Design and Synthesis of 4-(Heterocyclic Substituted Amino)-1H-Pyrazole-3-Carboxamide Derivatives and Their Potent Activity against Acute Myeloid Leukemia (AML)
Abstract
:1. Introduction
2. Results and Discussion
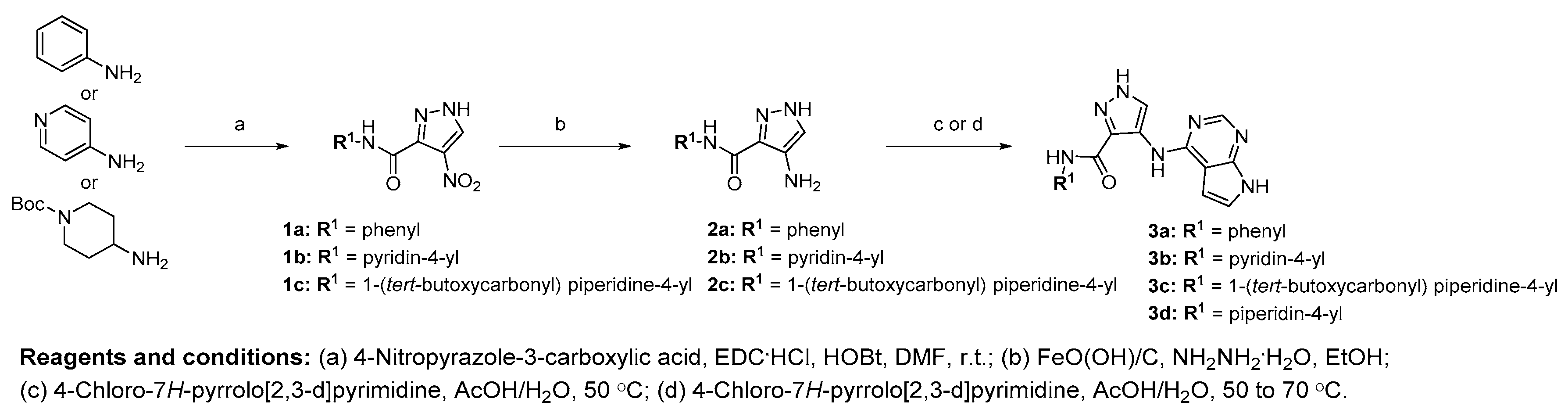
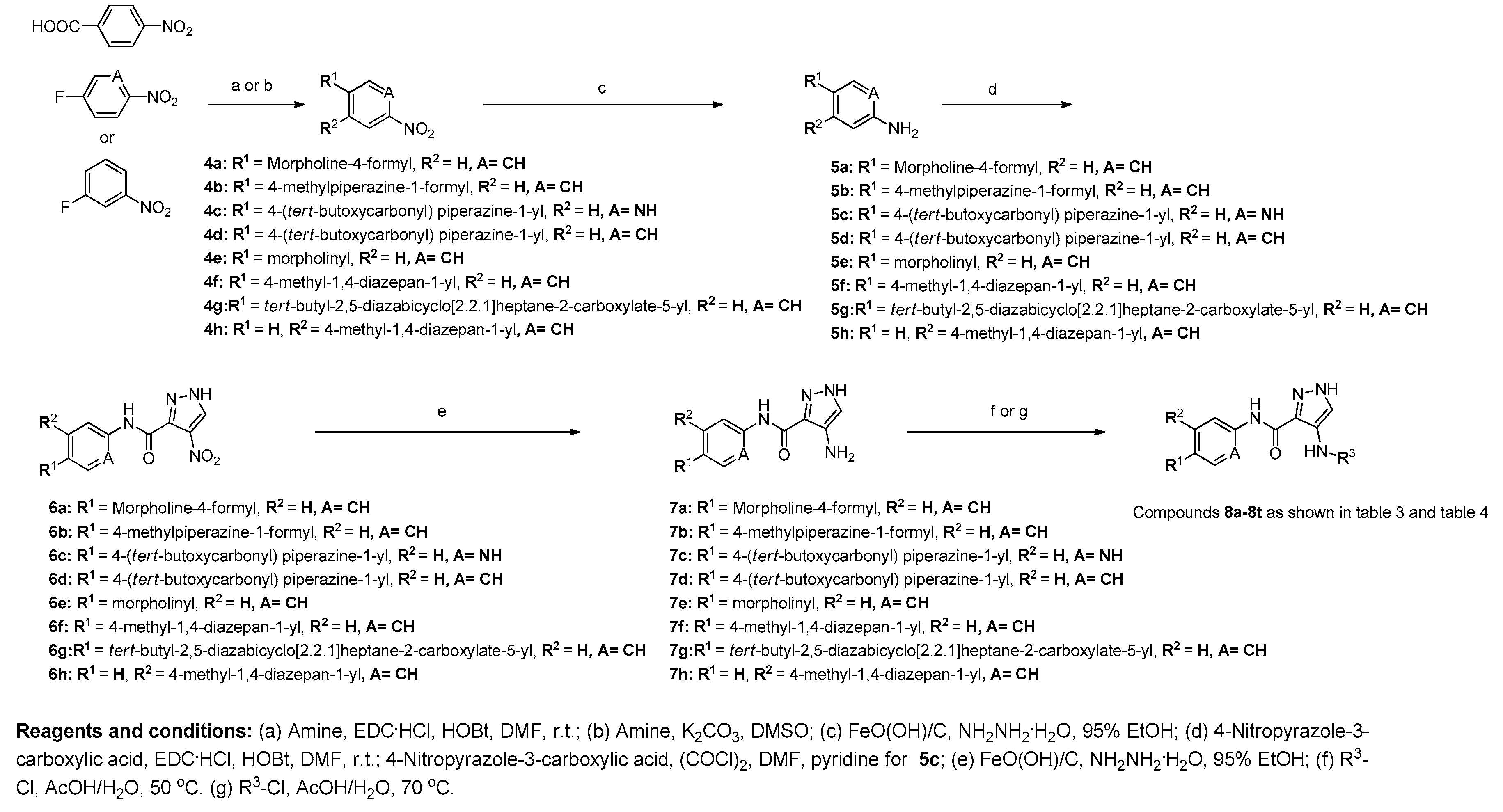
2.1. Chemistry
2.2. Structure-Activity Relationship Study
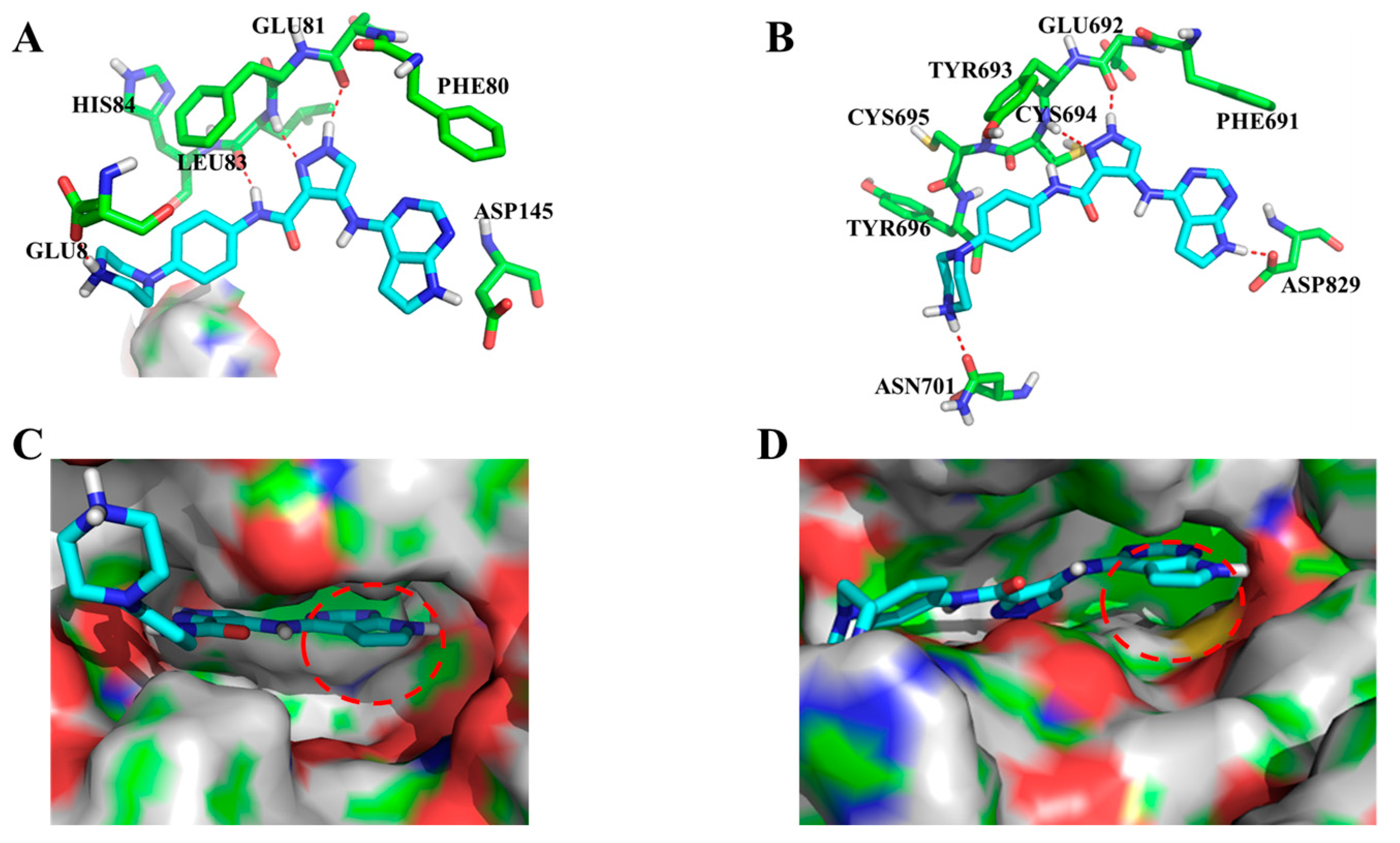
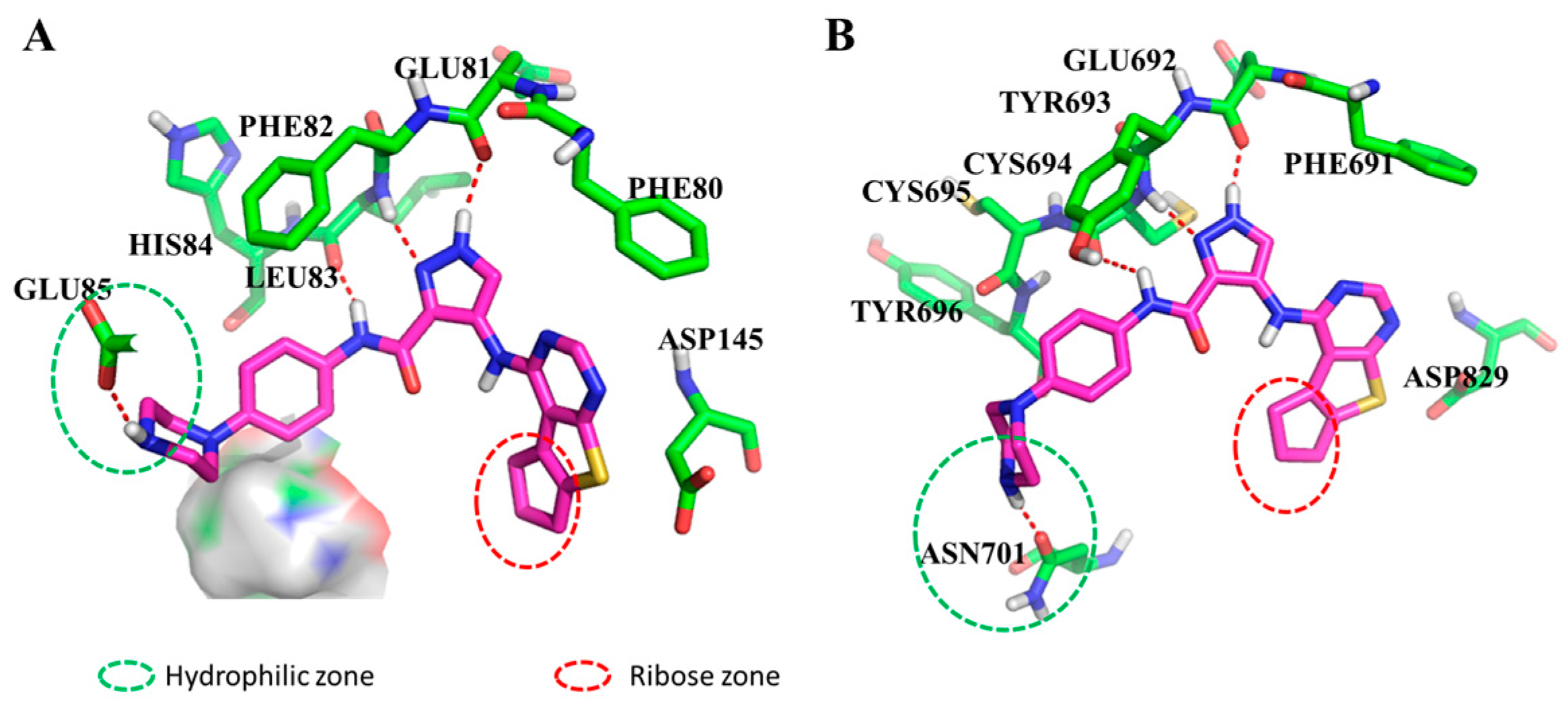
2.3. Molecular Modeling of Compound 8t with CDK2 and FLT3
2.4. Kinase Profiling
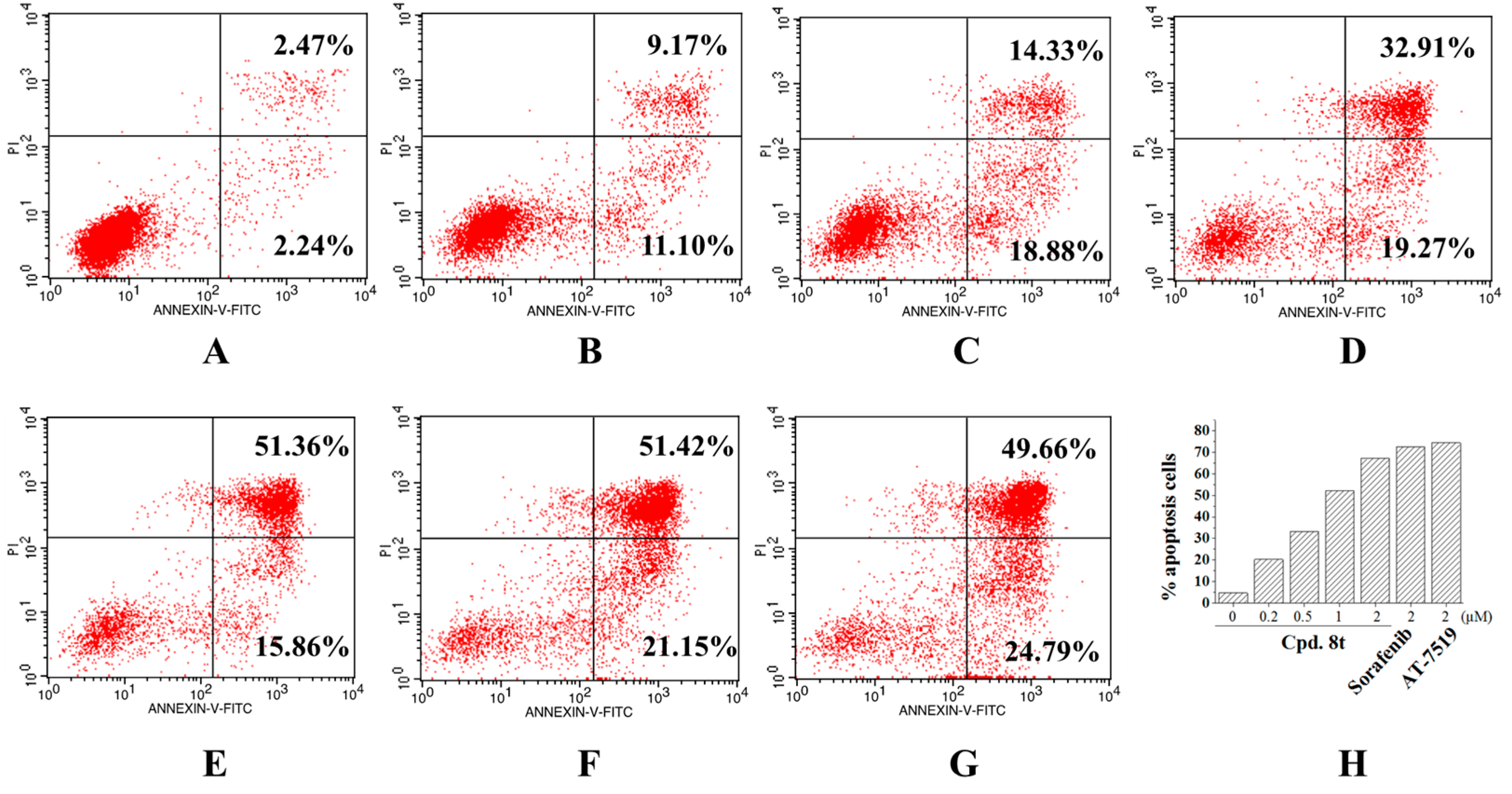
2.5. In vitro Cell Assays
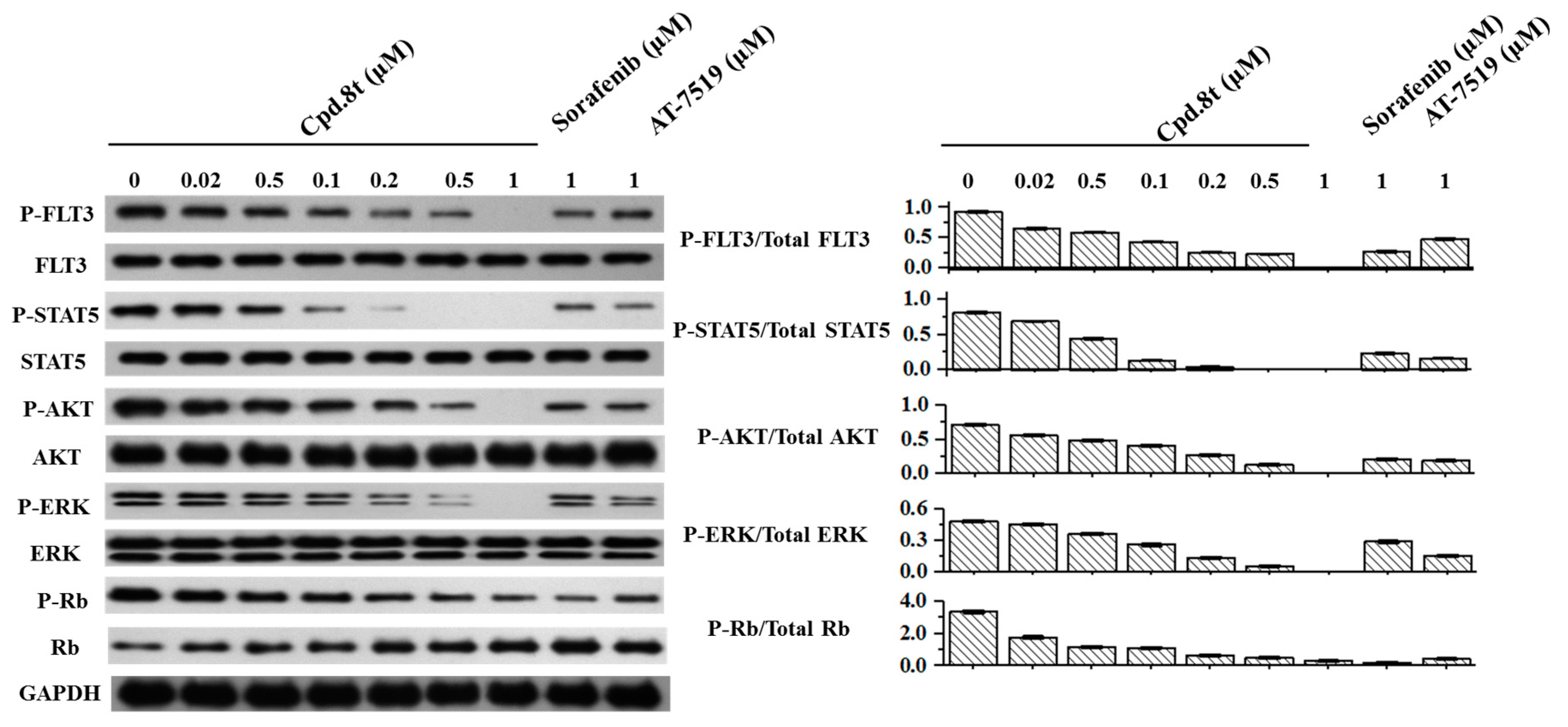
2.6. Cellular Mode of Action
3. Materials and Methods
3.1. Procedure A For the Synthesis of Compounds 4a and 4b
3.2. Procedure B For the Synthesis of Compounds 4c–4h
3.3. Procedure C For the Synthesis of Compounds 2a–2c, 5a–5h, and 7a–7h
3.4. Procedure D for the Synthesis of Compounds 1a–1c and 6a–6h
3.5. Procedure E for the Synthesis of Compounds 3a–3c, 8a–8g, and 8r
3.6. Procedure F for the Synthesis of Compounds 3d, 8h–8q and 8s–8t
3.7. Kinase Inhibition Assay
3.8. Cell Growth Inhibition Assay
3.9. Cell Apoptosis Assay
3.10. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FLT3 | Fms-like receptor tyrosine kinase 3 |
| AML | acute myeloid leukemia |
| ALL | acute lymphoblastic leukemia |
| ITD | internal-tandem duplication |
| TKD | tyrosine kinase domain |
| CDK | cyclin-dependent kinases |
References
- Khwaja, A.; Bjorkholm, M.; Gale, R.E.; Levine, R.L.; Jordan, C.T.; Ehninger, G.; Bloomfield, C.D.; Estey, E.; Burnett, A.; Cornelissen, J.J.; et al. Acute Myeloid Leukaemia. Nat. Rev. Dis. Primers 2016, 19, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, D.G.; Griffin, J.D. Role of Flt3 in Leukemia. Curr. Opin. Hematol. 