Adiponectin Reverses the Hypothalamic Microglial Inflammation during Short-Term Exposure to Fat-Rich Diet
Abstract
1. Introduction
2. Results
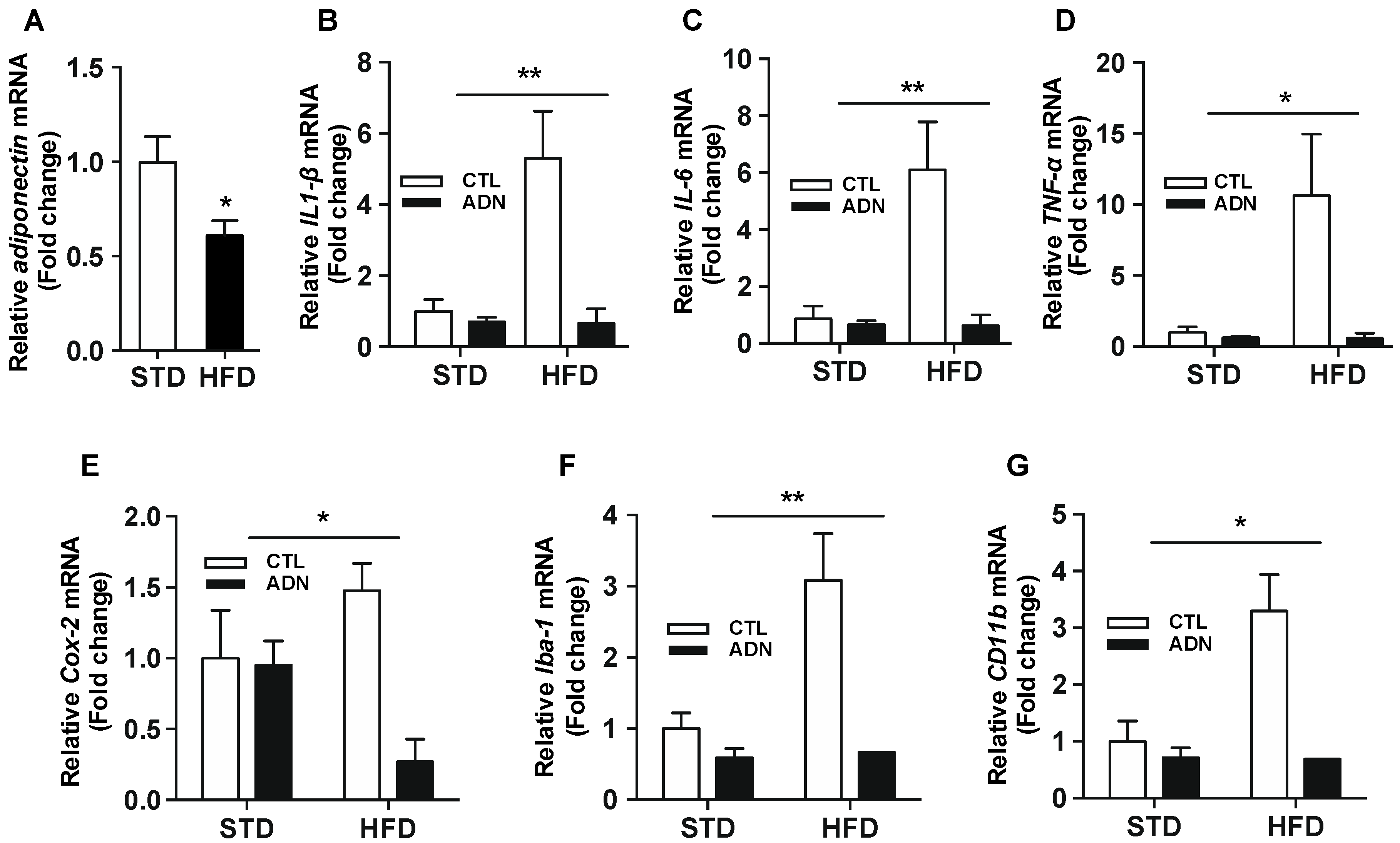
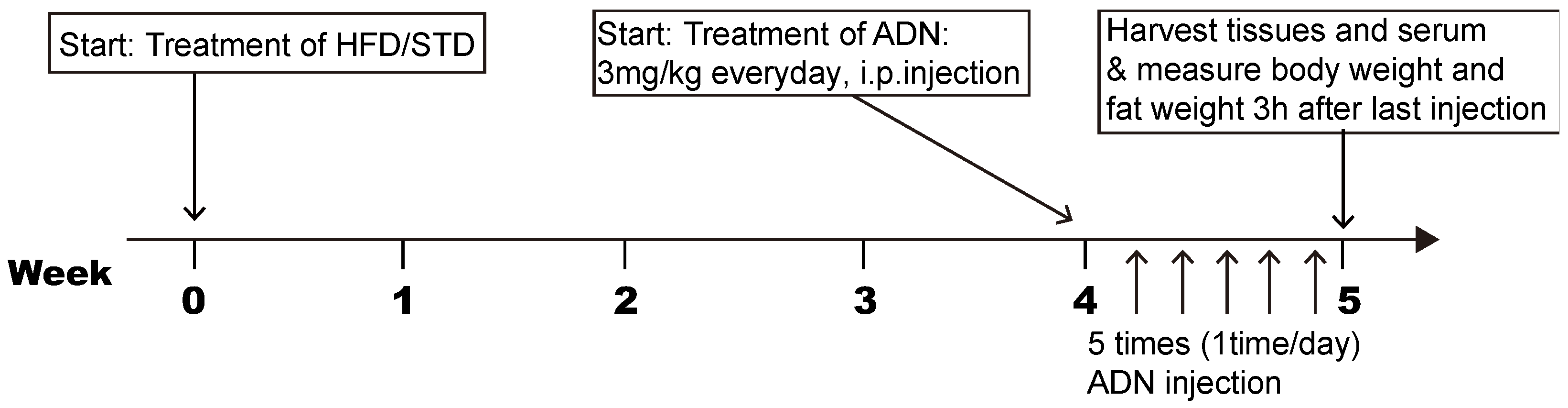
2.1. Adiponectin Reverses the Hypothalamic Inflammation Induced by Short-Term Exposure to High Fat Diet
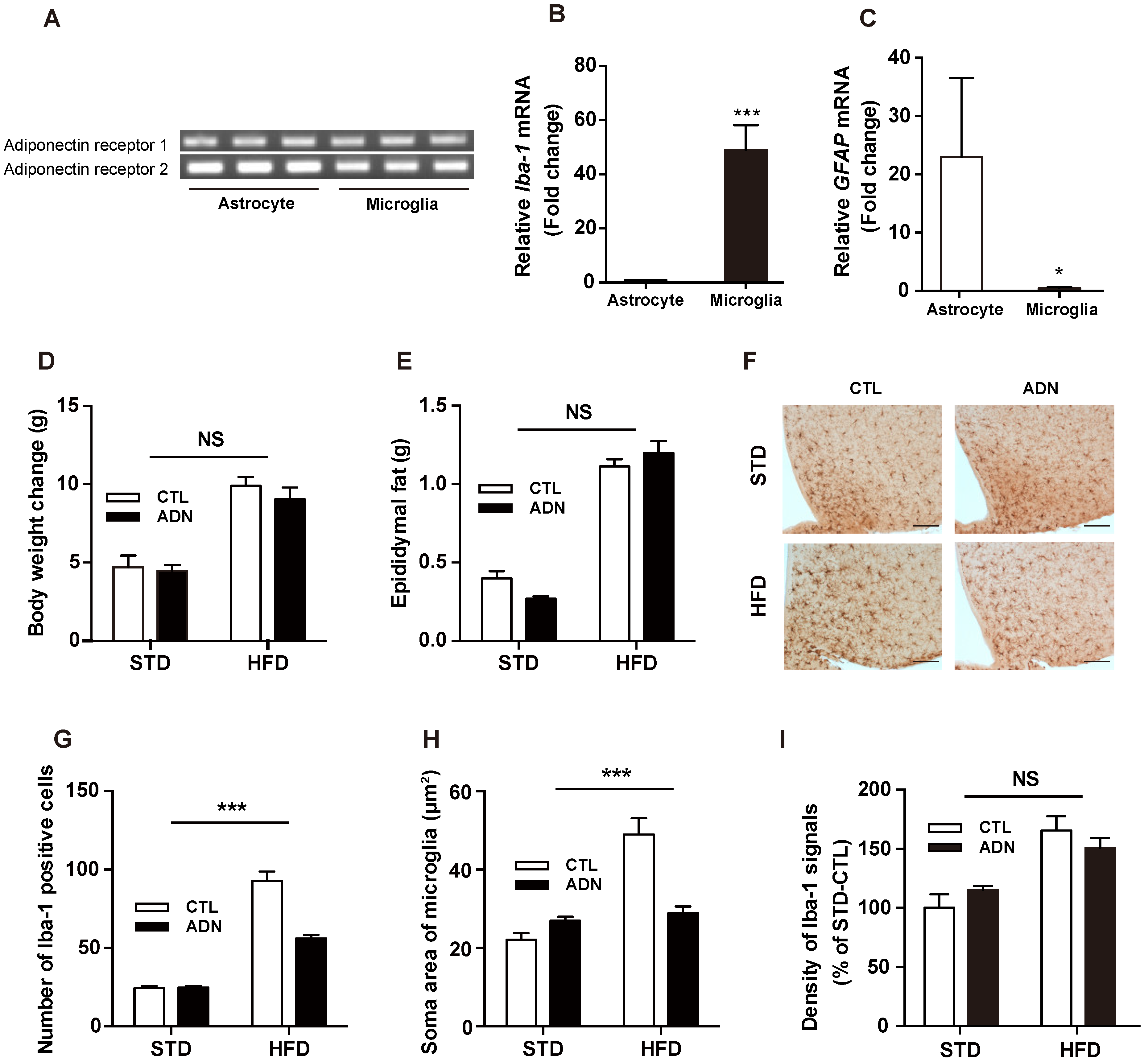
2.2. Adiponectin Suppresses the Microglial Activation Induced by Short-Term Exposure to HFD
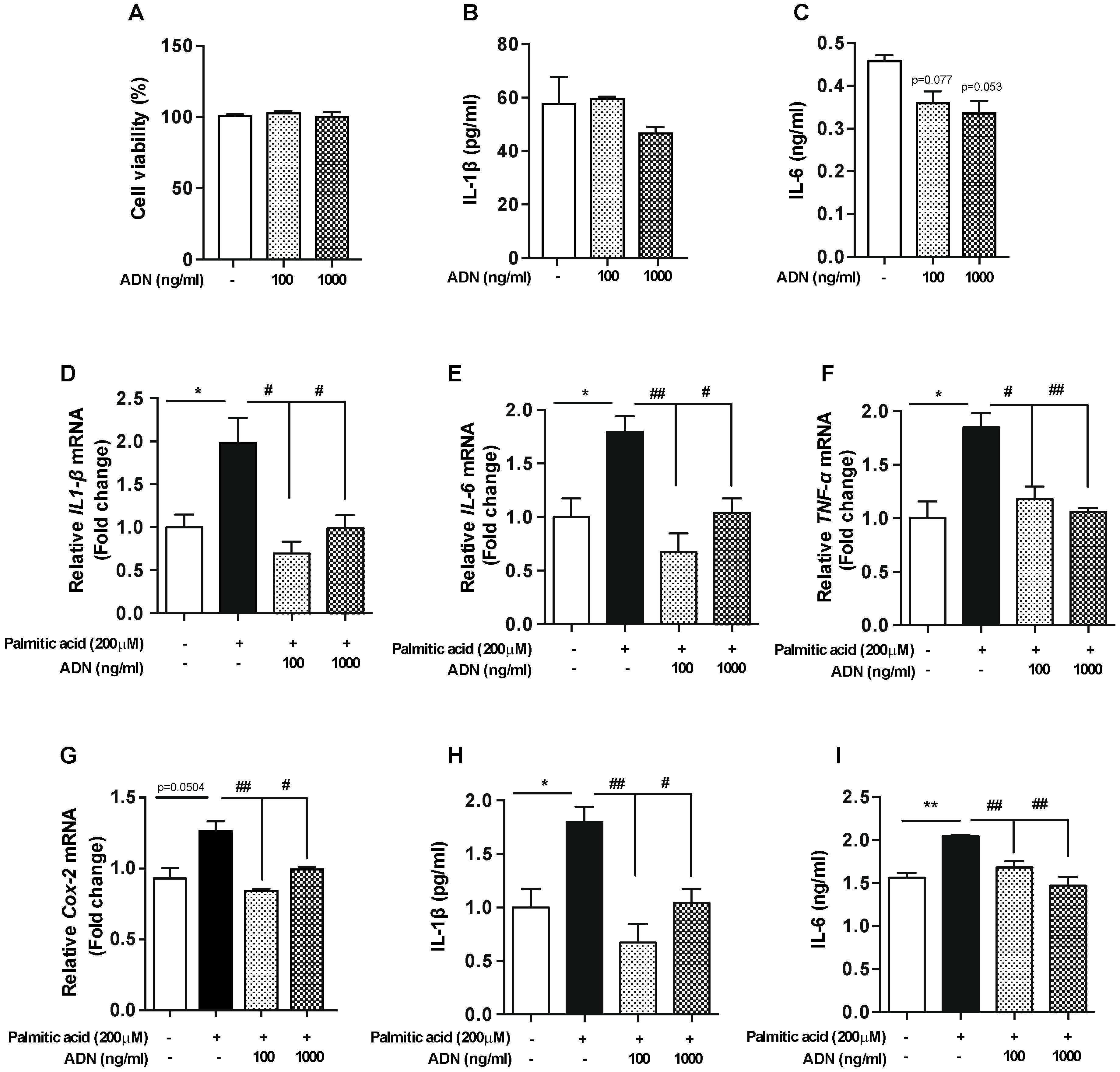
2.3. Adiponectin Improves Palmitic Acid-Induced Inflammatory Responses in the Microglial Cells
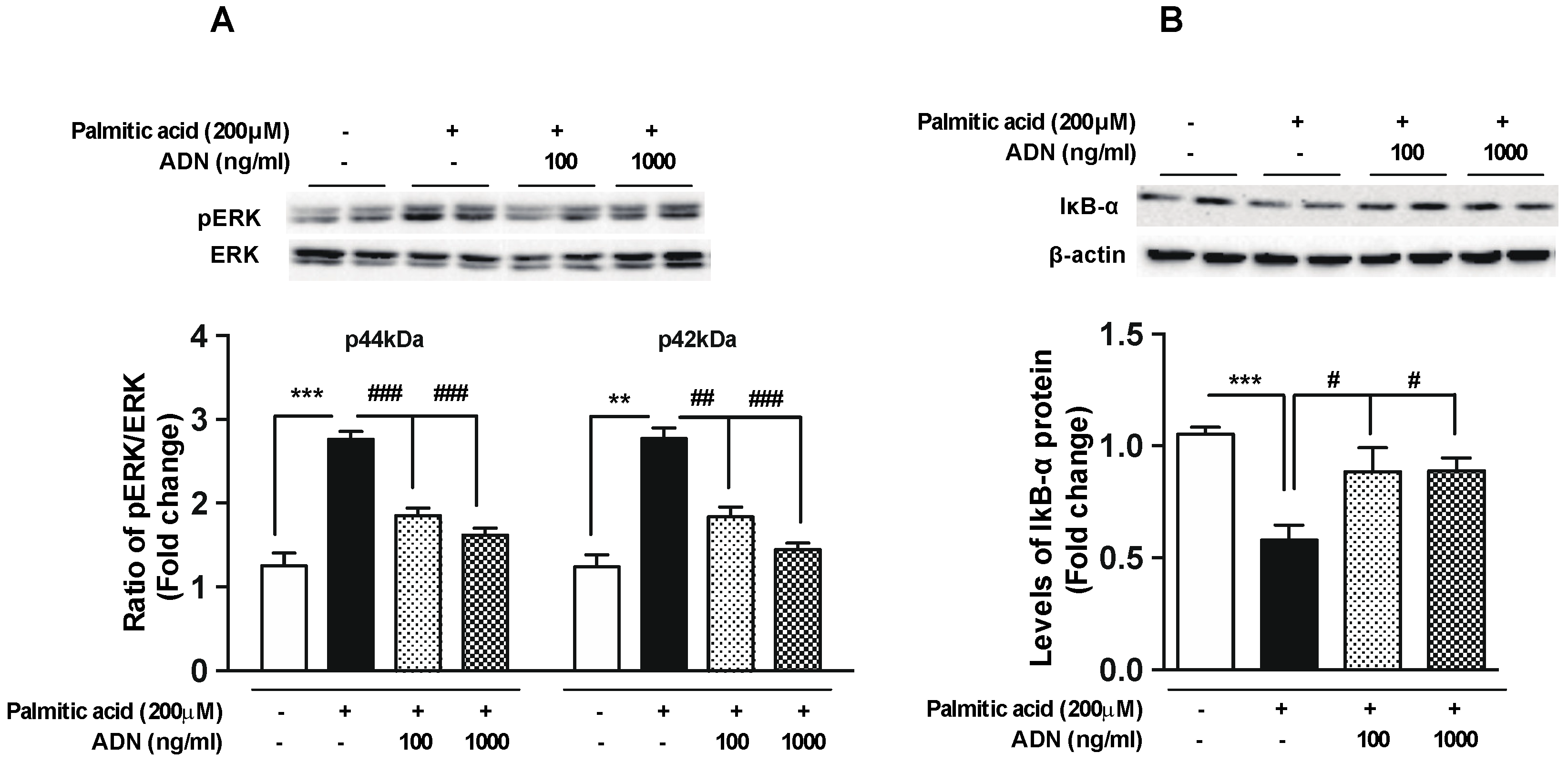
2.4. Adiponectin Reverses the Palmitic Acid-Induced Alterations in the Intracellular Signaling Molecules Involved in Inflammation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Immunohistochemistry
4.3. Cell Culture and Treatments
4.4. Primary Astrocyte Culture
4.5. Quantitative Real-Time PCR (qRT-PCR)
4.6. Measurement of Cytokine Levels
4.7. Immunoblot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Posey, K.A.; Clegg, D.J.; Printz, R.L.; Byun, J.; Morton, G.J.; Vivekanandan-Giri, A.; Pennathur, S.; Baskin, D.G.; Heinecke, J.W.; Woods, S.C.; et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1003–E1012. