Microcalcifications Drive Breast Cancer Occurrence and Development by Macrophage-Mediated Epithelial to Mesenchymal Transition
Abstract
1. Introduction
2. Results
2.1. Morphological Classification of Breast Lesions
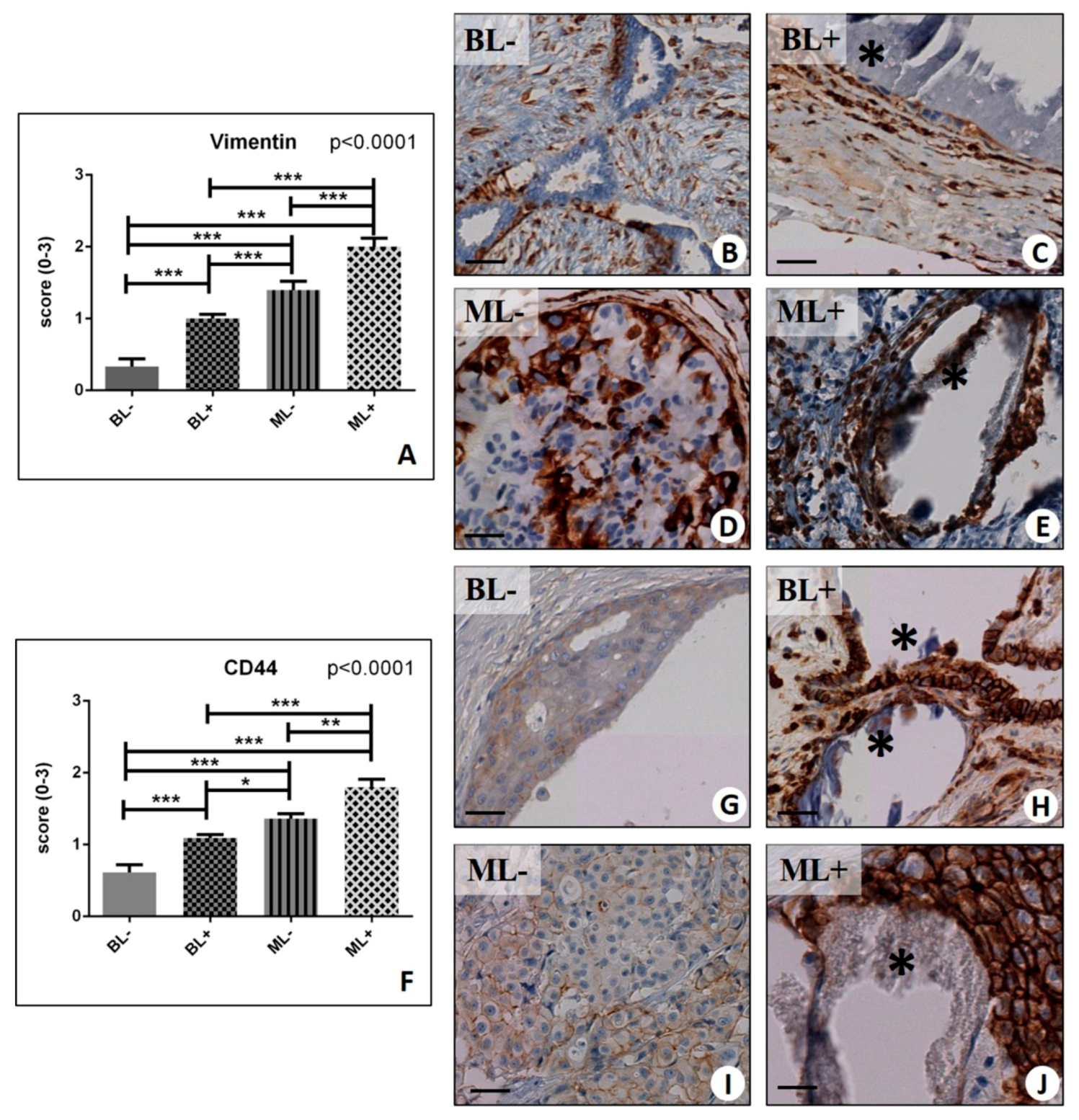
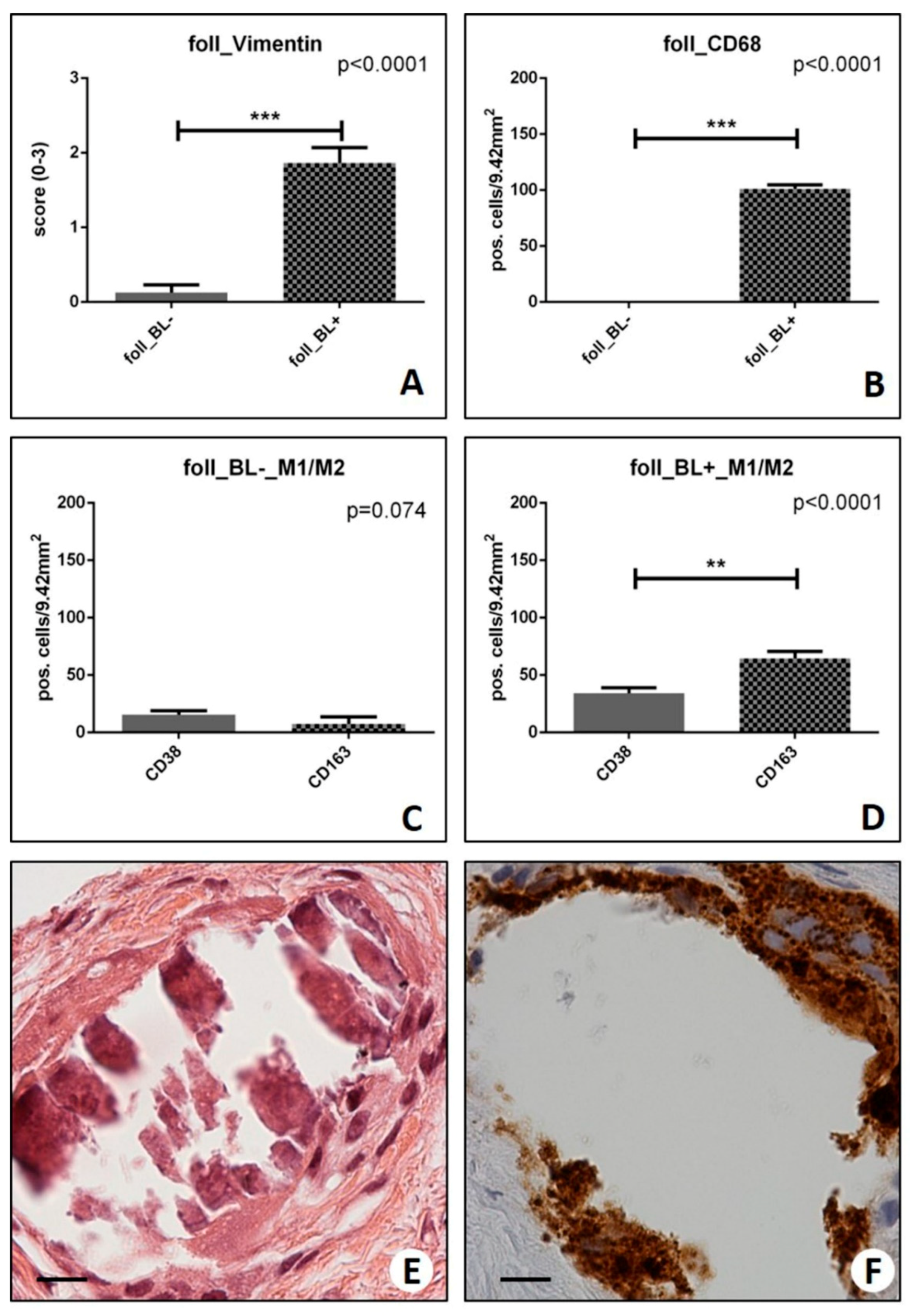
2.2. Assessment of the Mesenchymal Phenotype in Breast Lesions
2.3. Identification of Osteoblast-Like Cells in Breast Lesions: BOLCs
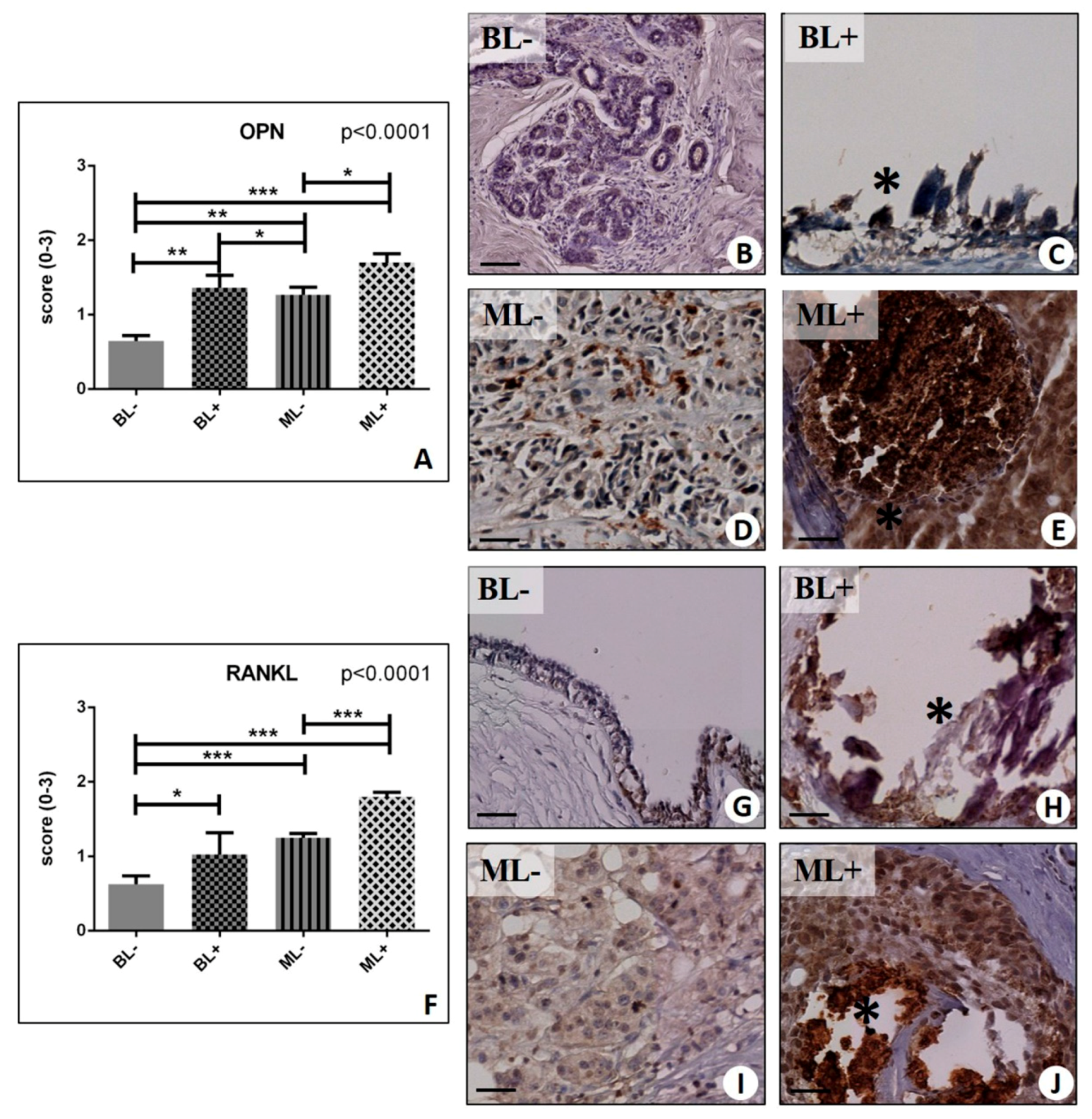
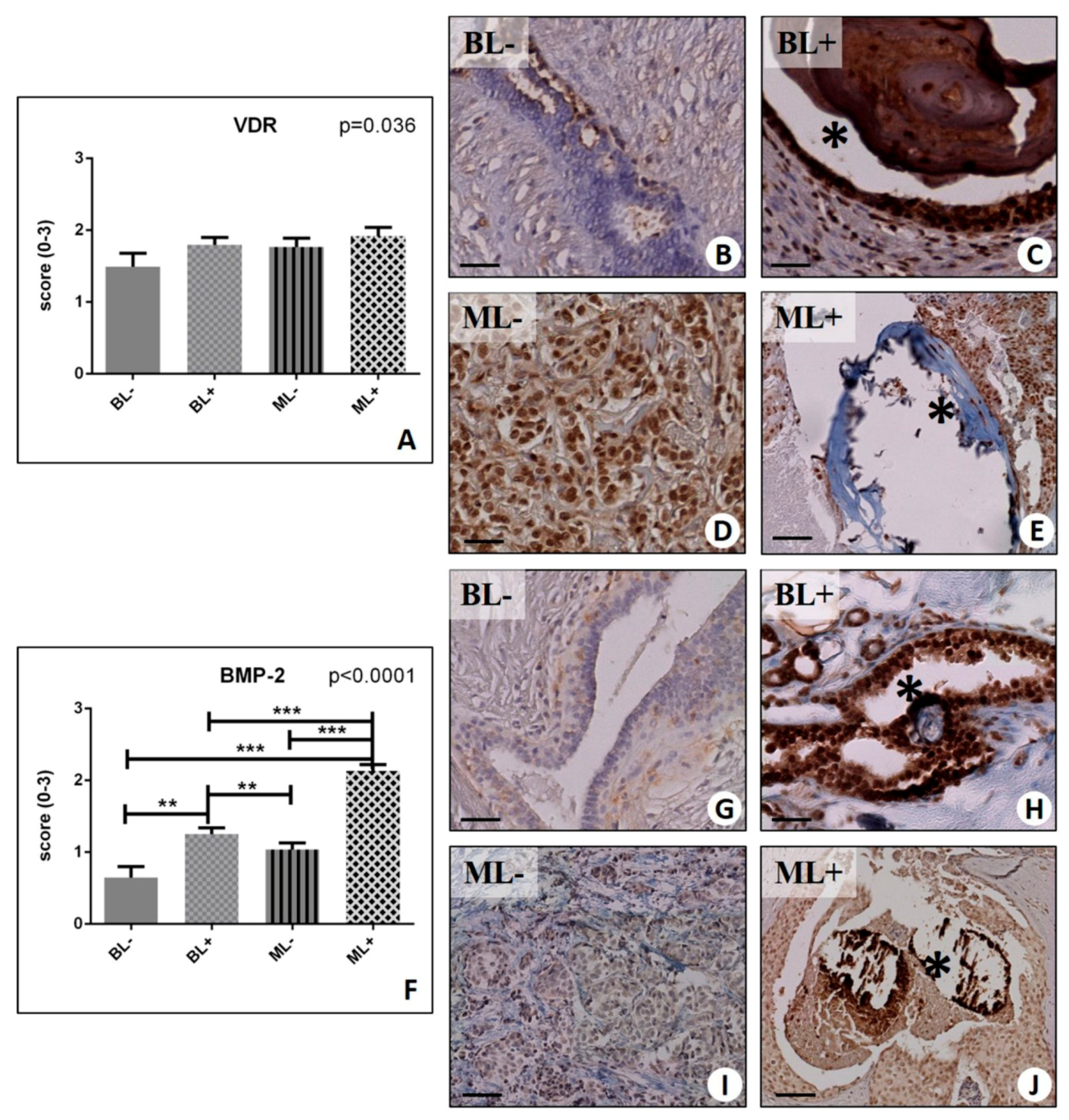
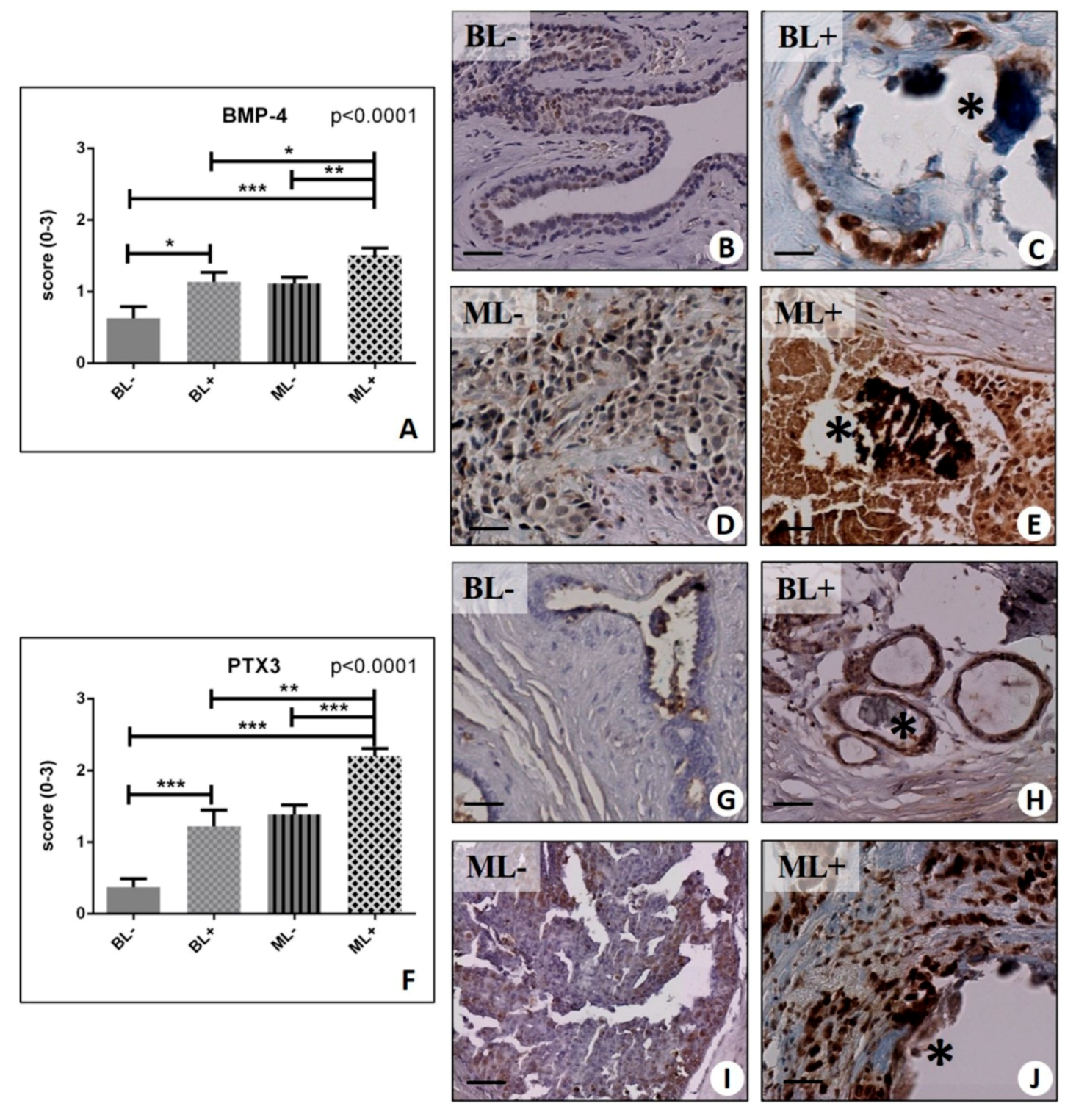
2.4. Osteoblastic Differentiation and Mineralization in Breast Lesions: A Home for BOLCs
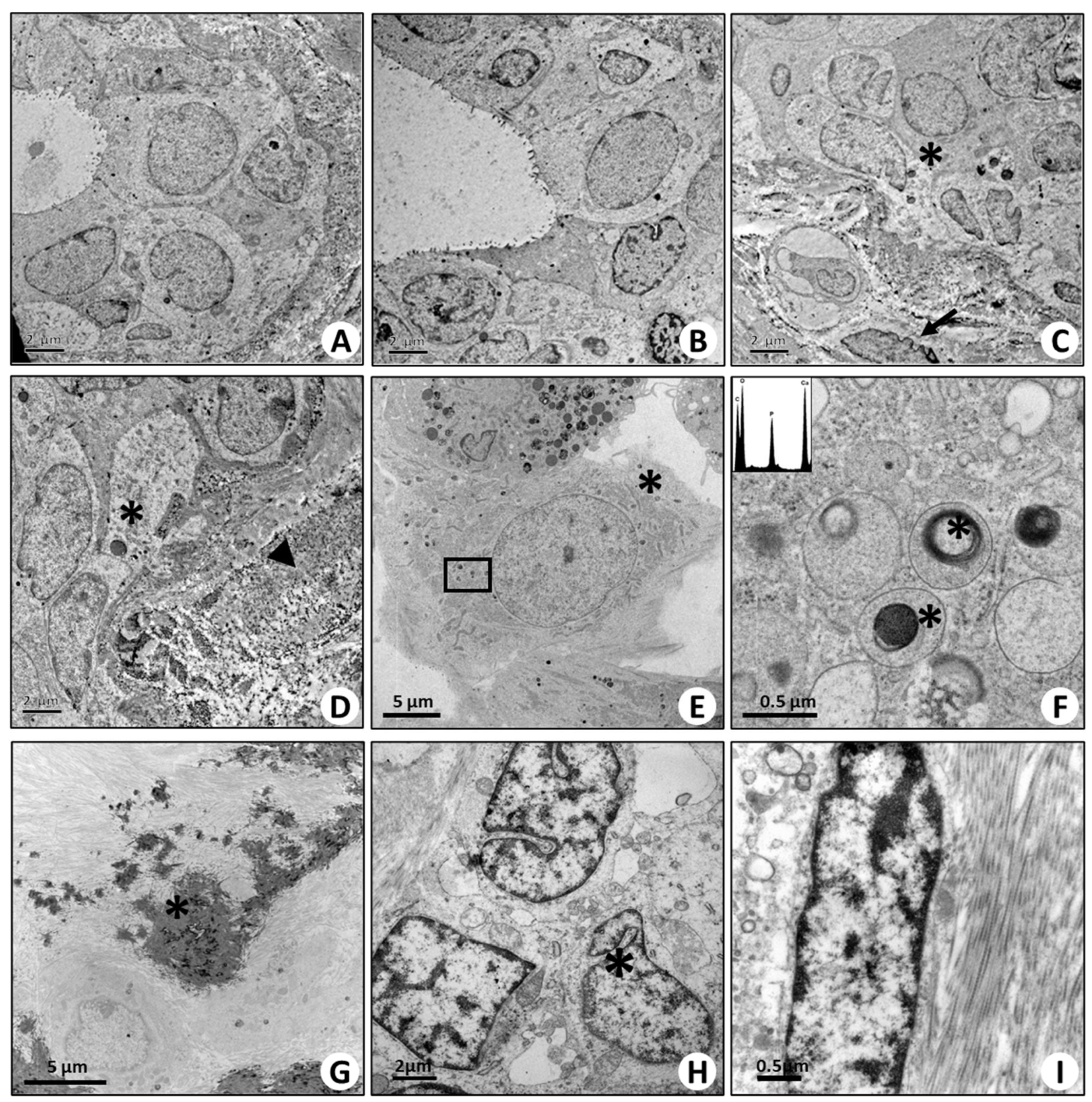
2.5. Ultrastructural Characterization of BOLCs
2.6. In Vitro Model of BOLCs Development
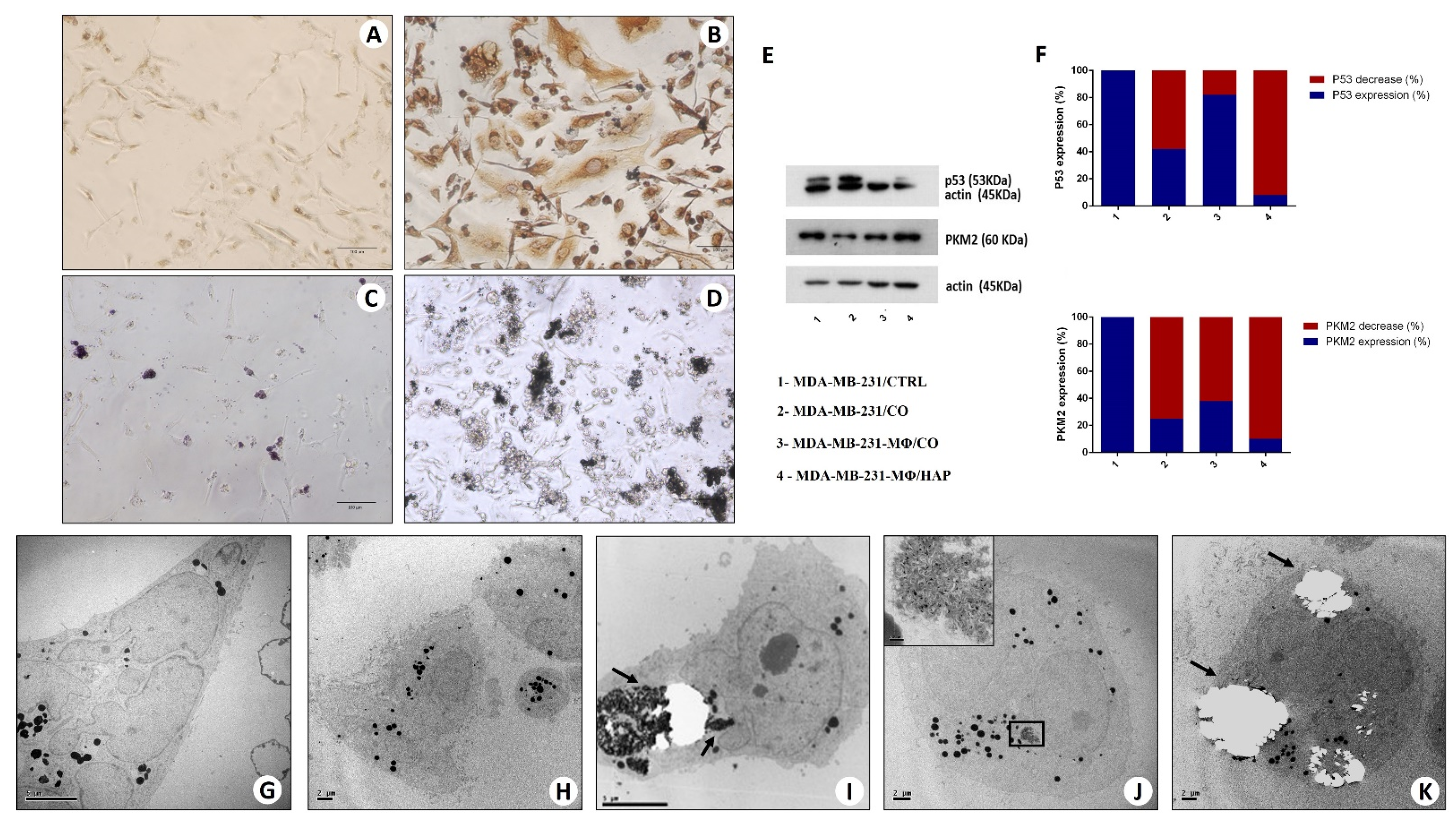
2.6.1. Morphological Aspect (Optical and Transmission Electron Microscopy)
2.6.2. Western Blot Analysis
2.6.3. Transmission Electron Microscopy and Energy Dispersive X-ray (EDX) Microanalysis
2.6.4. Progression of Breast Lesions with Microcalcifications: Morphological Classification
2.6.5. Elemental Analysis of Microcalcifications
2.6.6. The Link among Microcalcifications, Macrophages, and the EMT Phenomenon
3. Discussion
4. Material and Methods
4.1. Breast Sample Collection
4.2. Histology
4.3. Immunohistochemistry of the Paraffin Sections
4.4. Transmission Electron Microscopy (TEM) of Breast Tissues
4.5. Energy Dispersive X-ray (EDX) Microanalysis
4.6. Calcium Oxalate Synthesis
4.7. Cell Culture
4.8. Monocyte Isolation
4.9. In Vitro Model for the Development of “Osteoblast-Like Cells”
4.10. Protein Extraction and Western Blot Analysis
4.11. Cell Culture Immunohistochemistry
4.12. TEM and EDX Analysis of Cell Cultures
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMPs | Bone Morphogenetics Proteins |
| BOLCs | Breast Osteoblast-Like Cells |
| CO | calcium oxalate |
| EDX | Energy dispersive x-ray |
| EMT | epithelial to mesenchymal transition |
| FBS | fetal bovine serum |
| H&E | hematoxylin and eosin |
| HA | Hydroxyapatite |
| HPBMs | Human peripheral blood monocytes |
| Mg-HAp | Magnesium-substituted hydroxyapatite |