2002, 9, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting Flt3 Mutations in Aml: Review of Current Knowledge and Evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Rosnet, O.; Buhring, H.J.; Marchetto, S.; Rappold, I.; Lavagna, C.; Sainty, D.; Arnoulet, C.; Chabannon, C.; Kanz, L.; Hannum, C.; et al. Human Flt3/Flk2 Receptor Tyrosine Kinase Is Expressed at the Surface of Normal and Malignant Hematopoietic Cells. Leukemia 1996, 10, 238–248. [Google Scholar] [CrossRef]
- Gilliland, D.G.; Griffin, J.D. The Roles of Flt3 in Hematopoiesis and Leukemia. Blood 2002, 100, 1532–1542. [Google Scholar] [CrossRef]
- Frohling, S.; Schlenk, R.F.; Breitruck, J.; Benner, A.; Kreitmeier, S.; Tobis, K.; Dohner, H.; Dohner, K. Prognostic Significance of Activating Flt3 Mutations in Younger Adults (16 to 60 Years) with Acute Myeloid Leukemia and Normal Cytogenetics: A Study of the Aml Study Group Ulm. Blood 2002, 100, 4372–4380. [Google Scholar] [CrossRef]
- Fathi, A.T.; Chen, Y.B. The Role of Flt3 Inhibitors in the Treatment of Flt3-Mutated Acute Myeloid Leukemia. Eur. J. Haematol. 2017, 98, 330–336. [Google Scholar] [CrossRef]
- Tse, K.F.; Mukherjee, G.; Small, D. Constitutive Activation of Flt3 Stimulates Multiple Intracellular Signal Transducers and Results in Transformation. Leukemia 2000, 14, 1766–1776. [Google Scholar] [CrossRef]
- Takahashi, S. Downstream Molecular Pathways of Flt3 in the Pathogenesis of Acute Myeloid Leukemia: Biology and Therapeutic Implications. J. Hematol. Oncol. 2011, 4, 13. [Google Scholar] [CrossRef]
- Garcia, J.S.; Stone, R.M. The Development of Flt3 Inhibitors in Acute Myeloid Leukemia. Hematol. Oncol. Clin. North. Am. 2017, 31, 663–680. [Google Scholar] [CrossRef]
- Wu, M.; Li, C.T.; Zhu, X.P. Flt3 Inhibitors in Acute Myeloid Leukemia. J. Hematol. Oncol. 2018, 11, 133–144. [Google Scholar] [CrossRef]
- Weisberg, E.; Roesel, J.; Furet, P.; Bold, G.; Imbach, P.; Florsheimer, A.; Caravatti, G.; Jiang, J.; Manley, P.; Ray, A.; et al. Antileukemic Effects of Novel First- and Second-Generation Flt3 Inhibitors: Structure-Affinity Comparison. Genes Cancer 2010, 10, 1021–1032. [Google Scholar] [CrossRef]