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Guyenet, S.J.; Dorfman, M.D.; Wisse, B.E.; Schwartz, M.W. Hypothalamic inflammation: Marker or mechanism of obesity pathogenesis? Diabetes 2013, 62, 2629–2634. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017, 26, 185–197. [Google Scholar] [CrossRef]
- Koch, C.E.; Lowe, C.; Legler, K.; Benzler, J.; Boucsein, A.; Böttiger, G.; Grattan, D.R.; Williams, L.M.; Tups, A. Central adiponectin acutely improves glucose tolerance in male mice. Endocrinology 2014, 155, 1806–1816. [Google Scholar] [CrossRef]
- Liu, Y.; Turdi, S.; Park, T.; Morris, N.J.; Deshaies, Y.; Xu, A.; Sweeney, G. Adiponectin corrects high-fat diet-induced disturbances in muscle metabolomic profile and whole-body glucose homeostasis. Diabetes 2013, 62, 743–752. [Google Scholar] [CrossRef]
- Song, J.; Lee, J.E. Adiponectin as a new paradigm for approaching Alzheimer’s disease. Anat. Cell Biol. 2013, 46, 229–234. [Google Scholar] [CrossRef]
- Chan, K.-H.; Lam, K.S.-L.; Cheng, O.-Y.; Kwan, J.S.-C.; Ho, P.W.-L.; Cheng, K.K.-Y.; Chung, S.K.; Ho, J.W.-M.; Guo, V.Y.; Xu, A. Adiponectin is protective against oxidative stress induced cytotoxicity in amyloid-beta neurotoxicity. PLoS ONE 2012, 7, e52354. [Google Scholar] [CrossRef]
- Chen, B.; Liao, W.-Q.; Xu, N.; Xu, H.; Wen, J.-Y.; Yu, C.-A.; Liu, X.-Y.; Li, C.-L.; Zhao, S.-M.; Campbell, W. Adiponectin protects against cerebral ischemia-reperfusion injury through anti-inflammatory action. Brain Res. 2009, 1273, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, X.; Huang, H.; Ding, N.; Zhang, S.; Hutchinson, S.Z.; Zhang, X. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS ONE 2014, 9, e94545. [Google Scholar] [CrossRef] [PubMed]
- Naitoh, R.; Miyawaki, K.; Harada, N.; Mizunoya, W.; Toyoda, K.; Fushiki, T.; Yamada, Y.; Seino, Y.; Inagaki, N. Inhibition of GIP signaling modulates adiponectin levels under high-fat diet in mice. Biochem. Biophys. Res. Commun. 2008, 376, 21–25. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Kanazawa, H.; Sasaki, Y.; Kohsaka, S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J. Cell Sci. 2000, 113, 1–12. [Google Scholar]
