Anti-Inflammatory and Anti-Hyperuricemic Functions of Two Synthetic Hybrid Drugs with Dual Biological Active Sites
Abstract
:1. Introduction
2. Results
2.1. Acute Toxicity Analysis of Compounds A and B
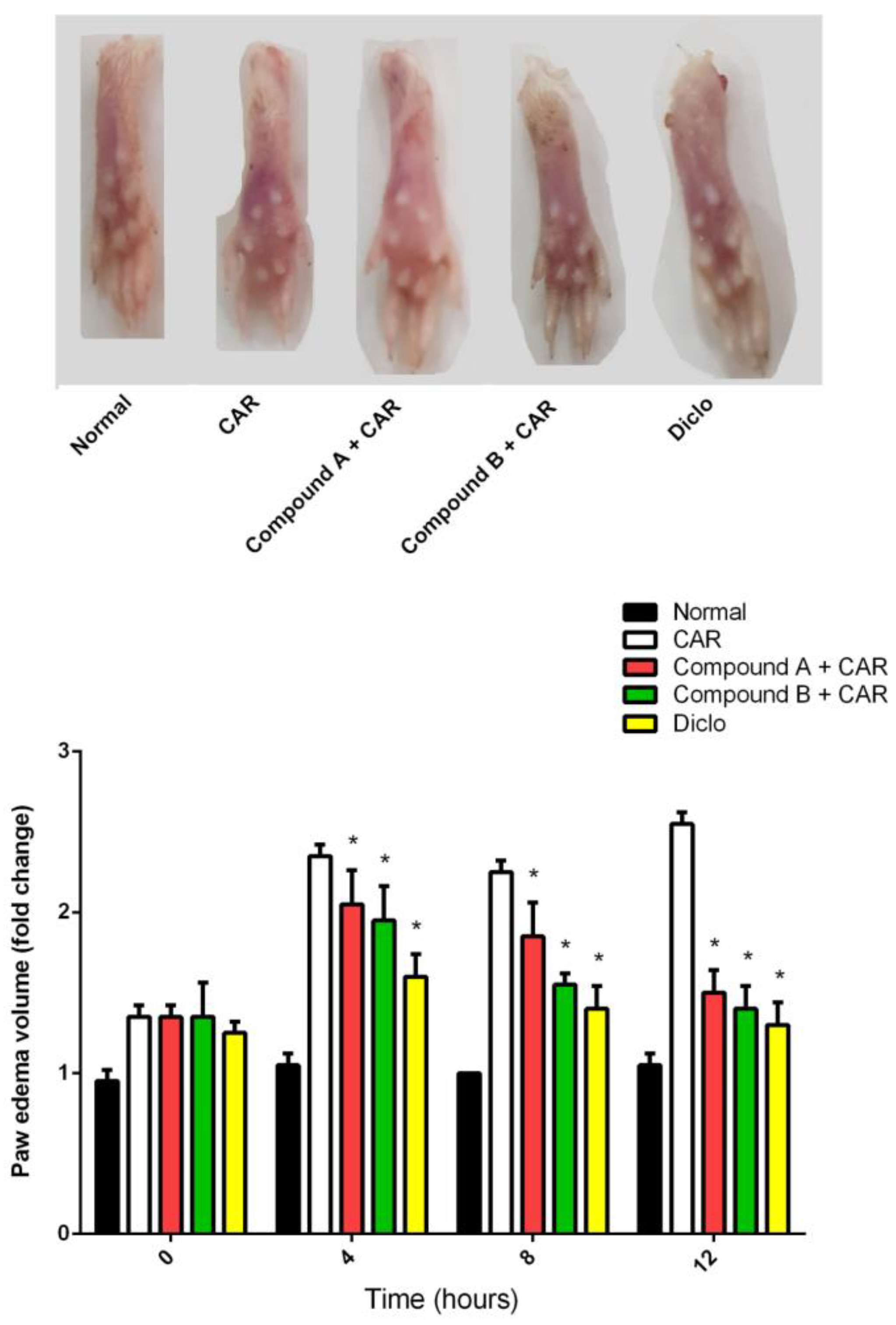
2.2. Effects of Different Compounds on CAR-Induced Paw Edema
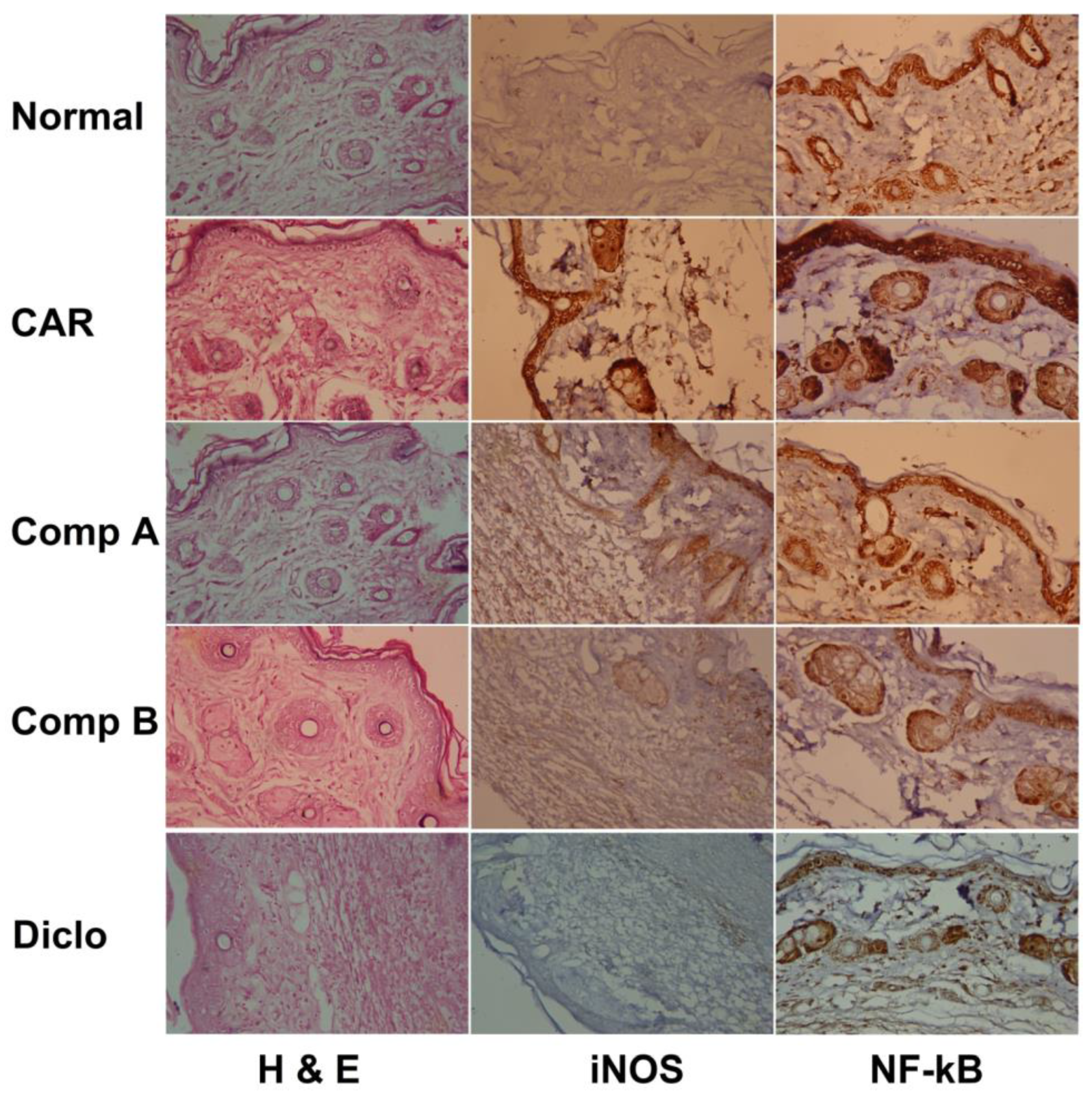
2.3. Effects of Different Compounds on CAR-Induced Histopathological Changes
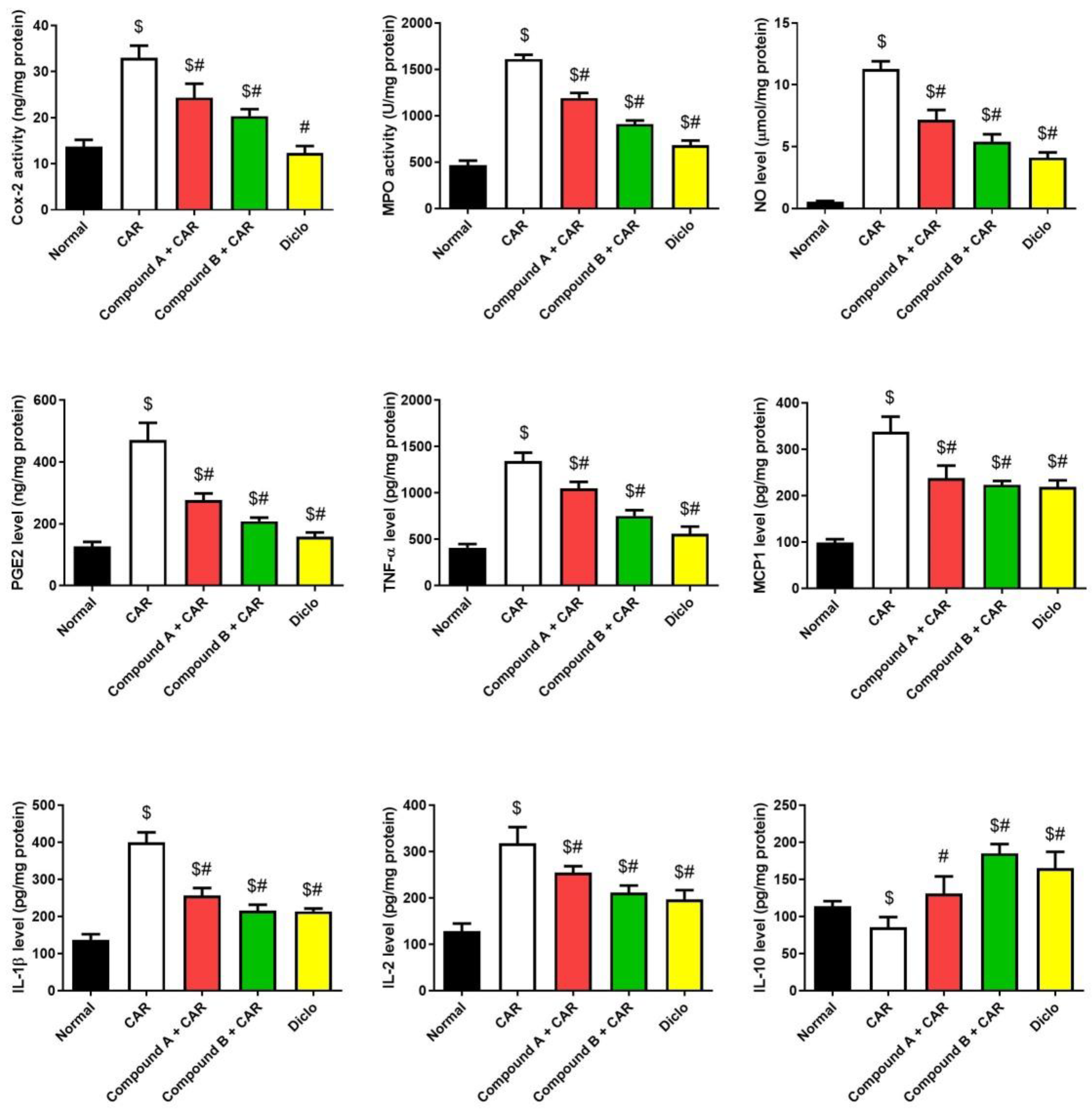
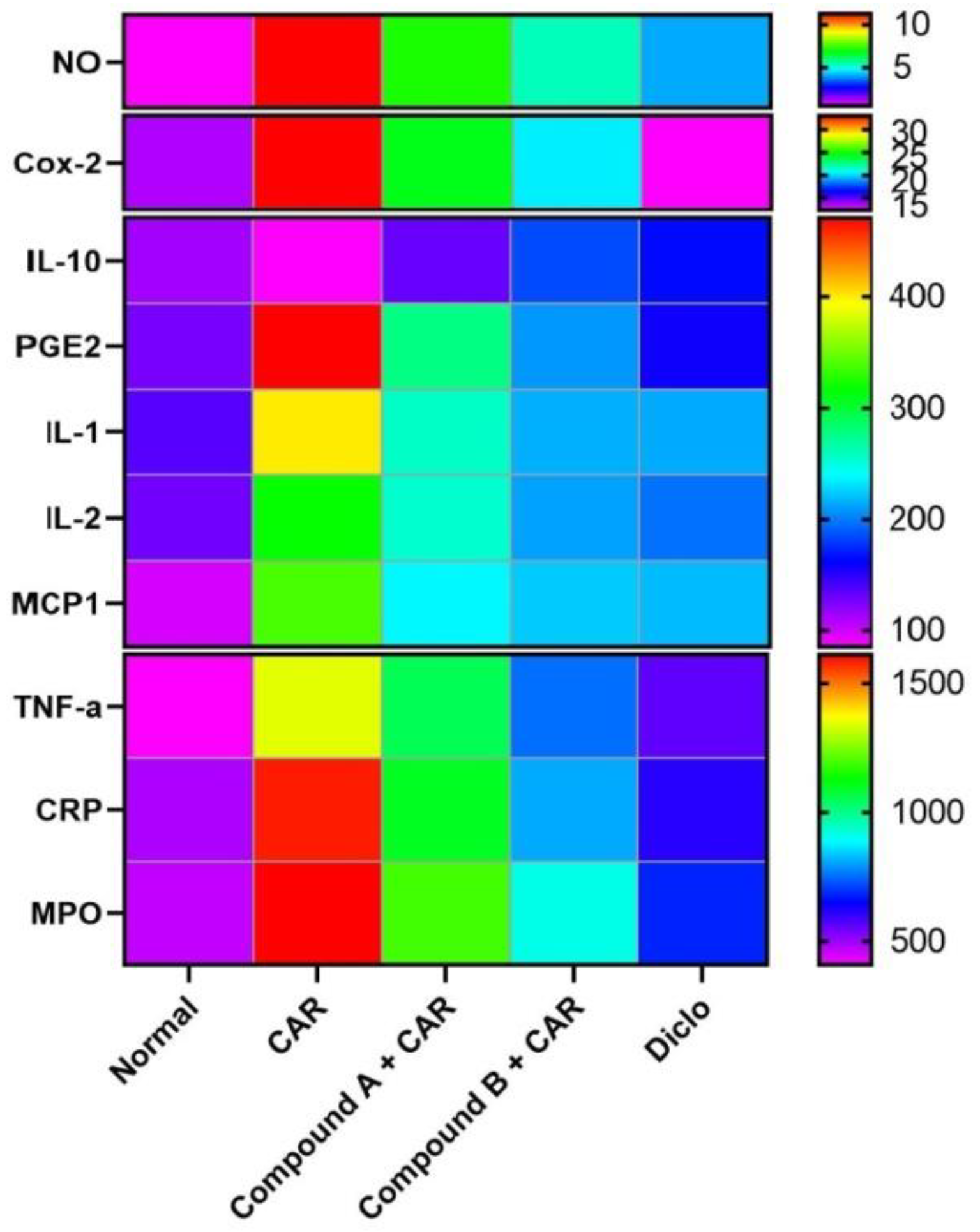
2.4. Effects of Different Compounds on CAR-Induced Inflammation
2.5. Xanthine Oxidase Inhibition Properties
3. Materials and Methods
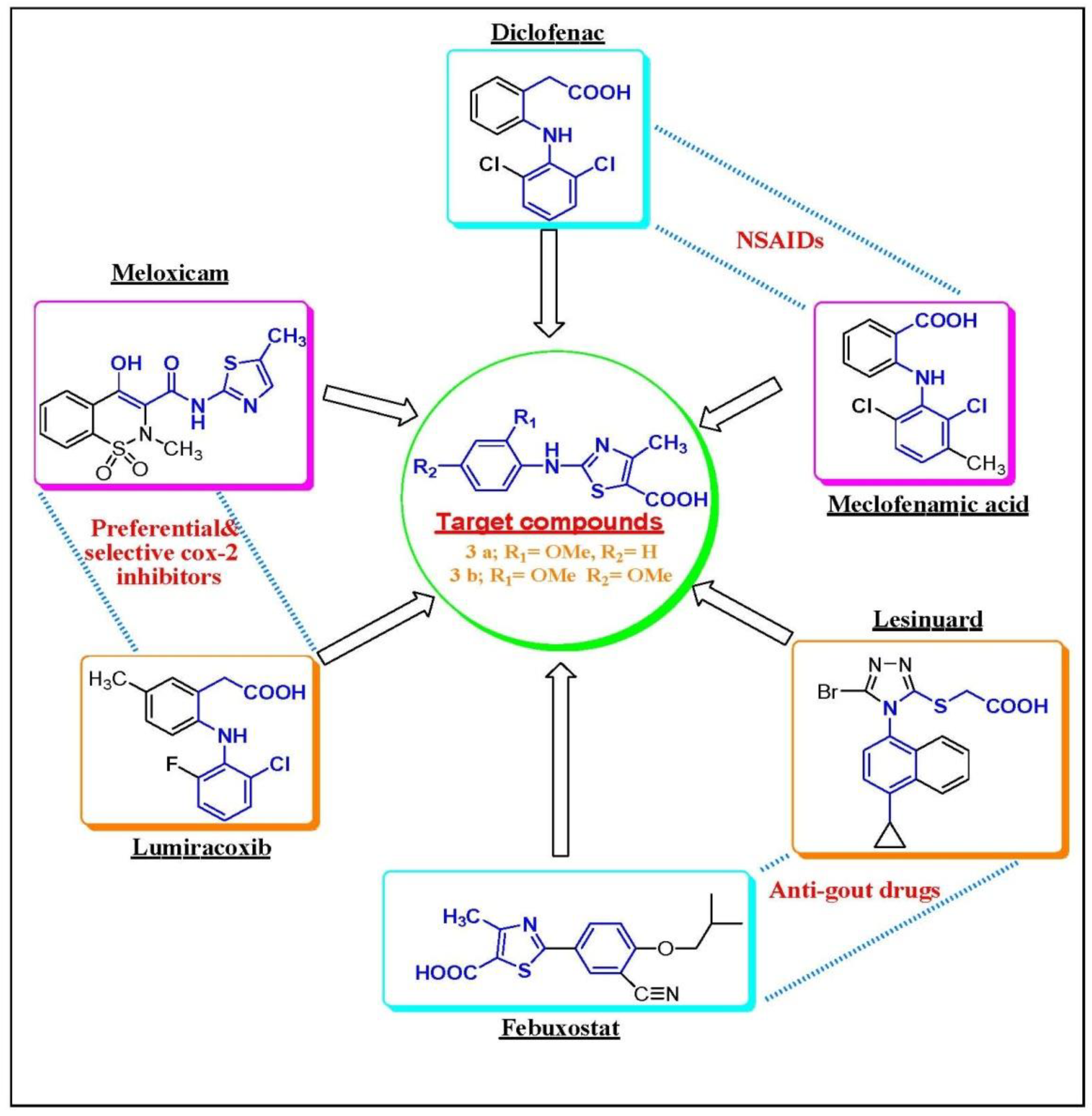
3.1. Chemistry Rationale, Synthesis and Analysis
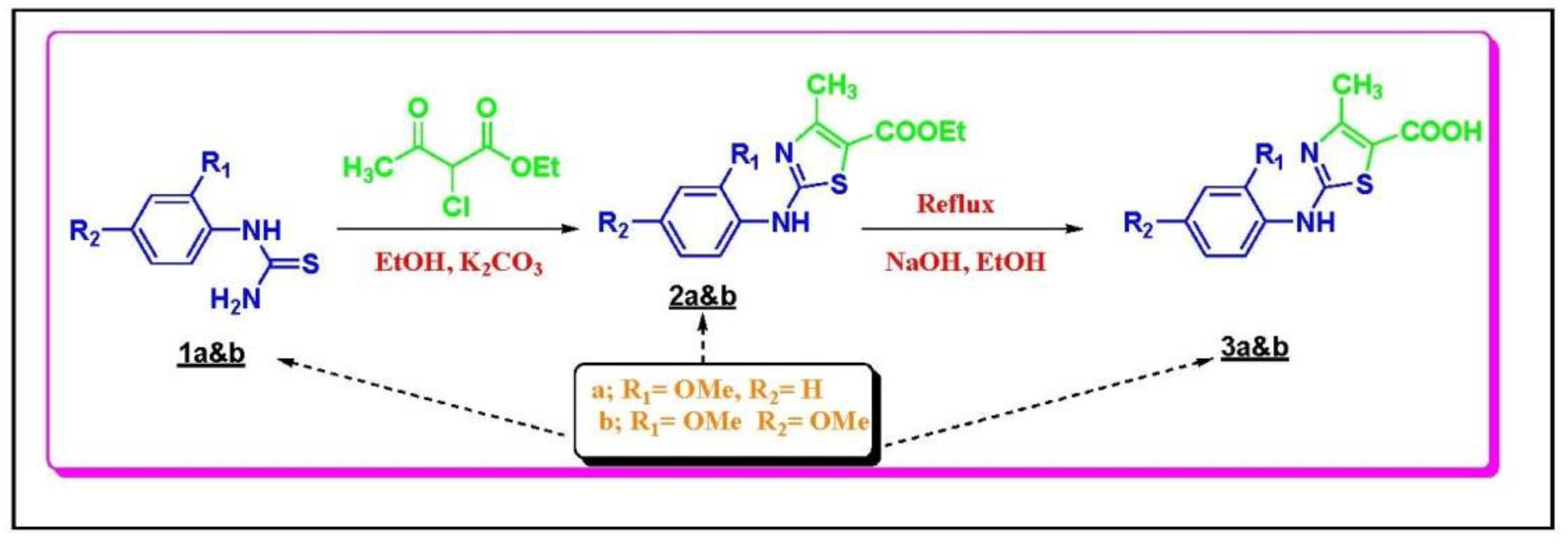
3.1.1. Chemistry Rationale