| OPG | osteoprotegerin |
| OPN | Osteopontin |
| RANKL | Receptor activator of nuclear factor kappa-Β ligand |
| VDR | Vitamin D receptor |
| PTX3 | Pentraxin-related protein 3 |
| BMP-2 | Bone Morphogenetic Protein-2 |
| BMP-4 | Bone Morphogenetic Protein-4 |
| TEM | Transmission electron microscopy |
References
- Bonfiglio, R.; Scimeca, M.; Urbano, N.; Bonanno, E.; Schillaci, O. Breast microcalcifications: Biological and diagnostic perspectives. Future Oncol. 2018, 14, 3097–3099. [Google Scholar] [CrossRef] [PubMed]
- Tabar, L.; Tony Chen, H.H.; Amy Yen, M.F.; Tot, T.; Tung, T.H.; Chen, L.S.; Chiu, Y.H.; Duffy, S.W.; Smith, R.A. Mammographic tumor features can predict long-term outcomes reliably in women with 1-14-mm invasive breast carcinoma. Cancer 2004, 101, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A. Age of diagnosis, tumor size, and survival after breast cancer: Implications for mammographic screening. Breast Cancer Res. Treat. 2011, 128, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, C.; Bicchierai, G.; De Benedetto, D.; Boeri, C.; Vanzi, E.; Miele, V.; Cirone, D.; Nori, J. Role of preoperative breast dual-energy contrast-enhanced digital mammography in ductal carcinoma in situ. Breast J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Menezes, G.L.; Winter-Warnars, G.A.; Koekenbier, E.L.; Groen, E.J.; Verkooijen, H.M.; Pijnappel, R. MSimplifying Breast Imaging Reporting and Data System classification of mammograms with pure suspicious calcifications. J. Med. Screen. 2018, 25, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Frappart, L.; Boudeulle, M.; Boumendil, J.; Lin, H.C.; Martinon, I.; Palayer, C.; Mallet-Guy, Y.; Raudrant, D.; Bremond, A.; Rochet, Y.; et al. Structure and composition of microcalcifications in benign and malignant lesions of the breast: Study by light microscopy, transmission and scanning electron microscopy, microprobe analysis, and X-ray diffraction. Hum. Pathol. 1984, 15, 880–889. [Google Scholar] [CrossRef]