- Levis, M. Midostaurin Approved for Flt3-Mutated Aml. Blood 2017, 129, 3403–3406. [Google Scholar] [CrossRef]
- Sudhindra, A.; Smith, C.C. Flt3 Inhibitors in Aml: Are We There Yet? Curr. Hematol. Malig. Rep. 2014, 9, 174–185. [Google Scholar] [CrossRef]
- Zappone, E.; Defina, M.; Aprile, L.; Bartalucci, G.; Gozzetti, A.; Bocchia, M. Flt3 Inhibitors in the Management of Acute Myeloid Leukemia. Anti-Cancer Agents Med. Chem. 2017, 17, 1028–1032. [Google Scholar] [CrossRef]
- Elshoury, A.; Przespolewski, A.; Baron, J.; Wang, E.S. Advancing Treatment of Acute Myeloid Leukemia: The Future of Flt3 Inhibitors. Expert Rev. Anticancer Ther. 2019, 19, 273–286. [Google Scholar] [CrossRef]
- Dhillon, S. Gilteritinib: First Global Approval. Drugs 2019, 79, 331–339. [Google Scholar] [CrossRef]
- Mori, M.; Kaneko, N.; Ueno, Y.; Yamada, M.; Tanaka, R.; Saito, R.; Shimada, I.; Mori, K.; Kuromitsu, S. Gilteritinib, a Flt3/Axl Inhibitor, Shows Antileukemic Activity in Mouse Models of Flt3 Mutated Acute Myeloid Leukemia. Investig. New Drugs 2017, 35, 556–565. [Google Scholar] [CrossRef]
- Zarrinkar, P.P.; Gunawardane, R.N.; Cramer, M.D.; Gardner, M.F.; Brigham, D.; Belli, B.; Karaman, M.W.; Pratz, K.W.; Pallares, G.; Chao, Q.; et al. Ac220 Is a Uniquely Potent and Selective Inhibitor of Flt3 for the Treatment of Acute Myeloid Leukemia (Aml). Blood 2009, 114, 2984–2992. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell Cycle, Cdks and Cancer: A Changing Paradigm. Nat. Rev. Cancer 2019, 9, 153–166. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Blaser, B.W.; Duchemin, A.M.; Kusewitt, D.F.; Liu, T.; Caligiuri, M.A.; Briesewitz, R. Pharmacologic Inhibition of Cdk4/6: Mechanistic Evidence for Selective Activity or Acquired Resistance in Acute Myeloid Leukemia. Blood 2007, 110, 2075–2083. [Google Scholar] [CrossRef]
