Adaptive Laboratory Evolution of Cupriavidus necator H16 for Carbon Co-Utilization with Glycerol
Abstract
:1. Introduction
1.1. Glycerol in Industrial Biotechnology
1.2. Cupriavidus necator H16 Can Produce Polyhydroxyalkanoate from Glycerol
2. Results and Discussion
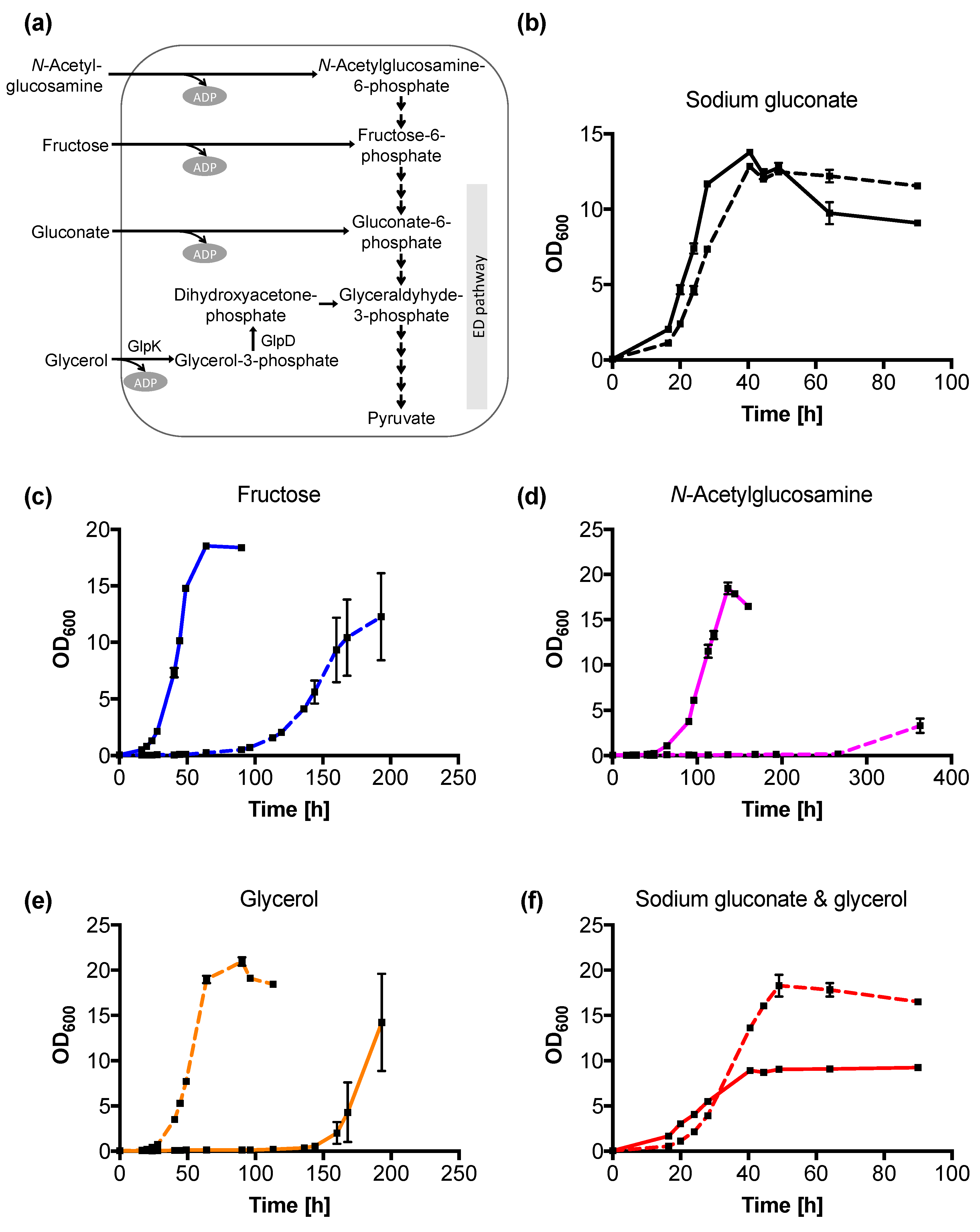
2.1. Glycerol Metabolism in C. necator H16 Wild-Type
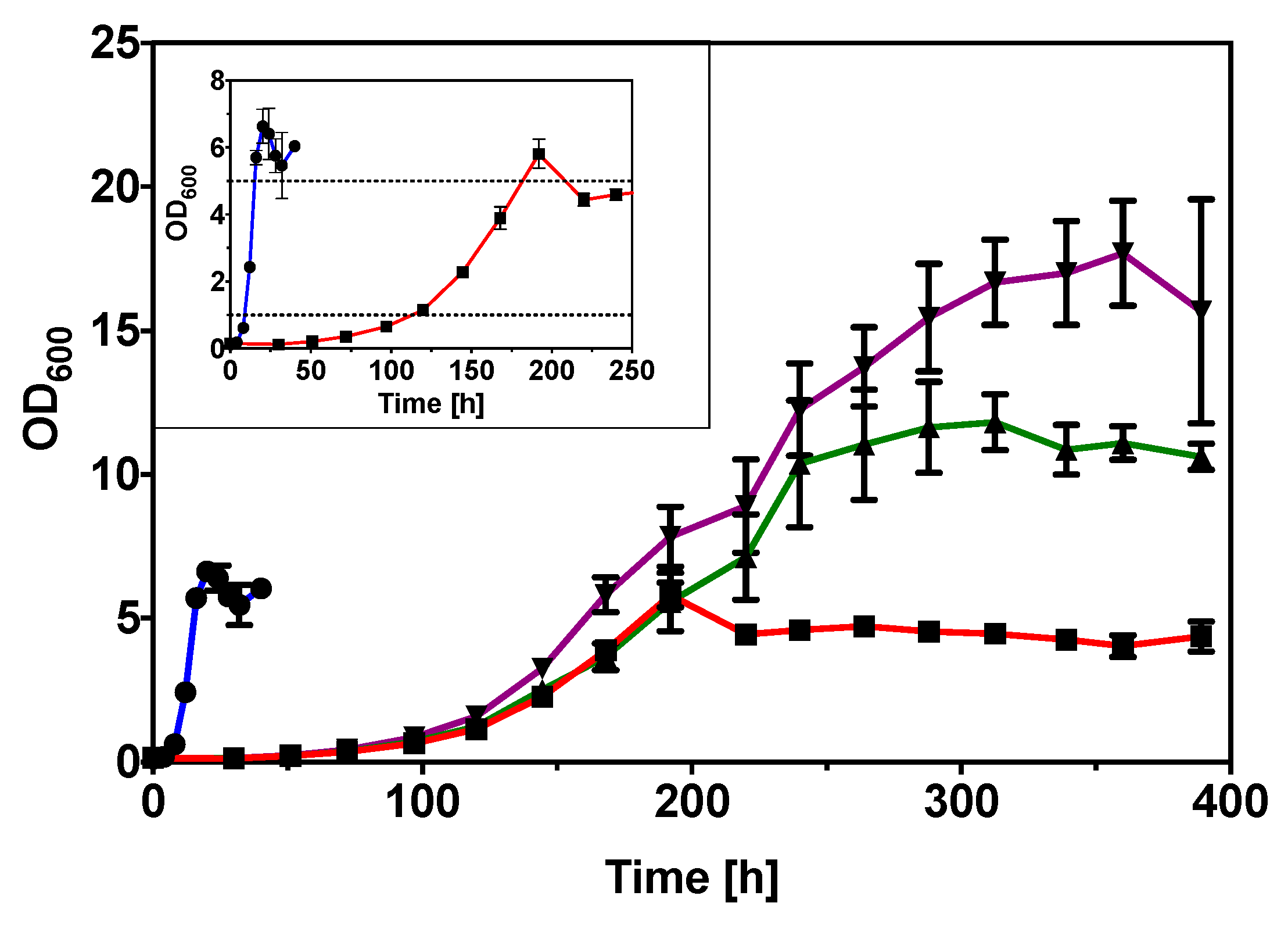
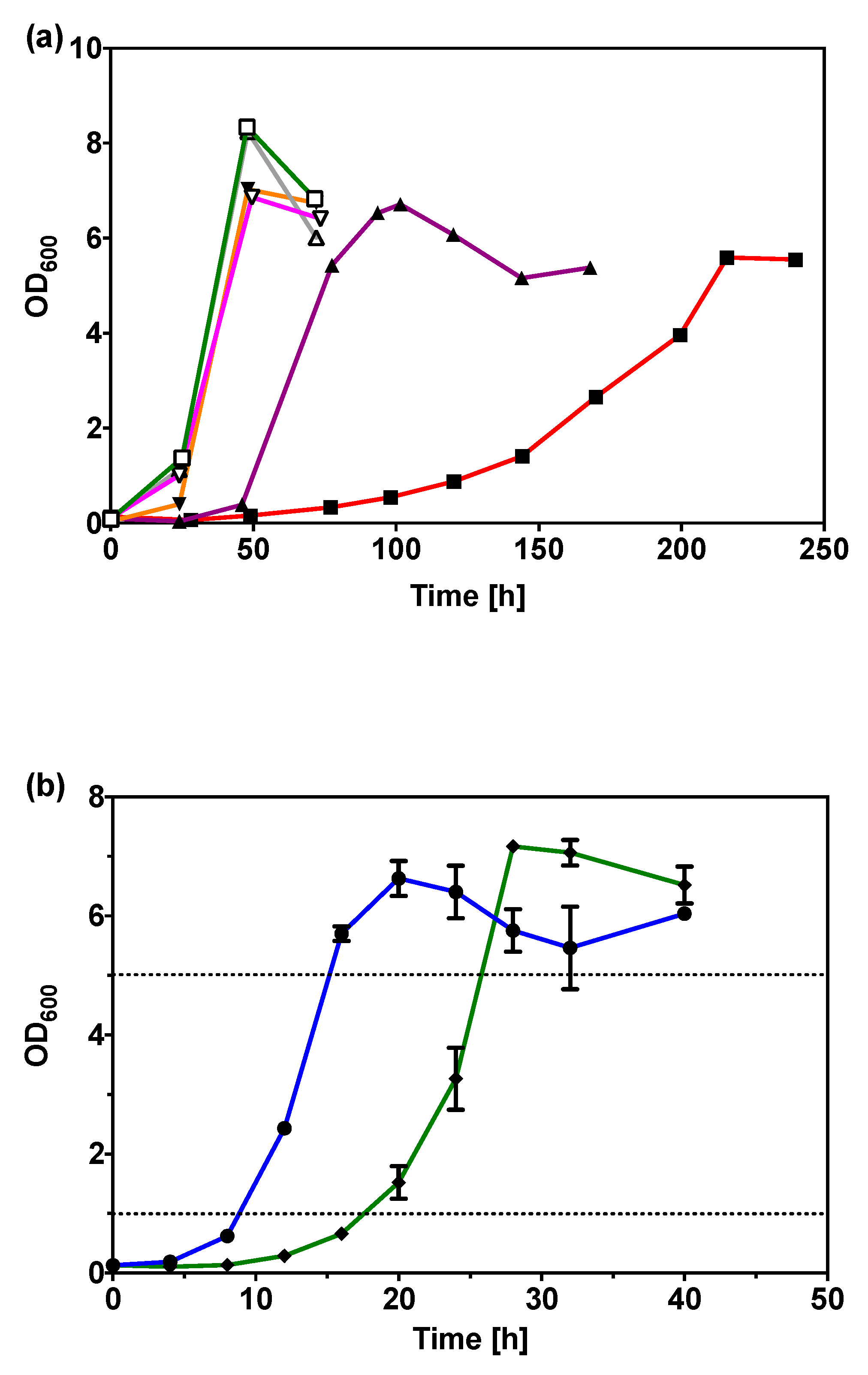
2.2. Improved Glycerol Utilization in C. necator H16 by Adaptive Evolution
2.3. C. necator H16 Variant v6C6 Co-Utilizes Glycerol and Gluconate
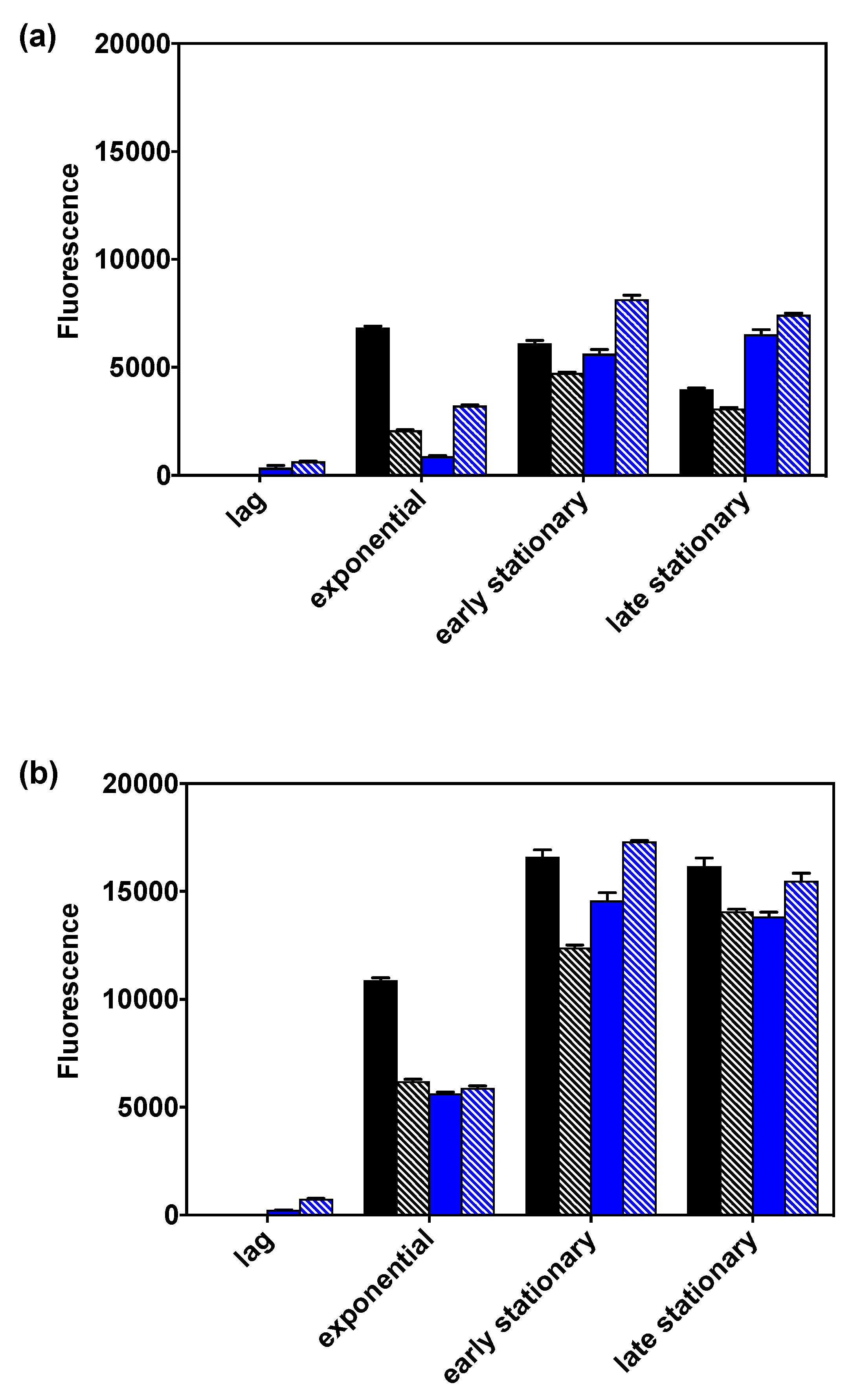
2.4. Variant v6C6 Has Decreased N-acetylglucosamine and Fructose Utilization
2.5. Variant v6C6 Produced More PHB from Glycerol
2.6. Variant v6C6 Showed Superior Growth in Crude Glycerol
2.7. Genome Sequencing of Improved Glycerol Variants
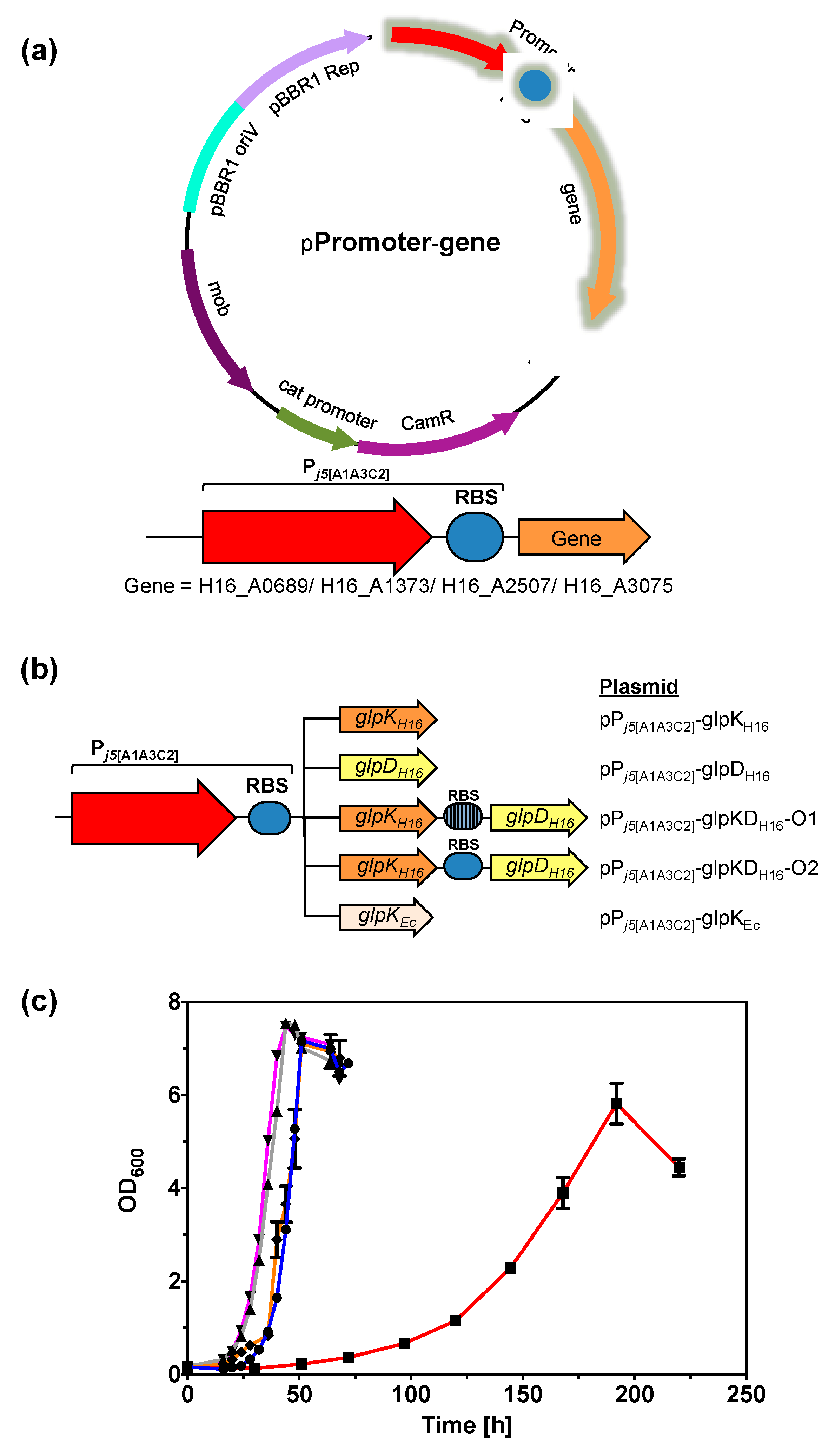
2.8. Expression of GlpK Enhances the Glycerol Metabolic Pathway
3. Methods
3.1. Bacterial Strains, Plasmids, and Cultivation Conditions
3.2. Adaptive Evolution of C. necator H16 for Enhanced Growth in Glycerol
3.3. Confirmation of Improved Glycerol-Utilizing Phenotype
3.4. Carbon Utilization of C. necator H16 Wild-Type and v6C6 in Gluconate–Glycerol Medium
3.5. Growth of C. necator H16 Wild-Type and v6C6 in Different Carbon Sources
3.6. Analysis of PHB Content Using the Nile Red Assay
3.7. Growth of C. necator H16 in Crude Glycerol (Sweetwater)
3.8. Genome Sequencing
3.9. Expression of GlpK and/or glpD Genes in C. necator H16–Plasmid Construction and Bacterial Cultivation
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| PHB | polyhydroxybutyrate |
| MSM | mineral salts medium |