- Scimeca, M.; Giannini, E.; Antonacci, C.; Pistolese, C.A.; Spagnoli, L.G.; Bonanno, E. Microcalcifications in breast cancer: An active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer 2014, 23, 286. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, R.; Scimeca, M.; Toschi, N.; Pistolese, C.A.; Giannini, E.; Antonacci, C.; Tancredi, V.; Tarantino, U.; Albonici, L.; Bonanno, E. Radiological, Histological and Chemical Analysis of Breast Microcalcifications: Diagnostic Value and Biological Significance. J. Mammary Gland Biol. Neoplasia 2018, 23, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Tabar, L.; Tot, T.; Fann, C.Y.; Yen, A.M.; Chen, S.L.; Chiu, S.Y.; Ku, M.M.; Hsu, C.Y.; Beckmann, K.R.; et al. Imaging Biomarkers as Predictors for Breast Cancer Death. J. Oncol. 2019, 2019, 2087983. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Urbano, N.; Bonfiglio, R.; Schillaci, O.; Bonanno, E. Breast osteoblast-like cells: A new biomarker for the management of breast cancer. Br. J. Cancer 2018, 119, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.F.; Jenkinson, A.; Pohl, K.; O’Brien, F.J.; Morgan, M.P. Osteomimicry of mammary adenocarcinoma cells in vitro; increased expression of bone matrix proteins and proliferation within a 3D collagen environment. PLoS ONE 2012, 7, e41679. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.P.; Cooke, M.M.; McCarthy, G.M. Microcalcifications associated with breast cancer: An epiphenomenon or biologically significant feature of selected tumors? J. Mammary Gland Biol. Neoplasia 2005, 10, 181–187. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, S.; Morgan, M.P. Deposition of calcium in an in vitro model of human breast tumour calcification reveals functional role for ALP activity, altered expression of osteogenic genes and dysregulation of the TRPM7 ion channel. Sci. Rep. 2019, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Green, A.R.; Soria, D.; Stephen, J.; Powe, D.G.; Nolan, C.C.; Kunkler, I.; Thomas, J.; Kerr, G.R.; Jack, W.; Cameron, D.; et al. Nottingham Prognostic Index Plus: Validation of a clinical decision making tool in breast cancer in an independent series. J. Pathol. Clin. Res. 2016, 2, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, J.V. Breast Cancer Screening, Mammography, and Other Modalities. Clin. Obstet. Gynecol. 2016, 59, 688–709. [Google Scholar] [CrossRef] [PubMed]