- Wang, Y.; Zhi, Y.L.; Jin, Q.M.; Lu, S.; Lin, G.W.; Yuan, H.L.; Yang, T.T.; Wang, Z.W.; Yao, C.; Ling, J.; et al. Discovery of 4-((7H-Pyrrolo[2,3-D]Pyrimidin-4-Yl)Amino)-N-(4-((4-Methylpiperazin-1-Yl)Methyl)Phenyl)-1H-Pyrazole-3-Carboxamide (Fn-1501), an Flt3- and Cdk-Kinase Inhibitor with Potentially High Efficiency against Acute Myelocytic Leukemia. J. Med. Chem. 2018, 61, 1499–1518. [Google Scholar] [CrossRef]
- Hatcher, J.M.; Weisberg, E.; Sim, T.; Stone, R.M.; Liu, S.Y.; Griffin, J.D.; Gray, N.S. Discovery of a Highly Potent and Selective Indenoindolone Type 1 Pan-Flt3 Inhibitor. ACS Med. Chem. Lett. 2016, 7, 476–481. [Google Scholar] [CrossRef]
- Kiyoi, H. Flt3 Inhibitors: Recent Advances and Problems for Clinical Application. Nagoya J. Med. Sci. 2015, 77, 7–17. [Google Scholar]
- Auclair, D.; Miller, D.; Yatsula, V.; Pickett, W.; Carter, C.; Chang, Y.; Zhang, X.; Wilkie, D.; Burd, A.; Shi, H.; et al. Antitumor Activity of Sorafenib in Flt3-Driven Leukemic Cells. Leukemia 2007, 21, 439–445. [Google Scholar] [CrossRef]
- Squires, M.S.; Cooke, L.; Lock, V.; Qi, W.Q.; Lewis, E.J.; Thompson, N.T.; Lyons, J.F.; Mahadevan, D. At7519, a Cyclin-Dependent Kinase Inhibitor, Exerts Its Effects by Transcriptional Inhibition in Leukemia Cell Lines and Patient Samples. Mol. Cancer Ther. 2010, 9, 920–928. [Google Scholar] [CrossRef]
- Yang, W.M.; Chen, Y.D.; Zhou, X.; Gu, Y.Z.; Qian, W.Q.; Zhang, F.; Han, W.; Lu, T.; Tang, W.F. Design, Synthesis and Biological Evaluation of Bis-Aryl Ureas and Amides Based on 2-Amino-3-Purinylpyridine Scaffold as Dfg-out B-Raf Kinase Inhibitors. Eur. J. Med. Chem. 2015, 89, 581–596. [Google Scholar] [CrossRef]
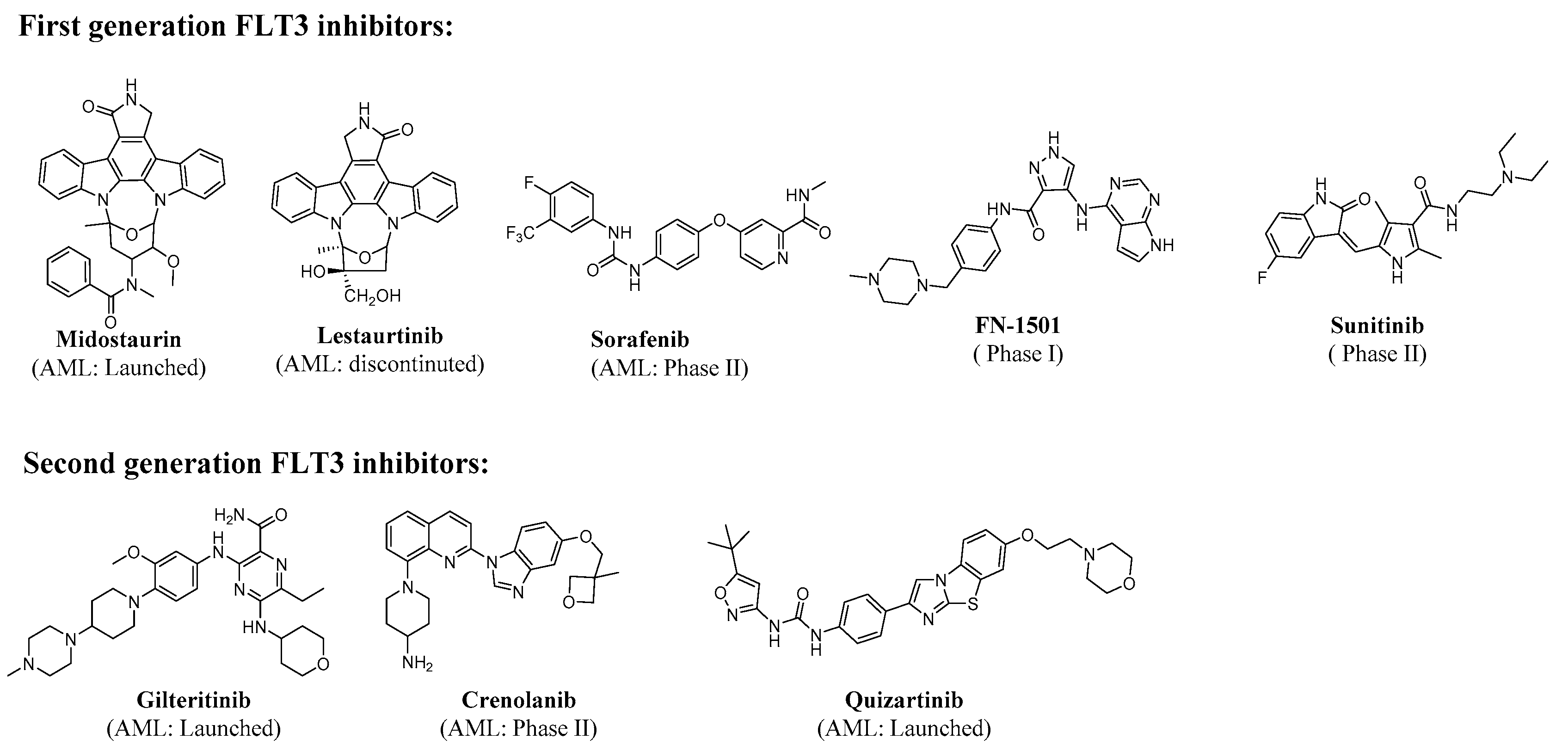
| FLT3 Inhibitors | Selectivity | Generation | Targets | Phases of Development (AML) | |
|---|---|---|---|---|---|