| GlpK | glycerol kinase |
| GlpD | glycerol dehydrogenase |
| rbs | ribosome binding site |
| DHAP | dihydroxyacetone-phosphate |
References
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—a byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Matthews, S.; Belcher, J.D.; Tee, K.L.; Girvan, H.M.; McLean, K.J.; Rigby, S.E.J.; Levy, C.W.; Leys, D.; Parker, D.A.; Blankley, R.T.; et al. Catalytic determinants of alkene production by the cytochrome P450 peroxygenase OleTJE. J. Biol. Chem. 2017, 292, 5128–5143. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Gray, V.; Rumbold, K. Microbial utilization of crude glycerol for the production of value-added products. J. Ind. Microbiol. Biotechnol. 2012, 39, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, A.; Zhao, C.; Budin-Verneuil, A.; Sauvageot, N.; Giard, J.C.; Auffray, Y.; Hartke, A. Glycerol is metabolized in a complex and strain-dependent manner in Enterococcus faecalis. J. Bacteriol. 2010, 192, 779–785. [Google Scholar] [CrossRef]
- Joseph, B.; Przybilla, K.; Stühler, C.; Schauer, K.; Slaghuis, J.; Fuchs, T.M.; Goebel, W. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J. Bacteriol. 2006, 188, 556–568. [Google Scholar] [CrossRef]
- Hames, C.; Halbedel, S.; Hoppert, M.; Frey, J.; Stülke, J. Glycerol metabolism is important for cytotoxicity of mycoplasma pneumoniae. J. Bacteriol. 2009, 191, 747–753. [Google Scholar] [CrossRef]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome sequence of the bioplastic-producing "Knallgas" bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef]