- Verdial, F.C.; Etzioni, R.; Duggan, C.; Anderson, B.O. Demographic changes in breast cancer incidence, stage at diagnosis and age associated with population-based mammographic screening. J. Surg. Oncol. 2017, 115, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Antonacci, C.; Toschi, N.; Giannini, E.; Bonfiglio, R.; Buonomo, C.O.; Pistolese, C.A.; Tarantino, U.; Bonanno, E. Breast Osteoblast-like Cells: A Reliable Early Marker for Bone Metastases fFrom Breast Cancer. Clin. Breast Cancer 2018, 18, e659–e669. [Google Scholar] [CrossRef] [PubMed]
- Singhai, R.; Patil, V.W.; Jaiswal, S.R.; Patil, S.D.; Tayade, M.B.; Patil, A.V. E-Cadherin as a diagnostic biomarker in breast cancer. N. Am. J. Med. Sci. 2011, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Pravettoni, G.; Galimberti, V.; Veronesi, P. Clinical implication of E-cadherin deficiency in lobular breast cancer. Breast Cancer Res. Treat. 2019, 173, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Hollestelle, A.; Peeters, J.K.; Smid, M.; Timmermans, M.; Verhoog, L.C.; Westenend, P.J.; Heine, A.A.; Chan, A.; Sieuwerts, A.M.; Wiemer, E.A.; et al. Loss of E-cadherin is not a necessity for epithelial to mesenchymal transition in human breast cancer. Breast Cancer Res. Treat. 2013, 138, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Canas-Marques, R.; Schnitt, S.J. E-cadherin immunohistochemistry in breast pathology: Uses and pitfalls. Histopathology 2016, 68, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Nagi, C.; Guttman, M.; Jaffer, S.; Qiao, R.; Keren, R.; Triana, A.; Li, M.; Godbold, J.; Bleiweiss, I.J.; Hazan, R.B. N-cadherin expression in breast cancer: Correlation with an aggressive histologic variant--invasive micropapillary carcinoma. Breast Cancer Res. Treat. 