Neuroplasticity in Cholinergic Projections from the Basal Forebrain to the Basolateral Nucleus of the Amygdala in the Kainic Acid Model of Temporal Lobe Epilepsy
Abstract
1. Introduction
2. Results
2.1. Monitoring for Spontaneous Seizures
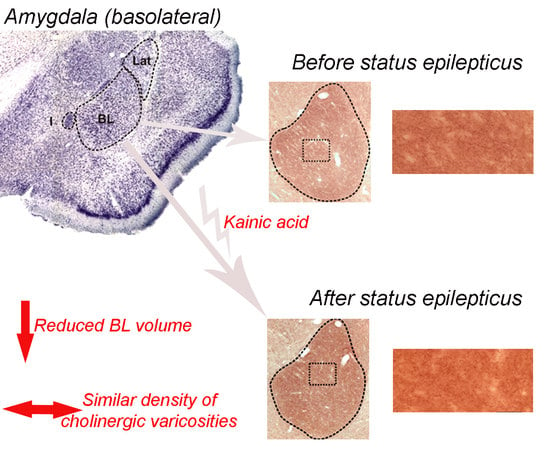
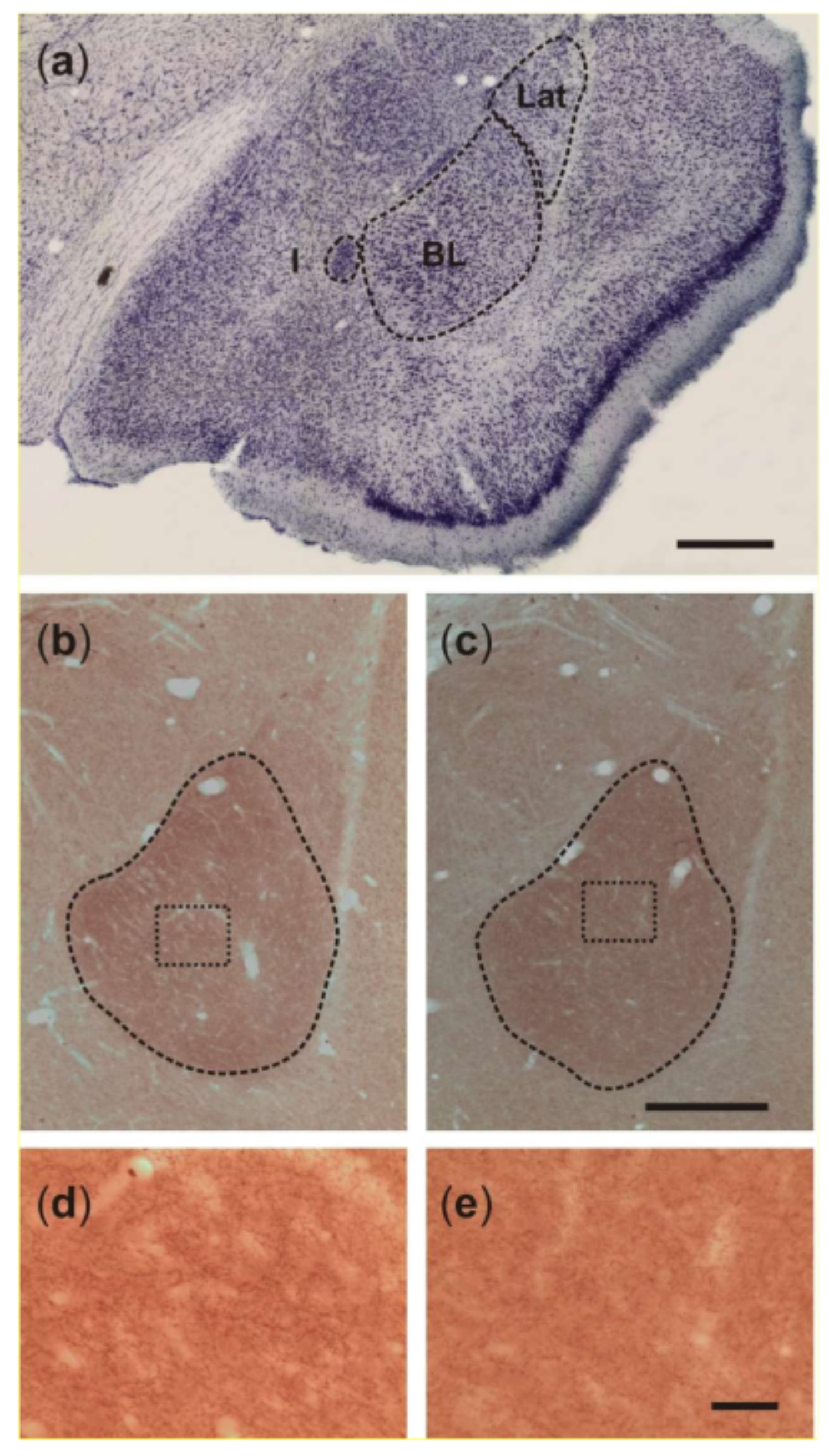
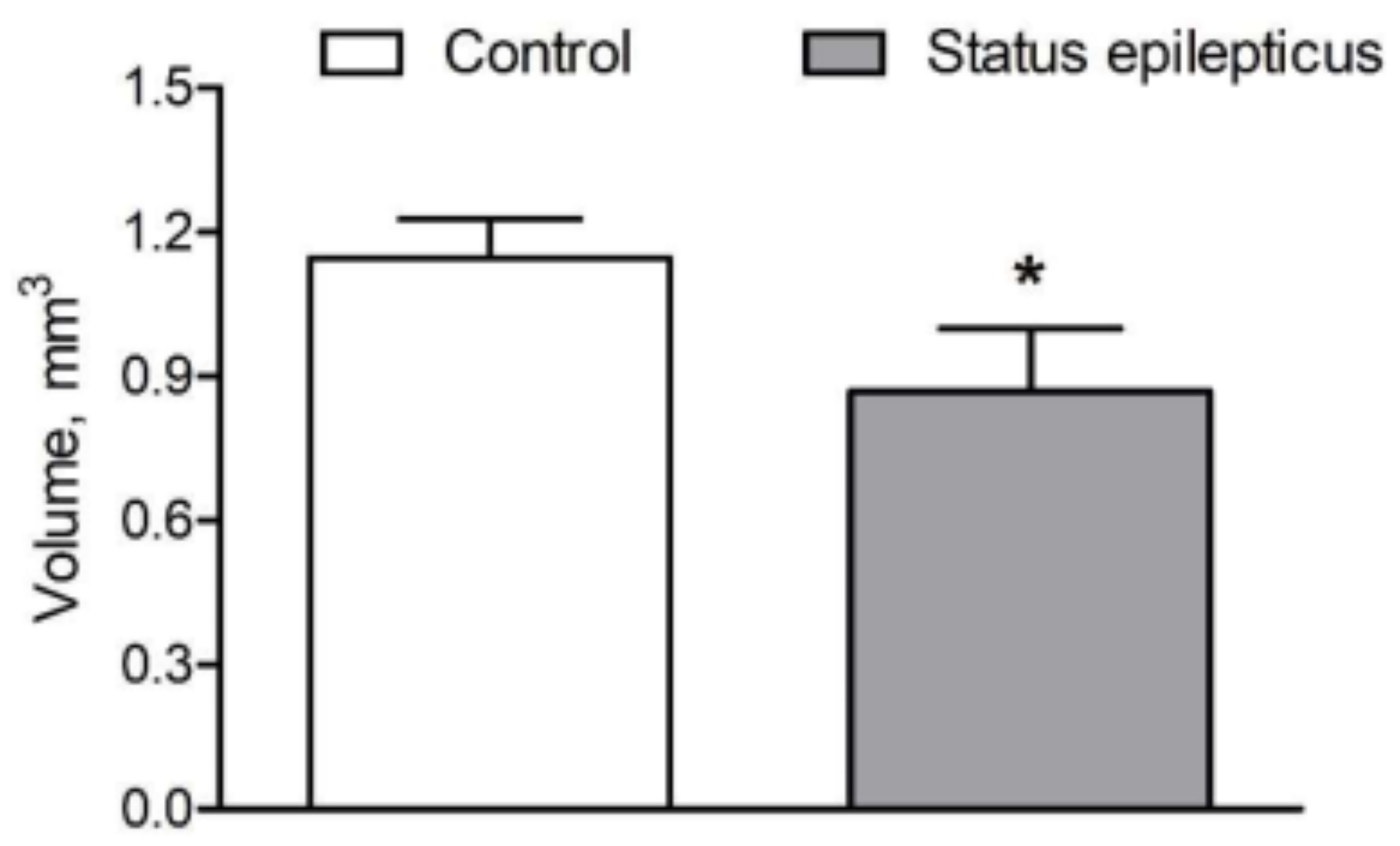
2.2. Basolateral Nucleus Volume
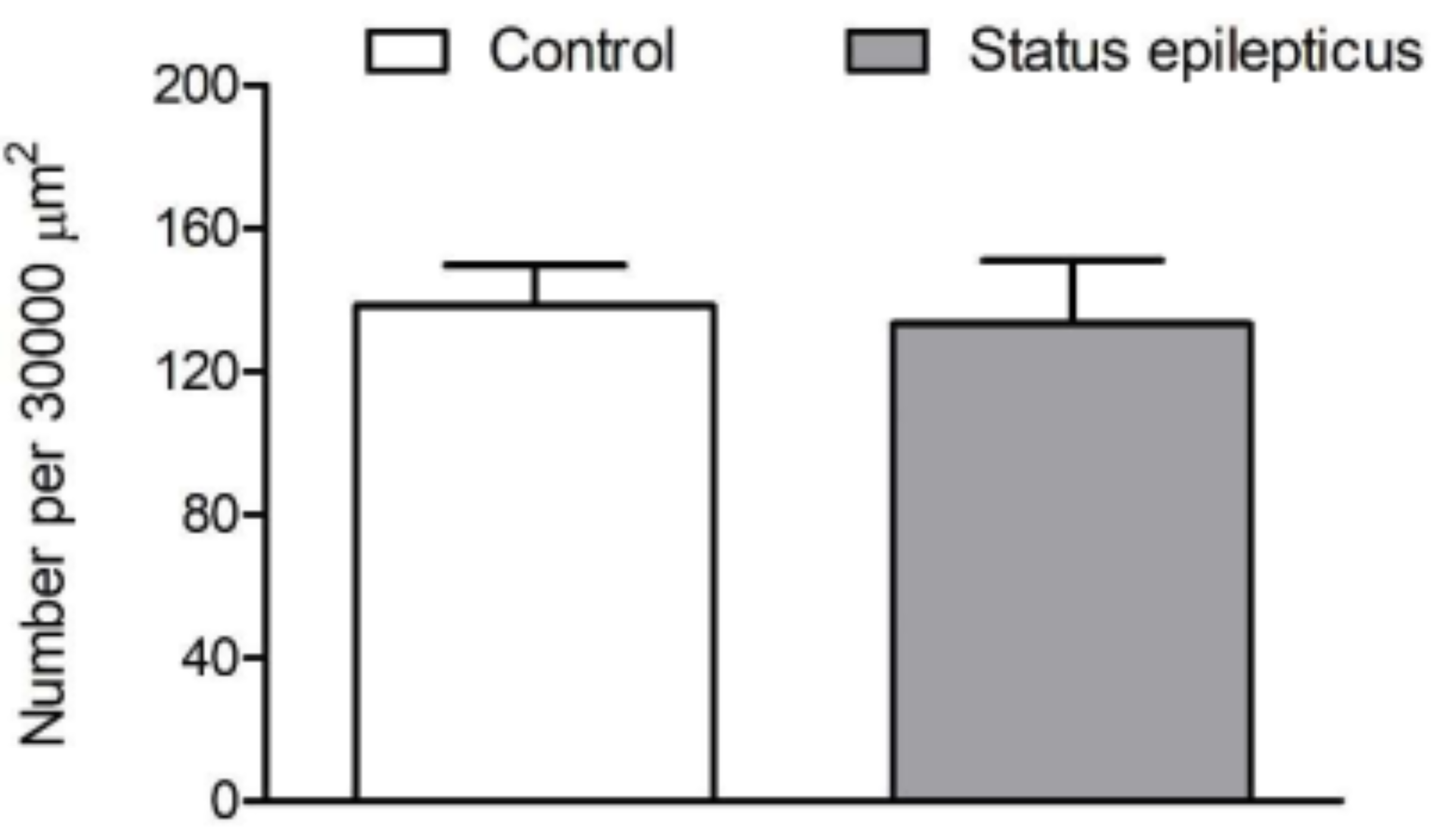
2.3. Density of VAChT-Immunoreactive Fiber Varicosities
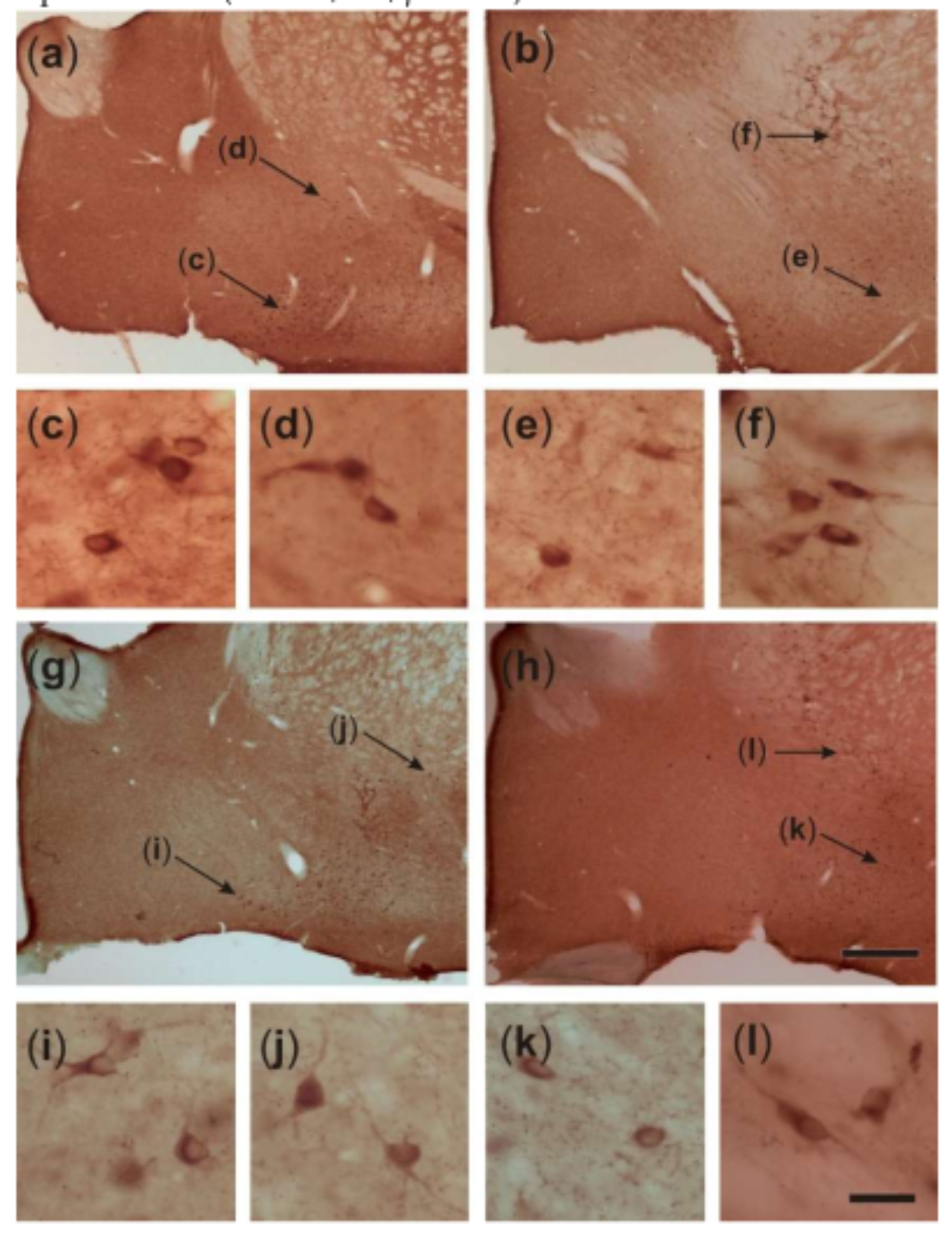
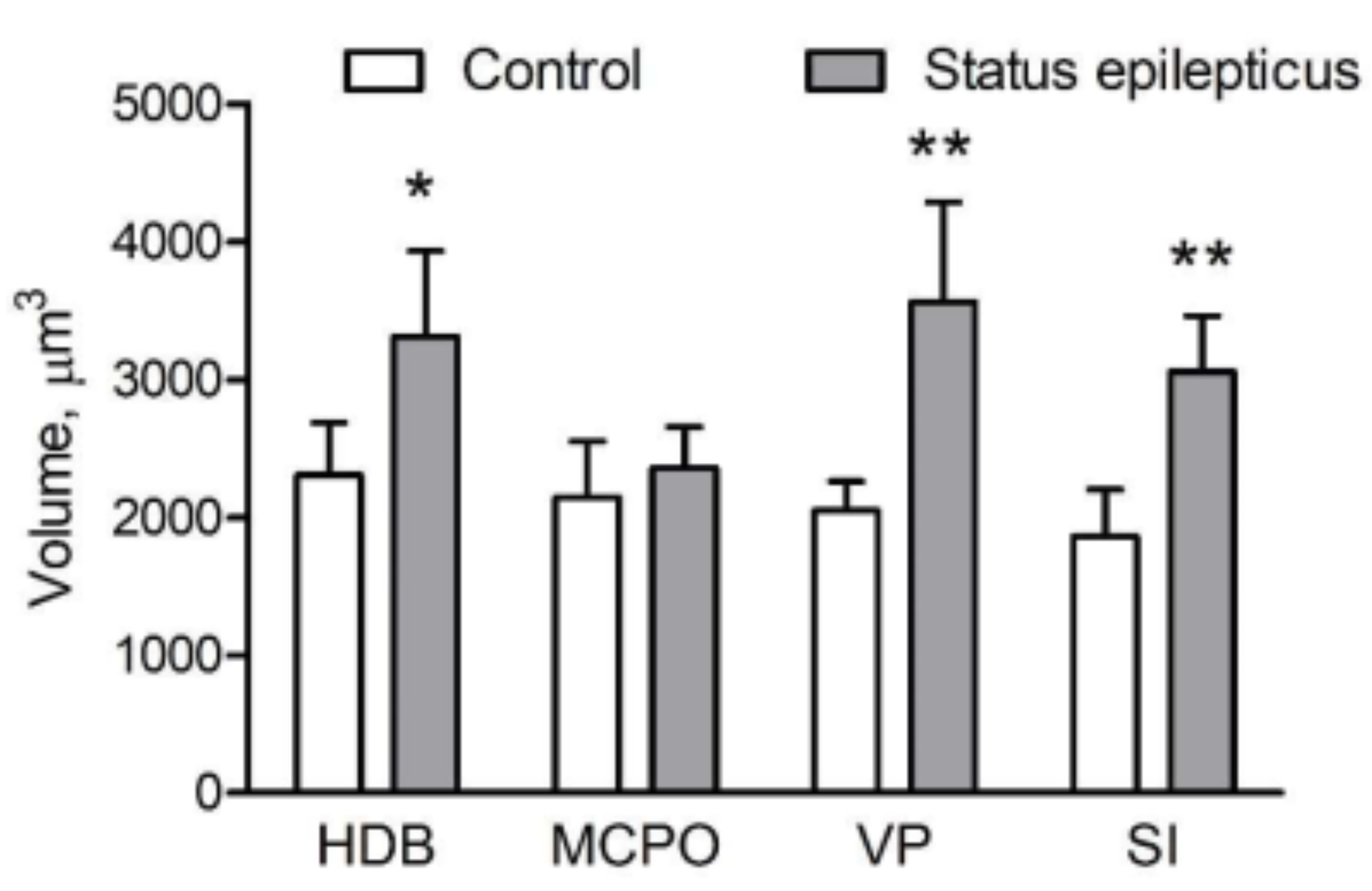
2.4. Somatic Volume of VAChT-Immunoreactive Cells
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Animals and Treatments
4.3. Immunohistochemistry and Histology
4.4. Density of VAChT-Immunoreactive Varicosities
4.5. Estimation of BL Volume
4.6. Estimation of Somatic Volume of VAChT-Immunoreactive Neurons
4.7. Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TLE | temporal lobe epilepsy |
| KA | kainic acid |
| SE | status epilepticus |
| LD | linear dichroism |
| MS | medial septum |
| VDB | vertical limb of the diagonal band of Broca |
| BL | basolateral amygdaloid nucleus |
| VAChT | vesicular acetylcholine transporter |
| HDB | horizontal limb of the diagonal band of Broca |
| VP | ventral pallidum |
| SI | substantia innominata |
| MCPO | magnocellular preoptic nucleus |
| PBS | phosphate buffered saline |
| NGS | normal goat serum |
References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Moshé, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef]
- Beghi, E. Addressing the burden of epilepsy: Many unmet needs. Pharm. Res. 2016, 107, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Bansal, A.; Dua, T.; Hill, S.R.; Moshe, S.L.; Mantel-Teeuwisse, A.K.; Saxena, S. Mapping the availability, price, and affordability of antiepileptic drugs in 46 countries. Epilepsia 2012, 53, 962–969. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Hauser, W.A.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Thurman, D.J.; Logroscino, G.; Beghi, E.; Hauser, W.A.; Hesdorffer, D.C.; Newton, C.R.; Scorza, F.A.; Sander, J.W.; Tomson, T. The burden of premature mortality of epilepsy in high-income countries: A systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia 2017, 58, 17–26. [Google Scholar] [CrossRef]