- Raberg, M.; Volodina, E.; Lin, K.; Steinbüchel, A. Ralstonia eutropha H16 in progress: Applications beside PHAs and establishment as production platform by advanced genetic tools. Crit. Rev. Biotechnol. 2018, 38, 494–510. [Google Scholar] [CrossRef]
- Johnson, A.O.; Gonzalez-Villanueva, M.; Tee, K.L.; Wong, T.S. An engineered constitutive promoter set with broad activity range for Cupriavidus necator H16. ACS Synth. Biol. 2018, 7, 1918–1928. [Google Scholar] [CrossRef]
- Volodina, E.; Raberg, M.; Steinbüchel, A. Engineering the heterotrophic carbon sources utilization range of Ralstonia eutropha H16 for applications in biotechnology. Crit. Rev. Biotechnol. 2016, 36, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Claret, C.; Bories, A.; Soucaille, P. Glycerol inhibition of growth and dihydroxyacetone production by Gluconobacter Oxydans. Curr. Microbiol. 1992, 25, 149–155. [Google Scholar] [CrossRef]
- Roger, V.; Fonty, G.; Andre, C.; Gouet, P. Effects of glycerol on the growth, adhesion, and cellulolytic activity of rumen cellulolytic bacteria and anaerobic fungi. Curr. Microbiol. 1992, 25, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska-Powalowska, D. The effect of high concentrations of glycerol on the growth, metabolism and adaptation capacity of Clostridium butyricum DSP1. Electron. J. Biotechnol. 2015, 18, 128–133. [Google Scholar] [CrossRef]
- Rittmann, D.; Lindner, S.N.; Wendisch, V.F. Engineering of a glycerol utilization pathway for amino acid production by Corynebacterium glutamicum. Appl. Environ. Microbiol. 2008, 74, 6216–6222. [Google Scholar] [CrossRef]