2005, 94, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ilić, I.R.; Petrović, A.; Živković, V.V.; Randjelović, P.J.; Stojanović, N.M.; Radulović, N.S.; Randjelović, D.; Ilić, R.S. Immunohistochemical features of multifocal and multicentric lobular breast carcinoma. Adv. Med. Sci. 2017, 62, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Delion, M.; Braux, J.; Jourdain, M.L.; Guillaume, C.; Bour, C.; Gangloff, S.; Pimpec-Barthes, F.L.; Sermet-Gaudelus, I.; Jacquot, J.; Velard, F. Overexpression of RANKL in osteoblasts: A possible mechanism of susceptibility to bone disease in cystic fibrosis. J. Pathol. 2016, 240, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.K. Role of osteopontin in modulation of hydroxyapatite formation. Calcif. Tissue Int. 2013, 93, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.B.; Wang, M.; Yan, C.; Oloyede, A. Molecular dynamics simulation of mechanical behavior of osteopontin-hydroxyapatite interfaces. J. Mech. Behav. Biomed. Mater. 2014, 36, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, Y.; Udagawa, N.; Horibe, K.; Mizoguchi, T.; Yamamoto, Y.; Nakamura, T.; Hosoya, A.; Kato, S.; Suda, T.; Takahashi, N. VDR in Osteoblast-Lineage Cells Primarily Mediates Vitamin D Treatment-Induced Increase in Bone Mass by Suppressing Bone Resorption. J. Bone Miner. Res. 2017, 32, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Mishina, Y. New insights on the roles of BMP signaling in bone-A review of recent mouse genetic studies. Biofactors 2011, 37, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Salustri, A.; Bonanno, E.; Nardozi, D.; Rao, C.; Piccirilli, E.; Feola, M.; Tancredi, V.; Rinaldi, A.; Iolascon, G.; et al. Impairment of PTX3 expression in osteoblasts: A key element for osteoporosis. Cell Death Dis. 2017, 8, e3125. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Feola, M.; Celi, M.; Scimeca, M. PTX3: A new mediator of bone metabolism and osteoporosis. Muscles Ligaments Tendons J. 2017, 7, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Grčević, D.; Sironi, M.; Valentino, S.; Deban, L.; Cvija, H.; Inforzato, A.; Kovačić, N.; Katavić, V.; Kelava, T.; Kalajzić, I.; et al. The Long Pentraxin 3 Plays a Role in Bone Turnover and Repair. Front. Immunol. 2018, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Sintiprungrat, K.; Thongboonkerd, V. Secreted products of macrophages exposed to calcium oxalate crystals induce epithelial mesenchymal transition of renal tubular cells via RhoA-dependent TGF-β1 pathway. Cell Biochem. Biophys. 