- Tanaka, Y.; Aleksunes, L.M.; Yeager, R.L.; Gyamfi, M.A.; Esterly, N.; Guo, G.L.; Klaassen, C.D. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 2008, 325, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef]
- Tu, T.H.; Kim, H.; Yang, S.; Kim, J.K.; Kim, J.G. Linoleic acid rescues microglia inflammation triggered by saturated fatty acid. Biochem. Biophys. Res. Commun. 2019, 513, 1–6. [Google Scholar] [CrossRef]
- Jiang, B.; Xu, S.; Hou, X.; Pimentel, D.R.; Brecher, P.; Cohen, R.A. Temporal control of NF-kappaB activation by ERK differentially regulates interleukin-1beta-induced gene expression. J. Biol. Chem. 2004, 279, 1323–1329. [Google Scholar] [CrossRef]
- Dumitru, C.D.; Ceci, J.D.; Tsatsanis, C.; Kontoyiannis, D.; Stamatakis, K.; Lin, J.H.; Patriotis, C.; Jenkins, N.A.; Copeland, N.G.; Kollias, G.; et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 2000, 103, 1071–1083. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: Key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Shadid, S.; Koutsari, C.; Jensen, M.D. Direct free fatty acid uptake into human adipocytes in vivo: Relation to body fat distribution. Diabetes 2007, 56, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ali, V.; Pinkney, J.H.; Coppack, S.W. Adipose tissue as an endocrine and paracrine organ. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 1145–1158. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.J.; Porte, D.; Schwartz, M.W. Signals that regulate food intake and energy homeostasis. Science 1998, 280, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Whitehead, J.P.; Richards, A.A.; Hickman, I.J.; Macdonald, G.A.; Prins, J.B. Adiponectin—A key adipokine in the metabolic syndrome. Diabetes Obes. Metab. 2006, 8, 264–280. [Google Scholar] [CrossRef]
- Ng, R.; Chan, K.-H. Potential neuroprotective effects of adiponectin in Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 592. [Google Scholar] [CrossRef]
- Graeber, M.B.; Li, W.; Rodriguez, M.L. Role of microglia in CNS inflammation. FEBS Lett. 2011, 585, 3798–3805. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [PubMed]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M. Hypothalamic dysfunction in obesity. Proc. Nutr. Soc. 2012, 71, 521–533. [Google Scholar] [CrossRef] [PubMed]
- André, C.; Guzman-Quevedo, O.; Rey, C.; Rémus-Borel, J.; Clark, S.; Castellanos-Jankiewicz, A.; Ladeveze, E.; Leste-Lasserre, T.; Nadjar, A.; Abrous, D.N.; et al. Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes 2017, 66, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Cahill, F.; Amini, P.; Wadden, D.; Khalili, S.; Randell, E.; Vasdev, S.; Gulliver, W.; Sun, G. Short-Term overfeeding increases circulating adiponectin independent of obesity status. PLoS ONE 2013, 8, e74215. [Google Scholar] [CrossRef]
| High-Fat Diet (5.24 kcal/g) | Standard Diet (3.85 kcal/g) | |||
|---|---|---|---|---|
| g% | kcal% | g% | kcal% | |
| Protein | 26.2 | 20 | 19.2 | 20 |
| Carbohydrate | 26.3 | 20 | 67.3 | 70 |
| Fat | 34.9 | 60 | 4.3 | 10 |
| g | kcal | g | kcal | |
| Casein80-mesh | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 |
| Cornstarch | 0 | 0 | 315 | 1260 |
| Maltodextrin 10 | 125 | 500 | 35 | 140 |
| Sucrose | 68.8 | 275.2 | 350 | 1400 |
| Cellulose BW200 | 50 | 0 | 50 | 0 |
| Soybean oil | 25 | 225 | 25 | 225 |
| Lard | 245 | 2205 | 20 | 180 |
| Mineral mix S10026 | 10 | 0 | 10 | 0 |
| Dicalcium phosphate | 13 | 0 | 13 | 0 |
| Calcium carbonate | 5.5 | 0 | 5.5 | 0 |
| Potassium citrate | 16.5 | 0 | 16.5 | 0 |
| Vitamin mix V10001 | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2 | 0 | 2 | 0 |