- Meiswinkel, T.M.; Rittmann, D.; Lindner, S.N.; Wendisch, V.F. Crude glycerol-based production of amino acids and putrescine by Corynebacterium glutamicum. Bioresour. Technol. 2013, 145, 254–258. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.Q.; Zhang, T.; Fan, L.H.; Tan, T.W. Multi-modular engineering of 1,3-propanediol biosynthesis system in Klebsiella pneumoniae from co-substrate. Appl. Microbiol. Biotechnol. 2017, 101, 647–657. [Google Scholar] [CrossRef]
- Dragosits, M.; Mattanovich, D. Adaptive laboratory evolution—principles and applications for biotechnology. Microb. Cell Fact. 2013, 12, 64. [Google Scholar] [CrossRef]
- Herring, C.D.; Raghunathan, A.; Honisch, C.; Patel, T.; Applebee, M.K.; Joyce, A.R.; Albert, T.J.; Blattner, F.R.; van den Boom, D.; Cantor, C.R.; et al. Comparative genome sequencing of Escherichia coli allows observation of bacterial evolution on a laboratory timescale. Nat. Genet. 2006, 38, 1406–1412. [Google Scholar] [CrossRef]
- Schlegel, H.G.; Kaltwasser, H.; Gottschalk, G. A submersion method for culture of hydrogen-oxidizing bacteria: Growth physiological studies. Arch. Mikrobiol 1961, 38, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.; Rehner, R.; Kienle, A.; Grammel, H. Rapid selection of glucose-utilizing variants of the polyhydroxyalkanoate producer Ralstonia eutropha H16 by incubation with high substrate levels. Lett. Appl. Microbiol. 2012, 54, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Wang, Y.; Tao, F.; Li, L.; Ma, C.; Xu, P. Co-utilization of glycerol and lignocellulosic hydrolysates enhances anaerobic 1,3-propanediol production by Clostridium diolis. Sci. Rep. 2016, 6, 19044. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, S.; Komon-Janczara, E.; Glibowska, A.; Kuzdralinski, A.; Czernecki, T.; Targonski, Z. A co-utilization strategy to consume glycerol and monosaccharides by Rhizopus strains for fumaric acid production. AMB Express. 