| Sunitinib (Type I) | Non-selective | First-generation | c-KIT, KDR, PDGFR, and FLT3 | Phase II | |
| Lestaurtinib (Type I) | Non-selective | First-generation | Mutant and wild-type FLT3, JAK2, and FLT3 | Phase II | |
| Midostaurin (Type I) | Non-selective | First-generation | FLT3, FLT3-ITD and FLT3-TKD | Launched | |
| Sorafenib (Type II) | Non-selective | First-generation | RAF-1, VEGFR, PDGFR, c-KIT and FLT3 | Phase II | |
| FN-1501 (Type I) | Non-selective | First-generation | FLT3, CDKs | Phase I 1 | |
| Quizartinib (Type II) | Selective | Second-generation | PDGFR, c-KIT, FLT3, CSF-1R and RET | Launched (In Japan) | |
| Gilteritinib (Type II) | Selective | Second-generation | FLT3 and AXL | Launched | |
| Crenolanib (Type II) | Selective | Second-generation | FLT3 and PDGFR α/β | Phase III | |
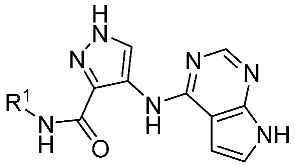
| Cpd. | R 1 | IC50 (nM) 1 | ||
|---|---|---|---|---|
| CDK2 | CDK4 | FLT3 | ||
| FN-1501 |  | 2.33 ± 0.02 | 1.02 ± 0.16 | 0.39 ± 0.07 |
| 3a | 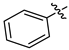 | 0.20 ± 0.01 | 34.13 ± 0.94 | 5.10 ± 0.46 |
| 3b | 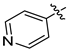 | 4.31 ± 0.91 | 54.24 ± 1.26 | 5.83 ± 0.74 |
| 3c | 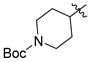 | 32.81 ± 1.34 | 87.07 ± 1.26 | 88.76 ± 1.06 |
| 3d | 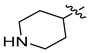 | 63.21 ± 0.91 | 77.37 ± 1.10 | 74.30 ± 1.21 |
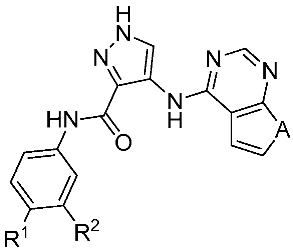
| Cpd. | Structure | IC50 (nM) 1 | IC50 (nM) 2 | ||||
|---|---|---|---|---|---|---|---|
| R 1 | R 2 | A | CDK2 | CDK4 | FLT3 | MV4-11 | |
| FN-1501 | 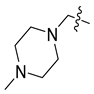 | H | NH | 2.33 ± 0.02 | 1.02 ± 0.16 | 0.39 ± 0.07 | 9 ± 0.27 |
| 8a |  | H | S | 10.39 ± 0.41 | 32.99 ± 0.94 | 17.81 ± 0.89 | 33.10 ± 0. 17 |
| 8b |  | H | S | 8.42 ± 0.52 | 30.14 ± 0.99 | 19.18 ± 0.18 | 35.21 ± 0.83 |
| 8c |  | H | S | 3.51 ± 0.19 | 2.41 ± 0.21 | 0.176 ± 0.09 | 4.28 ± 0.35 |