- Mula, M.; Sander, J.W. Psychosocial aspects of epilepsy: A wider approach. Br. J. Psychiatry Open 2016, 2, 270–274. [Google Scholar] [CrossRef]
- Clossen, B.L.; Reddy, D.S. Novel therapeutic approaches for disease-modification of epileptogenesis for curing epilepsy. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1519–1538. [Google Scholar] [CrossRef]
- Łukawski, K.; Andres-Mach, M.; Czuczwar, M.; Łuszczki, J.J.; Kruszyński, K.; Czuczwar, S.J. Mechanisms of epileptogenesis and preclinical approach to antiepileptogenic therapies. Pharm. Rep. 2018, 70, 284–293. [Google Scholar] [CrossRef]
- Ravizza, T.; Vezzani, A. Pharmacological targeting of brain inflammation in epilepsy: Therapeutic perspectives from experimental and clinical studies. Epilepsia Open 2018, 3, 133–142. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Aronica, E.; Vezzani, A.; Ravizza, T. Review: Neuroinflammatory pathways as treatment targets and biomarker candidates in epilepsy: Emerging evidence from preclinical and clinical studies. Neuropathol. Appl. Neurobiol. 2018, 44, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Klement, W.; Garbelli, R.; Zub, E.; Rossini, L.; Tassi, L.; Girard, B.; Blaquiere, M.; Bertaso, F.; Perroy, J.; de Bock, F.; et al. Seizure progression and inflammatory mediators promote pericytosis and pericyte-microglia clustering at the cerebrovasculature. Neurobiol. Dis. 2018, 113, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Behr, C. Epidemiology of epilepsy. Rev. Neurol. Paris 2016, 172, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Trevick, S. The Epidemiology of Global Epilepsy. Neurol. Clin. 2016, 34, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Thom, M.; Bertram, E.H. Temporal lobe epilepsy. Handb. Clin. Neurol. 2012, 107, 225–240. [Google Scholar] [PubMed]
- Ben-Ari, Y.; Tremblay, E.; Ottersen, O.P.; Meldrum, B.S. The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res. 1980, 191, 79–97. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Limbic seizure and brain damage produced by kainic acid: Mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 1985, 14, 375–403. [Google Scholar] [CrossRef]
- Cronin, J.; Dudek, F.E. Chronic seizures and collateral sprouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res. 1988, 474, 181–184. [Google Scholar] [CrossRef]