2013, 67, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Bonfiglio, R.; Urbano, N.; Cerroni, C.; Anemona, L.; Montanaro, M.; Fazi, S.; Schillaci, O.; Mauriello, A.; Bonanno, E. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urol. Oncol. 2019, 37, 297.e19–297.e31. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Orlandi, A.; Terrenato, I.; Bischetti, S.; Bonanno, E. Assessment of metal contaminants in non-small cell lung cancer by EDX microanalysis. Eur. J. Histochem. 2014, 58, 2403. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. 2018, 62, 2841. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Millan, A.; Conte, A. Production of calcium oxalate monohydrate, dihydrate or trihydrate. A comparative study. Urol Res. 1990, 18, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Urbano, N.; Scimeca, M.; Bonanno, E.; Schillaci, O. 99mTc sestamibi SPECT: A possible tool for early detection of breast cancer lesions with high bone metastatic potential. Future Oncol. 2019, 15, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Bonfiglio, R.; Montanaro, M.; Bonanno, E. Osteoblast-like cells in human cancers: New cell type and reliable markers for bone metastasis. Future Oncol. 2018, 14, 9–11. [Google Scholar] [CrossRef] [PubMed]
| Patients | Lesion_1 | Microcalfification | Lesion_2 (Follow-Up) | Microcalcification |
|---|---|---|---|---|
| 1 | fibrocystic mastopathies | Calcium Oxalate | Ductal in situ carcinoma | Hydroxyapatite |
| 2 | fibroadenomas | Calcium Oxalate | Infiltrating carcinoma G3 | Hydroxyapatite-Mg |
| 3 | fibroadenomas | Hydroxyapatite | Ductal in situ carcinoma | Hydroxyapatite |
| 4 | fibroadenomas | Calcium Oxalate | Infiltrating carcinoma G2 | Hydroxyapatite-Mg |
| 5 | fibrocystic mastopathies | Calcium Oxalate | Infiltrating carcinoma G3 | Hydroxyapatite-Mg |
| 6 | fibrocystic mastopathies | Calcium Oxalate | Infiltrating carcinoma G2 | Hydroxyapatite |
| 7 | fibroadenomas | Calcium Oxalate | Infiltrating carcinoma G1 | Hydroxyapatite |