2018, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Mukoyama, M.; Orita, I.; Nakamura, S. Enhancement of glycerol utilization ability of Ralstonia eutropha H16 for production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 7559–7568. [Google Scholar] [CrossRef]
- Kaddor, C.; Steinbuchel, A. Effects of homologous phosphoenolpyruvate-carbohydrate phosphotransferase system proteins on carbohydrate uptake and poly(3-Hydroxybutyrate) accumulation in Ralstonia eutropha H16. Appl. Environ. Microbiol. 2011, 77, 3582–3590. [Google Scholar] [CrossRef]
- Tyo, K.E.; Zhou, H.; Stephanopoulos, G.N. High-throughput screen for poly-3-hydroxybutyrate in Escherichia coli and Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 2006, 72, 3412–3417. [Google Scholar] [CrossRef]
- Lee, S.Y. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996, 14, 431–438. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32, W327–W331. [Google Scholar] [CrossRef]
- Anderson, M.J.; DeLabarre, B.; Raghunathan, A.; Palsson, B.O.; Brunger, A.T.; Quake, S.R. Crystal structure of a hyperactive Escherichia coli glycerol kinase mutant Gly230 --> Asp obtained using microfluidic crystallization devices. Biochemistry 2007, 46, 5722–5731. [Google Scholar] [CrossRef]
- Yeh, J.I.; Charrier, V.; Paulo, J.; Hou, L.; Darbon, E.; Claiborne, A.; Hol, W.G.; Deutscher, J. Structures of enterococcal glycerol kinase in the absence and presence of glycerol: Correlation of conformation to substrate binding and a mechanism of activation by phosphorylation. Biochemistry 2004, 43, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Chou, K.; Orita, I.; Suzuki, Y.; Nakamura, S.; Fukui, T. Detection of phase-dependent transcriptomic changes and Rubisco-mediated CO2 fixation into poly (3-hydroxybutyrate) under heterotrophic condition in Ralstonia eutropha H16 based on RNA-seq and gene deletion analyses. BMC Microbiol. 