- Hellier, J.L.; Patrylo, P.R.; Buckmaster, P.S.; Dudek, F.E. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: Assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998, 31, 73–84. [Google Scholar] [CrossRef]
- Dudek, F.E.; Clark, S.; Williams, P.A.; Grabenstatter, H.L. Kainate-Induced Status Epilepticus: A Chronic Model of Acquired Epilepsy. In Models of Seizures and Epilepsy; Sutula, T., Pitkanen, A., Eds.; Elsevier: New York, NY, USA, 2005; pp. 415–432. [Google Scholar]
- Lothman, E.W.; Collins, R.C. Kainic acid induced limbic seizures: Metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res. 1981, 218, 299–318. [Google Scholar] [CrossRef]
- Schwob, J.E.; Fuller, T.; Price, J.L.; Olney, J.W. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: A histological study. Neuroscience 1980, 5, 991–1014. [Google Scholar] [CrossRef]
- Victor Nadler, J. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981, 29, 2031–2042. [Google Scholar] [CrossRef]
- Du, F.; Eid, T.; Lothman, E.W.; Kohler, C.; Schwarcz, R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J. Neurosci. 1995, 15, 6301–6313. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Makiura, Y.; Guilhem, D.; Sørensen, J.C.; Onteniente, B. Correlated axonal sprouting and dendritic spine formation during kainate-induced neuronal morphogenesis in the dentate gyrus of adult mice. Exp. Neurol. 1997, 145, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ryley Parrish, R.; Albertson, A.J.; Buckingham, S.C.; Hablitz, J.J.; Mascia, K.L.; Davis Haselden, W.; Lubin, F.D. Status epilepticus triggers early and late alterations in brain-derived neurotrophic factor and NMDA glutamate receptor Grin2b DNA methylation levels in the hippocampus. Neuroscience 2013, 248, 602–619. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.S.; Mirsattari, S.M.; Leung, L.S. Resting state functional network disruptions in a kainic acid model of temporal lobe epilepsy. Neuroimage Clin. 2017, 13, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.I.; Valente, M.C.; Andrade, P.A.; Maia, G.H.; Lukoyanov, N.V. Reorganization of the septohippocampal cholinergic fiber system in experimental epilepsy. J. Comp. Neurol. 2017, 525, 2690–2705. [Google Scholar] [CrossRef]