3.1.2. Chemical Synthesis
3.1.3. General Procedure for the Preparation of Compounds 2A&B
3.1.4. General Procedure for the Preparation of Compounds 3A&B
3.2. Acute Toxicity Study
3.3. Carrageenan (CAR)-Induced Paw Edema
3.4. Myeloperoxidase (MPO) Activity Assay
3.5. Measurement of Nitrite/Nitrate Levels
3.6. Cytokine and Mediator Analyses
3.7. In Vitro Analysis of XOD Inhibitory Activity
3.8. Histopathological and Immunohistochemical Analysis
3.9. Quantitative Real-Time PCR
3.10. Statistical Analyses
4. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.H.; Blesso, C.N. Dietary and endogenous sphingolipid metabolism in chronic inflammation. Nutrients 2017, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- De Rosset, L.; Strutz, K.L. Developmental origins of chronic inflammation: A review of the relationship between birth weight and C-reactive protein. Ann. Epidemiol. 2015, 25, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Al-Quraishy, S.; Moneim, A.E.A. Ziziphus spina-christi leaf extract pretreatment inhibits liver and spleen injury in a mouse model of sepsis via anti-oxidant and anti-inflammatory effects. Inflammopharmacology 2018, 26, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.K.; Lirk, P.; Tan, C.H.; Seymour, R.A. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin. Med. Res. 2007, 5, 19–34. [Google Scholar] [CrossRef] [PubMed]
- McCarberg, B.; Gibofsky, A. Need to develop new nonsteroidal anti-inflammatory drug formulations. Clin. Ther. 2012, 34, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lv, Y.; Lei, Y.; Liu, D.; Feng, Y.; Zhao, J.; Chen, S.; Meng, F.; Wang, S. Design, synthesis and biological evaluation of 1-hydroxy-2-phenyl-4-pyridyl-1H-imidazole derivatives as xanthine oxidase inhibitors. Eur. J. Med. Chem. 2018, 146, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef] [PubMed]
- McGill, N.W. The epidemiology and treatment of gout. Open Access Rheumatol. 2011, 3, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Blom, P.; DE Kerpel, J.O.; Fourmaintraux, E.P.R.; Kaletta, T.J.; Leysen, D. 5-carboxamido substituted thiazole derivatives that interact with ion channels, in particular with ion channels from the Kv family. US20080125432A1, 29 May 2008. [Google Scholar]
- Gan, T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, I.; Pfeilschifter, J.; Geisslinger, G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001, 15, 2057–2072. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.I.; Amin, H.A. Mechanism of endothelial cyto-protective and thrombo-resistance effects of sildenafil, vardenafil and tadalafil in male rabbit. Arch. Med. Sci. 2015, 11, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Song, J.U.; Jang, J.W.; Kim, T.H.; Park, H.; Park, W.S.; Jung, S.H.; Kim, G.T. Structure-based design and biological evaluation of novel 2-(indol-2-yl) thiazole derivatives as xanthine oxidase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Smelcerovic, A.; Tomovic, K.; Smelcerovic, Z.; Petronijevic, Z.; Kocic, G.; Tomasic, T.; Jakopin, Z.; Anderluh, M. Xanthine oxidase inhibitors beyond allopurinol and