| FD and C dye | 0.05 | 0 | 0.05 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Tu, T.H.; Park, B.S.; Yang, S.; Kim, J.G. Adiponectin Reverses the Hypothalamic Microglial Inflammation during Short-Term Exposure to Fat-Rich Diet. Int. J. Mol. Sci. 2019, 20, 5738. https://doi.org/10.3390/ijms20225738
Lee H, Tu TH, Park BS, Yang S, Kim JG. Adiponectin Reverses the Hypothalamic Microglial Inflammation during Short-Term Exposure to Fat-Rich Diet. International Journal of Molecular Sciences. 2019; 20(22):5738. https://doi.org/10.3390/ijms20225738
Chicago/Turabian StyleLee, Hannah, Thai Hien Tu, Byong Seo Park, Sunggu Yang, and Jae Geun Kim. 2019. "Adiponectin Reverses the Hypothalamic Microglial Inflammation during Short-Term Exposure to Fat-Rich Diet" International Journal of Molecular Sciences 20, no. 22: 5738. https://doi.org/10.3390/ijms20225738
APA StyleLee, H., Tu, T. H., Park, B. S., Yang, S., & Kim, J. G. (2019). Adiponectin Reverses the Hypothalamic Microglial Inflammation during Short-Term Exposure to Fat-Rich Diet. International Journal of Molecular Sciences, 20(22), 5738. https://doi.org/10.3390/ijms20225738