- Soares, J.I.; Afonso, A.R.; Maia, G.H.; Lukoyanov, N.V. The pedunculopontine and laterodorsal tegmental nuclei in the kainate model of epilepsy. Neurosci. Lett. 2018, 672, 90–95. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Zigmond, R.E.; Shute, C.C.D.; Lewis, P.R. Regional distribution of choline acetyltransferase and acetylcholinesterase within the amygdaloid complex and stria terminalis system. Brain Res. 1977, 120, 435–445. [Google Scholar] [CrossRef]
- Carlsen, J.; Záborszky, L.; Heimer, L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: A combined retrograde fluorescent and immunohistochemical study. J. Comp. Neurol. 1985, 234, 155–167. [Google Scholar] [CrossRef]
- Li, R.; Nishijo, H.; Wang, Q.; Uwano, T.; Tamura, R.; Ohtani, O.; Ono, T. Light and electron microscopic study of cholinergic and noradrenergic elements in the basolateral nucleus of the rat amygdala: Evidence for interactions between the two systems. J. Comp. Neurol. 2001, 439, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Emre, M.; Heckers, S.; Mash, D.C.; Geula, C.; Mesulam, M.-M. Cholinergic innervation of the amygdaloid complex in the human brain and its alterations in old age and Alzheimer’s disease. J. Comp. Neurol. 1993, 336, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Jolkkonen, E.; Kemppainen, S. Anatomic heterogeneity of the rat amygdaloid complex. Folia Morphol. 2000, 59, 1–23. [Google Scholar]
- Ménard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2015, 321, 138–162. [Google Scholar] [CrossRef]
- Tuunanen, J.; Halonen, T.; Pitkänen, A. Status epilepticus causes selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. Eur. J. Neurosci. 1996, 8, 2711–2725. [Google Scholar] [CrossRef]
- Tuunanen, J.; Halonen, T.; Pitkänen, A. Decrease in somatostatin-immunoreactive neurons in the rat amygdaloid complex in a kindling model of temporal lobe epilepsy. Epilepsy Res. 1997, 26, 315–327. [Google Scholar] [CrossRef]
- Tuunanen, J.; Lukasiuk, K.; Halonen, T.; Pitkänen, A. Status epilepticus-induced neuronal damage in the rat amygdaloid complex: Distribution, time-course and mechanisms. Neuroscience 1999, 94, 473–495. [Google Scholar] [CrossRef]
- Tuunanen, J.; Pitkänen, A. Do seizures cause neuronal damage in rat amygdala kindling? Epilepsy Res. 2000, 39, 171–176. [Google Scholar] [CrossRef]
- Pitkänen, A.; Tuunanen, J.; Kälviäinen, R.; Partanen, K.; Salmenperä, T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998, 32, 233–253. [Google Scholar] [CrossRef]