2013, 13, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allaman, I.; Belanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, H.G.; Gottschalk, G.; Von Bartha, R. Formation and utilization of poly-beta-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature 1961, 191, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tee, K.L.; Grinham, J.; Othusitse, A.M.; Gonzalez-Villanueva, M.; Johnson, A.O.; Wong, T.S. An efficient transformation method for the bioplastic-producing "Knallgas" bacterium Ralstonia eutropha H16. Biotechnol. J. 2017. [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]


| Strain | Plasmid | Carbon Source | Specific Growth Rate (h−1) |
|---|---|---|---|
| H16 | n.a. | 0.59% (w/v) sodium gluconate | (2.4 ± 0.2) × 10−1 |
| H16 | n.a. | 0.5% (w/v) glycerol | (2.1 ± 0.1) × 10−2 |
| H16 | n.a. | 1.0% (w/v) glycerol | (2.0 ± 0.2) × 10−2 |
| H16 | n.a. | 2.0% (w/v) glycerol | (2.6 ± 0.2) × 10−2 |
| v6C6 | n.a. | 0.5% (w/v) glycerol | (2.0 ± 0.1) × 10−1 |
| v6C6 | n.a. | 1.0% (w/v) glycerol | (1.8 ± 0.1) × 10−1 |
| H16 | pPj5[A1A3C2]-glpKv6C6 | 1.0% (w/v) glycerol | (0.8 ± 0.0) × 10−1 |
| H16 | pPj5[A1A3C2]-glpKH16 | 0.5% (w/v) glycerol | (1.4 ± 0.1) × 10−1 |
| H16 | pPj5[A1A3C2]-glpDH16 | 0.5% (w/v) glycerol | - |
| H16 | pPj5[A1A3C2]-glpKDH16-O1 | 0.5% (w/v) glycerol | (1.3 ± 0.0) × 10−1 |
| H16 | pPj5[A1A3C2]-glpKDH16-O2 | 0.5% (w/v) glycerol | (1.4 ± 0.0) × 10−1 |
| H16 | pPj5[A1A3C2]-glpKEc | 0.5% (w/v) glycerol | (1.5 ± 0.1) × 10−1 |