| 8d |  | H | NH | 2.32 ± 0.014 | 5.32 ± 0.31 | 0.262 ± 0.01 | 9.5 ± 0.01 |
| 8e | H | 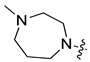 | NH | 5.49 ± 0.42 | 51.035 ± 0.88 | 2.71 ± 0.31 | 38.3 ± 1.21 |
| 8f | H | 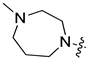 | S | 31.7 ± 0.55 | 67.28 ± 1.09 | 8.07 ± 0.21 | 54.15 ± 1.73 |
| 8g | 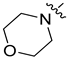 | H | NH | 3.74 ± 0.16 | 10.605 ± 0.24 | 1.945 ± 0.013 | 16.02 ± 0.43 |
| 8h | 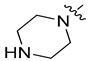 | H | NH | 0.282 ± 0.013 | 1.19 ± 0.09 | 0.038 ± 0.001 | 7.3 ± 0.33 |
| 8i | 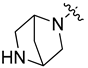 | H | S | 24.53 ± 0.57 | 9.165 ± 0.33 | 3.24 ± 0.14 | 27.21 ± 0.43 |
| 8j | 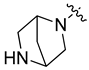 | H | NH | 9.64 ± 0.46 | 17.79 ± 0.82 | 2.81 ± 0.11 | 21.35 ± 0.56 |
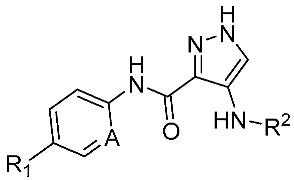
| Cpd. | Structure | IC50 (nM) 1 | IC50 (nM) 2 | ||||
|---|---|---|---|---|---|---|---|
| R 1 | A | R 2 | CDK2 | CDK4 | FLT3 | MV4-11 | |
| 8k | 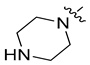 | N | 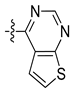 | 315.21 ± 2.30 | 22.3 ± 0.92 | 6.03 ± 0.16 | 51.09 ± 1.34 |
| 8l |  | N | 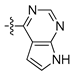 | 156.54 ± 3.22 | 23.11 ± 0.55 | 13.83 ± 0.37 | 133.50 ± 1.64 |
| 8m | 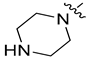 | N | 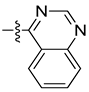 | 133.37 ± 1.74 | 17.53 ± 0.88 | 23.69 ± 0.65 | 143.50 ± 1.21 |
| 8n |  | N |  | 98.72 ± 1.33 | 4.85 ± 0.20 | 1.88 ± 0.09 | 19.92 ± 0.74 |
| 8o |  | N |  | 109.21 ± 1.01 | 1.81 ± 0.023 | 8.28 ± 0.12 | 45.44 ± 1.07 |
| 8p | 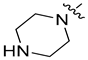 | N | 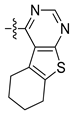 | 86.36 ± 1.36 | 3.81 ± 0.26 | 7.45 ± 0.19 | 27.04 ± 0. 31 |
| 8q |  | N | 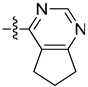 | 508.94 ± 10.33 | 123.37 ± 1.09 | 20.23 ± 0.35 | 458.32 ± 9.20 |
| 8r | 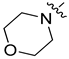 | CH |  | 9.29 ± 0.64 | 24.95 ± 0.29 | 3.80 ± 0.10 | 3.28 ± 0.19 |