- Pitkänen, A.; Nissinen, J.; Nairismägi, J.; Lukasiuk, K.; Gröhn, O.H.J.; Miettinen, R.; Kappinen, R. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog. Brain Res. 2002, 135, 67–83. [Google Scholar] [PubMed]
- Pirttila, T.R.M.; Pitkanen, A.; Tuunanen, J.; Kauppinen, R.A. Ex vivo MR microimaging of neuronal damage after kainate-induced status epilepticus in rat: Correlation with quantitative histology. Magn. Reson. Med. 2001, 46, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Narkilahti, S.; Pirttilä, T.J.; Lukasiuk, K.; Tuunanen, J.; Pitkänen, A. Expression and activation of caspase 3 following status epilepticus in the rat. Eur. J. Neurosci. 2003, 18, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Goddard, G.V.; McIntyre, D.C.; Leech, C.K. A permanent change in brain function resulting from daily electrical stimulation. Exp. Neurol. 1969, 25, 295–330. [Google Scholar] [CrossRef]
- McIntyre, D.C. The Kindling Phenomenon. In Models of Seizures and Epilepsy; Sutula, T., Pitkanen, A., Eds.; Elsevier: New York, NY, USA, 2005; pp. 351–363. [Google Scholar]
- Dutra Moraes, M.F.; Galvis-Alonso, O.Y.; Garcia-Cairasco, N. Audiogenic kindling in the Wistar rat: A potential model for recruitment of limbic structures. Epilepsy Res. 2000, 39, 251–259. [Google Scholar] [CrossRef]
- Galvis-Alonso, O.Y.; Cortes De Oliveira, J.A.; Garcia-Cairasco, N. Limbic epileptogenicity, cell loss and axonal reorganization induced by audiogenic and amygdala kindling in wistar audiogenic rats (WAR strain). Neuroscience 2004, 125, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Faingold, C.L. Brainstem Networks. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M., Olsen, R., Delgado-Escueta, A.V., Eds.; Contemporary Neurology Series; Oxford University Press: New York, NY, USA, 2012; Volume 80, pp. 257–271. [Google Scholar]
- Wieser, H.G. Mesial temporal lobe epilepsy versus amygdalar epilepsy: Late seizure recurrence after initially successful amygdalotomy and regained seizure control following hippocampectomy. Epileptic Disord. 2000, 2, 141–152. [Google Scholar]
- Cendes, F.; Andermann, F.; Gloor, P.; Lopes-Cendes, I.; Andermann, E.; Melanson, D.; Jones-Gotman, M.; Robitaille, Y.; Evans, A.; Peters, T. Atrophy of mesial structures in patients with temporal lobe epilepsy: Cause or consequence of repeated seizures? Ann. Neurol. 1993, 34, 795–801. [Google Scholar] [CrossRef]