| v6C6 | pPj5[A1A3C2]-glpKH16 | 0.5% (w/v) glycerol | (2.1 ± 0.1) × 10−1 |
| Strain | Crude Glycerol (% (v/v)) | Specific Growth Rate (h−1) |
|---|---|---|
| H16 | 1 | (2.3 ± 0.1) × 10−2 |
| H16 | 2 | (3.1 ± 0.2) × 10−2 |
| H16 | 4 | (4.8 ± 0.4) × 10−2 |
| v6C6 | 1 | (1.7 ± 0.5) × 10−1 |
| v6C6 | 2 | (2.2 ± 0.3) × 10−1 |
| v6C6 | 4 | (2.2 ± 0.1) × 10−1 |
| Chromosome | Gene Position | Mutation | Amino Acid Substitution | Strains with Mutation | Locus Tag | Product or Function (Protein ID) |
|---|---|---|---|---|---|---|
| 1 | 738,900 | T→A | M129K | v6C6 v6F8 v6G7 | H16_A0689 | Ornithine cyclodeaminase (WP_010813066.1) |
| 1 | 1,486,642 | G→C | S736R | v6C6 | H16_A1373 | Histidine kinase (WP_037024292.1) |
| 1 | 1,487,637 | Deletion (2 bp) | n.a. | v6F8 v6G7 | H16_A1373 | Histidine kinase (WP_037024292.1) |
| 1 | 1,864,239 | Insertion (42 bp) | n.a. | v6F8 v6G7 | n.a. | n.a. |
| 1 | 2,723,382 | G→C | W480S | v6C6 v6F8 v6G7 | H16_A2507 | Glycerol kinase (WP_011615701.1) |
| 1 | 3,328,089 | A→C | N261K | v6C6 v6F8 v6G7 | H16_A3075 | Dihydropyrimidinase (WP_041687507.1) |
| Bacterial Strains and Plasmids | Description | Reference or Source |
|---|---|---|
| Bacterial Strains | ||
| E. coli DH5α | Standard cloning strain | Lab collection |
| E. coli BW25113 | Wild-type | [35] |
| C. necator H16 | Wild-type | DSM 428 |
| v6C6 | Variant of C. necator H16 with improved glycerol utilization | This study |
| Plasmids | ||
| pBBR1c-RFP | CamR, mob, pBBR1 Rep, pBBR1 oriV, araC, PBAD, rfp | This study |
| pPj5[C2]-RFP | CamR, mob, pBBR1 Rep, pBBR1 oriV, Pj5[C2], rfp | This study |
| pPj5[A1A3C2]-glpKEc | CamR, mob, pBBR1 Rep, pBBR1 oriV, Pj5[A1A3C2], glpKEc | This study |