| 8s |  | CH |  | 5.43 ± 0.41 | 4.36 ± 0.19 | 0.82 ± 0.003 | 9.13 ± 0.11 |
| 8t |  | CH |  | 0.719 ± 0.064 | 0.770 ± 0.007 | 0.089 ± 0.013 | 1.22 ± 0.06 |
| Kinase | IC50 (nM) |
|---|---|
| Compound 8t | |
| CDK1/cyclin B | 138.03 ± 1.24 |
| CDK2/cyclin A | 2.56 ± 0.31 |
| CDK3/cyclin E | 6.88 ± 0.25 |
| CDK4/cyclin D1 | 0.78 ± 0.04 |
| CDK5/p35 | 9.64 ± 0.81 |
| CDK6/cyclin D1 | 0.59 ± 0.09 |
| CDK7/cyclin H | 21.31 ± 1.01 |
| CDK9/cyclin K | 14.20 ± 0.82 |
| ERK7/MAPK15 | 9.57 ± 0.23 |
| FLT1/VEGFR1 | 12.41 ± 0.19 |
| FLT3 | 0.035 ± 0.01 |
| FLT3 (D835Y) | 0.75 ± 0.04 |
| FLT3 (F594_R595insR) | 0.63 ± 0.09 |
| FLT3 (F594_R595insREY) | 1.38 ± 0.11 |
| FLT3 (ITD)-NPOS | 4.32 ± 0.23 |
| FLT3 (ITD)-W51 | 0.94 ± 0.09 |
| FLT3 (R595_E596insEY) | 1.59 ± 0.14 |
| FLT3 (Y591-V592insVDFREYEYD) | 0.66 ± 0.08 |
| FLT3 (ITD)-F691L | 0.60 ± 0.01 |
| FLT4/VEGFR3 | 4.16 ± 0.19 |
| GSK3β | 11.99 ± 1.20 |
| KDR/VEGFR2 | 8.32 ± 0.54 |
| Panel | Cell Line | GI50 (μM) | Panel | Cell Line | GI50 (μM) |
|---|---|---|---|---|---|
| Leukemia | CCRF-CEM | 0.22 | Melanoma | LOX IMVI | 0.20 |
| HL-60(TB) | 1.15 | MALME-3M | 0.24 | ||
| K-562 | 0.12 | M14 | 0.12 | ||
| MOLT-4 | 0.08 | MDA-MB-435 | 0.11 | ||
| RPMI-8226 | 0.89 | SK-MEL-2 | 1.16 | ||
| SR | 0.06 | SK-MEL-5 | 0.25 | ||
| Non-Small Cell Lung Cancer | A549/ATCC | 0.14 | SK-MEL-28 | 1.13 | |
| EKVX | 0.06 | UACC-257 | 0.48 | ||
| HOP-62 | 0.37 | UACC-62 | 1.09 | ||
| HOP-92 | 0.04 | Ovarian Cancer | IGROV1 | 0.12 | |
| NCI-H226 | 0.32 | OVCAR-3 | 0.23 | ||
| NCI-H23 | 0.26 | OVCAR-4 | 0.22 | ||
| NCI-H322M | 0.14 | OVCAR-5 | 0.27 | ||
| NCI-H460 | 0.06 | OVCAR-8 | 0.36 | ||
| NCI-H522 | 0.21 | NCI/ADR-RES | 1.48 | ||
| Colon Cancer | COLO 205 | 0.12 | SK-OV-3 | 0.26 | |
| HCC-2998 | 1.20 | Renal Cancer | 786-0 | 0.36 | |
| HCT-116 | 0.11 | A498 | 0.14 | ||
| HCT-15 | 0.22 | ACHN | 0.16 | ||
| HT29 | 0.26 | CAKI-1 | 0.08 | ||