- Cendes, F.; Andermann, F.; Dubeau, F.; Gloor, P.; Evans, A.; Jones-Gotman, M.; Olivier, A.; Andermann, E.; Robitaille, Y.; Lopes-Cendes, I.; et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: An MRI volumetric study. Neurology 1993, 43, 1083–1087. [Google Scholar] [CrossRef]
- Cendes, F.; Leproux, F.; Melanson, D.; Ethier, R.; Evans, A.; Peters, T.; Andermann, F. MRI of amygdala and hippocampus in temporal lobe epilepsy. J. Comput. Assist. Tomogr. 1993, 17, 206–210. [Google Scholar] [CrossRef]
- Cendes, F.; Andermann, F.; Gloor, P.; Evans, A.; Jones-Gotman, M.; Watson, C.; Melanson, D.; Olivier, A.; Peters, T.; Lopes-Cendes, I.; et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology 1993, 43, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Kälviäinen, R.; Salmenperä, T. Do recurrent seizures cause neuronal damage? A series of studies with MRI volumetry in adults with partial epilepsy. Prog. Brain Res. 2002, 135, 279–295. [Google Scholar] [PubMed]
- Salmenperä, T.; Kälviäinen, R.; Partanen, K.; Pitkänen, A. Hippocampal and amygdaloid damage in partial epilepsy. Epilepsy Res. 2002, 46, 69–82. [Google Scholar] [CrossRef]
- Bronen, R.A.; Fulbright, R.K.; Kim, J.H.; Spencer, S.S.; Spencer, D.D.; Al-Rodhan, N.R.F. Regional distribution of MR findings in hippocampal sclerosis. Am. J. Neuroradiol. 1995, 16, 1193–1200. [Google Scholar]
- Kälviäinen, R.; Salmenperä, T.; Partanen, K.; Vainio, P.; Riekkinen, P., Sr.; Pitkänen, A. MRI volumetry and T2 relaxometry of the amygdala in newly diagnosed and chronic temporal lobe epilepsy. Epilepsy Res. 1997, 28, 39–50. [Google Scholar] [CrossRef]
- Margerison, J.H.; Corsellis, J.A.N. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain 1966, 89, 499–530. [Google Scholar] [CrossRef]
- Hudson, L.P.; Munoz, D.G.; Miller, L.; McLachlan, R.S.; Girvin, J.P.; Blume, W.T. Amygdaloid sclerosis in temporal lobe epilepsy. Ann. Neurol. 1993, 33, 622–631. [Google Scholar] [CrossRef]
- Miller, L.A.; McLachlan, R.S.; Bouwer, M.S.; Hudson, L.P.; Munoz, D.G. Amygdalar sclerosis: Preoperative indicators and outcome after temporal lobectomy. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1099–1105. [Google Scholar] [CrossRef][Green Version]
- Callahan, P.M.; Paris, J.M.; Kathryn, C.; Shinnick-Gallagher, P. Decrease of GABA-immunoreactive neurons in the amygdala after electrical kindling in the rat. Brain Res. 1991, 555, 335–339. [Google Scholar] [CrossRef]