| pPj5[A1A3C2]-glpKH16 | CamR, mob, pBBR1 Rep, pBBR1 oriV, Pj5[A1A3C2], glpKH16 | This study |
| pPj5[A1A3C2]-glpDH16 | CamR, mob, pBBR1 Rep, pBBR1 oriV, Pj5[A1A3C2], glpDH16 | This study |
| pPj5[A1A3C2]-glpKDH16-O1 | CamR, mob, pBBR1 Rep, pBBR1 oriV, Pj5[A1A3C2], glpKDH16, native glpDH16 rbs preceding glpDH16 | This study |
| pPj5[A1A3C2]-glpKDH16-O2 | CamR, mob, pBBR1 Rep, pBBR1 oriV, Pj5[A1A3C2], glpKDH16, synthetic rbs preceding glpDH16 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Villanueva, M.; Galaiya, H.; Staniland, P.; Staniland, J.; Savill, I.; Wong, T.S.; Tee, K.L. Adaptive Laboratory Evolution of Cupriavidus necator H16 for Carbon Co-Utilization with Glycerol. Int. J. Mol. Sci. 2019, 20, 5737. https://doi.org/10.3390/ijms20225737
González-Villanueva M, Galaiya H, Staniland P, Staniland J, Savill I, Wong TS, Tee KL. Adaptive Laboratory Evolution of Cupriavidus necator H16 for Carbon Co-Utilization with Glycerol. International Journal of Molecular Sciences. 2019; 20(22):5737. https://doi.org/10.3390/ijms20225737
Chicago/Turabian StyleGonzález-Villanueva, Miriam, Hemanshi Galaiya, Paul Staniland, Jessica Staniland, Ian Savill, Tuck Seng Wong, and Kang Lan Tee. 2019. "Adaptive Laboratory Evolution of Cupriavidus necator H16 for Carbon Co-Utilization with Glycerol" International Journal of Molecular Sciences 20, no. 22: 5737. https://doi.org/10.3390/ijms20225737
APA StyleGonzález-Villanueva, M., Galaiya, H., Staniland, P., Staniland, J., Savill, I., Wong, T. S., & Tee, K. L. (2019). Adaptive Laboratory Evolution of Cupriavidus necator H16 for Carbon Co-Utilization with Glycerol. International Journal of Molecular Sciences, 20(22), 5737. https://doi.org/10.3390/ijms20225737