| 8 | fibroadenomas | Hydroxyapatite | Ductal in situ carcinoma | Hydroxyapatite |
| 9 | fibroadenomas | Calcium Oxalate | Infiltrating carcinoma G3 | Hydroxyapatite-Mg |
| 10 | fibrocystic mastopathies | Calcium Oxalate | Infiltrating carcinoma G3 | Hydroxyapatite-Mg |
| 11 | fibroadenomas | Calcium Oxalate | Ductal in situ carcinoma | Hydroxyapatite |
| 12 | fibroadenomas | Calcium Oxalate | Ductal in situ carcinoma | Hydroxyapatite |
| 13 | fibroadenomas | Calcium Oxalate | Infiltrating carcinoma G2 | Hydroxyapatite |
| 14 | fibroadenomas | Calcium Oxalate | Ductal in situ carcinoma | Hydroxyapatite |
| 15 | fibrocystic mastopathies | Hydroxyapatite | Ductal in situ carcinoma | Calcium Oxalate |
| 16 | fibroadenomas | / | Ductal in situ carcinoma | / |
| 17 | fibroadenomas | / | Ductal in situ carcinoma | / |
| 18 | fibrocystic mastopathies | / | Ductal in situ carcinoma | / |
| 19 | fibrocystic mastopathies | / | Infiltrating carcinoma G1 | / |
| 20 | fibroadenomas | / | Ductal in situ carcinoma | / |
| 21 | fibrocystic mastopathies | / | Infiltrating carcinoma G3 | / |
| 22 | fibroadenomas | / | Ductal in situ carcinoma | / |
| 23 | fibroadenomas | / | Infiltrating carcinoma G1 | / |
| 24 | fibrocystic mastopathies | / | Infiltrating carcinoma G2 | / |
| 25 | fibroadenomas | / | Ductal in situ carcinoma | / |
| 26 | fibrocystic mastopathies | / | Infiltrating carcinoma G3 | / |
| 27 | fibroadenomas | / | Ductal in situ carcinoma | / |
| 28 | fibroadenomas | / | Infiltrating carcinoma G1 | / |
| 29 | fibrocystic mastopathies | / | Ductal in situ carcinoma | / |
| 30 | fibroadenomas | / | Ductal in situ carcinoma | / |
| Antibody | Characteristics | Reaction Target | Dilution | Retrieval |
|---|---|---|---|---|
| anti-Vimentin | mouse monoclonal clone V9; Ventana, Tucson, AZ, USA | / | Pre-diluted | EDTA citrate pH 7.8 |
| anti-CD44 | rabbit clone SP37; Ventana, Tucson, AZ, USA | Internal region of human CD44 | Pre-diluted | EDTA citrate pH 8.0 |
| anti-OPN | Mouse monoclonal clone AE1/AE3/PCK26; Ventana, Tucson, AZ, USA | / | 1:100 | EDTA citrate pH 7.8 |
| anti-RANKL | rabbit monoclonal clone 12A668; AbCam, Cambridge, UK | Membrane bound form of RANKL. | 1:100 | Citrate pH 6.0 |