- Rainnie, D.G.; Asprodini, E.K.; Shinnick-Gallagher, P. Kindling-induced long-lasting changes in synaptic transmission in the basolateral amygdala. J. Neurophysiol. 1992, 67, 443–454. [Google Scholar] [CrossRef]
- Carlsen, J.; Heimer, L. A correlated light and electron microscopic immunocytochemical study of cholinergic terminals and neurons in the rat amygdaloid body with special emphasis on the basolateral amygdaloid nucleus. J. Comp. Neurol. 1986, 244, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.F.; Mascagni, F.; Zaric, V.; Mott, D.D.; McDonald, A.J. Localization of the M2 muscarinic cholinergic receptor in dendrites, cholinergic terminals, and noncholinergic terminals in the rat basolateral amygdala: An ultrastructural analysis. J. Comp. Neurol. 2016, 524, 2400–2417. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J.; Mascagni, F. Neuronal localization of M1 muscarinic receptor immunoreactivity in the rat amygdala. Neuroscience 2010, 215, 37–48. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J.; Mascagni, F. Neuronal localization of M2 muscarinic receptor immunoreactivity in the rat amygdala. Neuroscience 2011, 196, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Schliebs, R.; Zivin, M.; Steinbach, J.; Rothe, R. Changes in Cholinergic but Not in GABAergic Markers in Amygdala, Piriform Cortex, and Nucleus Basalis of the Rat Brain Following Systemic Administration of Kainic Acid. J. Neurochem. 1989, 53, 212–218. [Google Scholar] [CrossRef]
- Lukoyanov, N.V.; Sá, M.J.; Madeira, M.D.; Paula-Barbosa, M.M. Selective loss of hilar neurons and impairment of initial learning in rats after repeated administration of electroconvulsive shock seizures. Exp. Brain Res. 2004, 154, 192–200. [Google Scholar] [CrossRef]
- Woolf, N.J.; Eckenstein, F.; Butcher, L.L. Cholinergic systems in the rat brain: I. Projections to the limbic telencephalon. Brain Res. Bull. 1984, 13, 751–784. [Google Scholar] [CrossRef]
- Rao, Z.R.; Shiosaka, S.; Tohyama, M. Origin of cholinergic fibers in the basolateral nucleus of the amygdaloid complex by using sensitive double-labeling technique of retrograde biotinized tracer and immunocytochemistry. J. Hirnforsch. 1987, 28, 553–560. [Google Scholar]