| SW-620 | 0.12 | RXF 393 | 0.39 | ||
| KM12 | 0.17 | SN12C | 0.45 | ||
| CNS Cancer | SF-268 | 0.72 | TK-10 | 0.47 | |
| SF-295 | 0.27 | UO-31 | 0.40 | ||
| SF-539 | 0.39 | Breast Cancer | MCF7 | 0.16 | |
| SNB-19 | 0.24 | MDA-MB-231 | 1.93 | ||
| SNB-75 | 0.05 | HS 578T | 0.10 | ||
| U251 | 0.11 | BT-549 | 1.02 | ||
| Prostate Cancer | PC-3 | 0.18 | T-47D | 0.59 | |
| DU-145 | 0.30 | MDA-MB-468 | 0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, Y.; Wang, Z.; Yao, C.; Li, B.; Heng, H.; Cai, J.; Xiang, L.; Wang, Y.; Lu, T.; Lu, S. Design and Synthesis of 4-(Heterocyclic Substituted Amino)-1H-Pyrazole-3-Carboxamide Derivatives and Their Potent Activity against Acute Myeloid Leukemia (AML). Int. J. Mol. Sci. 2019, 20, 5739. https://doi.org/10.3390/ijms20225739
Zhi Y, Wang Z, Yao C, Li B, Heng H, Cai J, Xiang L, Wang Y, Lu T, Lu S. Design and Synthesis of 4-(Heterocyclic Substituted Amino)-1H-Pyrazole-3-Carboxamide Derivatives and Their Potent Activity against Acute Myeloid Leukemia (AML). International Journal of Molecular Sciences. 2019; 20(22):5739. https://doi.org/10.3390/ijms20225739
Chicago/Turabian StyleZhi, Yanle, Zhijie Wang, Chao Yao, Baoquan Li, Hao Heng, Jiongheng Cai, Li Xiang, Yue Wang, Tao Lu, and Shuai Lu. 2019. "Design and Synthesis of 4-(Heterocyclic Substituted Amino)-1H-Pyrazole-3-Carboxamide Derivatives and Their Potent Activity against Acute Myeloid Leukemia (AML)" International Journal of Molecular Sciences 20, no. 22: 5739. https://doi.org/10.3390/ijms20225739
APA StyleZhi, Y., Wang, Z., Yao, C., Li, B., Heng, H., Cai, J., Xiang, L., Wang, Y., Lu, T., & Lu, S. (2019). Design and Synthesis of 4-(Heterocyclic Substituted Amino)-1H-Pyrazole-3-Carboxamide Derivatives and Their Potent Activity against Acute Myeloid Leukemia (AML). International Journal of Molecular Sciences, 20(22), 5739. https://doi.org/10.3390/ijms20225739




