.; Hsieh, C.-K.; Kuo, C.-W.; Wang, F.-S. MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness. Int. J. Mol. Sci. 2019, 20, 5742. https://doi.org/10.3390/ijms20225742
Ko J-Y, Lian W-S, Tsai T-C, Chen Y-S, Hsieh C-K, Kuo C-W, Wang F-S. MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness. International Journal of Molecular Sciences. 2019; 20(22):5742. https://doi.org/10.3390/ijms20225742
Chicago/Turabian StyleKo, Jih-Yang, Wei-Shiung Lian, Tsai-Chen Tsai, Yu-Shan Chen, Chin-Kuei Hsieh, Chung-Wen Kuo, and Feng-Sheng Wang. 2019. "MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness" International Journal of Molecular Sciences 20, no. 22: 5742. https://doi.org/10.3390/ijms20225742
APA StyleKo, J.-Y., Lian, W.-S., Tsai, T.-C., Chen, Y.-S., Hsieh, C.-K., Kuo, C.-W., & Wang, F.-S. (2019). MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness. International Journal of Molecular Sciences, 20(22), 5742. https://doi.org/10.3390/ijms20225742