- Soares, J.I.; Da Costa, C.; Ferreira, M.H.; Andrade, P.A.; Maia, G.H.; Lukoyanov, N.V. Partial depletion of septohippocampal cholinergic cells reduces seizure susceptibility, but does not mitigate hippocampal neurodegeneration in the kainate model of epilepsy. Brain Res. 2019, 1717, 235–246. [Google Scholar] [CrossRef]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; Garber, J.C., Ed.; The National Academies Press: Washington, DC, USA, 2011; pp. 1–208. [Google Scholar]
- Lucchi, C.; Curia, G.; Vinet, J.; Gualtieri, F.; Bresciani, E.; Locatelli, V.; Torsello, A.; Biagini, G. Protective but Not Anticonvulsant Effects of Ghrelin and JMV-1843 in the Pilocarpine Model of Status epilepticus. PLoS ONE 2013, 8, e72716. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 1–209. [Google Scholar]
- Gundersen, H.J.G. The nucleator. J. Microsc. 1988, 151, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Geeraedts, L.M.G.; Nieuwenhuys, R.; Veening, J.G. Medial forebrain bundle of the rat: III. Cytoarchitecture of the rostral (telencephalic) part of the medial forebrain bundle bed nucleus. J. Comp. Neurol. 1990, 294, 507–536. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosal Lustosa, Í.; Soares, J.I.; Biagini, G.; Lukoyanov, N.V. Neuroplasticity in Cholinergic Projections from the Basal Forebrain to the Basolateral Nucleus of the Amygdala in the Kainic Acid Model of Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2019, 20, 5688. https://doi.org/10.3390/ijms20225688
Rosal Lustosa Í, Soares JI, Biagini G, Lukoyanov NV. Neuroplasticity in Cholinergic Projections from the Basal Forebrain to the Basolateral Nucleus of the Amygdala in the Kainic Acid Model of Temporal Lobe Epilepsy. International Journal of Molecular Sciences. 2019; 20(22):5688. https://doi.org/10.3390/ijms20225688
Chicago/Turabian StyleRosal Lustosa, Ítalo, Joana I. Soares, Giuseppe Biagini, and Nikolai V. Lukoyanov. 2019. "Neuroplasticity in Cholinergic Projections from the Basal Forebrain to the Basolateral Nucleus of the Amygdala in the Kainic Acid Model of Temporal Lobe Epilepsy" International Journal of Molecular Sciences 20, no. 22: 5688. https://doi.org/10.3390/ijms20225688
APA StyleRosal Lustosa, Í., Soares, J. I., Biagini, G., & Lukoyanov, N. V. (2019). Neuroplasticity in Cholinergic Projections from the Basal Forebrain to the Basolateral Nucleus of the Amygdala in the Kainic Acid Model of Temporal Lobe Epilepsy. International Journal of Molecular Sciences, 20(22), 5688. https://doi.org/10.3390/ijms20225688