| anti-VDR | rabbit polyclonal clone NBP1-19478; Novus Biologicals, Littleton, CO, USA | Full-length protein | 1:100 | Citrate pH 6.0 |
| anti-PTX3 | rat monoclonal clone MNB1; AbCam, Cambridge, UK | Recognizes the C-terminus of PTX3 | 1:100 | Citrate pH 6.0 |
| anti-BMP2 | rabbit clone N/A; Novus Biologicals, Littleton, CO, USA | Internal region of human BMP2 (within residues 250-350) | 1:500 | Citrate pH 6.0 |
| anti-BMP4 | rabbit polyclonal clone 3C11C7; Novus Biologicals, Littleton, CO, USA | / | 1:100 | Citrate pH 6.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scimeca, M.; Bonfiglio, R.; Menichini, E.; Albonici, L.; Urbano, N.; De Caro, M.T.; Mauriello, A.; Schillaci, O.; Gambacurta, A.; Bonanno, E. Microcalcifications Drive Breast Cancer Occurrence and Development by Macrophage-Mediated Epithelial to Mesenchymal Transition. Int. J. Mol. Sci. 2019, 20, 5633. https://doi.org/10.3390/ijms20225633
Scimeca M, Bonfiglio R, Menichini E, Albonici L, Urbano N, De Caro MT, Mauriello A, Schillaci O, Gambacurta A, Bonanno E. Microcalcifications Drive Breast Cancer Occurrence and Development by Macrophage-Mediated Epithelial to Mesenchymal Transition. International Journal of Molecular Sciences. 2019; 20(22):5633. https://doi.org/10.3390/ijms20225633
Chicago/Turabian StyleScimeca, Manuel, Rita Bonfiglio, Erika Menichini, Loredana Albonici, Nicoletta Urbano, Maria Teresa De Caro, Alessandro Mauriello, Orazio Schillaci, Alessandra Gambacurta, and Elena Bonanno. 2019. "Microcalcifications Drive Breast Cancer Occurrence and Development by Macrophage-Mediated Epithelial to Mesenchymal Transition" International Journal of Molecular Sciences 20, no. 22: 5633. https://doi.org/10.3390/ijms20225633
APA StyleScimeca, M., Bonfiglio, R., Menichini, E., Albonici, L., Urbano, N., De Caro, M. T., Mauriello, A., Schillaci, O., Gambacurta, A., & Bonanno, E. (2019). Microcalcifications Drive Breast Cancer Occurrence and Development by Macrophage-Mediated Epithelial to Mesenchymal Transition. International Journal of Molecular Sciences, 20(22), 5633. https://doi.org/10.3390/ijms20225633