febuxostat; an overview and selection of potential leads based on in silico calculated physico-chemical properties, predicted pharmacokinetics and toxicity. Eur. J. Med. Chem. 2017, 135, 491–516. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Kosti, D.A.; Dimitrijevi, D.S.; Stojanovi, G.S.; Pali, I.R.; Đorđević, A.S.; Ickovski, J.D. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Sweeney, A.P.; Wyllie, S.G.; Shalliker, R.A.; Markham, J.L. Xanthine oxidase inhibitory activity of selected Australian native plants. J. Ethnopharmacol. 2001, 75, 273–277. [Google Scholar] [CrossRef]



| Compound Dose (mg/kg bwt) | Liver (g) | Kidney (g) | Heart (mg) | Spleen (mg) | Stomach (g) | Testis (mg) | |
|---|---|---|---|---|---|---|---|
| Vehicle | 1.83 ± 0.21 | 0.73 ± 0.14 | 208.61 ± 27.36 | 146.34 ± 16.28 | 0.82 ± 0.19 | 192.71 ± 20.54 | |
| Compound A | 10 | 1.86 ± 0.18 | 0.73 ± 0.15 | 212.54 ± 31.05 | 151.28 ± 17.16 | 0.82 ± 0.20 | 201.34 ± 19.76 |
| 20 | 1.85 ± 0.23 | 0.74 ± 0.13 | 201.36 ± 25.48 | 155.68 ± 14.91 | 0.84 ± 0.18 | 206.15 ± 18.66 | |
| 40 | 1.87 ± 0.17 | 0.75 ± 0.17 | 218.64 ± 33.15 | 140.19 ± 20.38 | 0.85 ± 0.21 | 201.51 ± 20.69 | |
| 100 | 1.90 ± 0.25 * | 0.78 ± 0.16 | 201.43 ± 26.27 | 163.17 ± 18.06 * | 0.81 ± 0.21 | 226.17 ± 22.35 * | |
| Compound B | 10 | 1.84 ± 0.16 | 0.74 ± 0.17 | 209.14 ± 21.60 | 138.78 ± 15.67 | 0.83 ± 0.20 | 204.32 ± 16.87 |
| 20 | 1.87 ± 0.22 | 0.76 ± 0.19 | 215.32 ± 31.47 | 148.14 ± 20.13 | 0.84 ± 0.22 | 214.81 ± 20.39 | |
| 40 | 1.86 ± 0.20 | 0.76 ± 0.15 | 219.44 ± 24.61 | 151.32 ± 17.42 | 0.84 ± 0.21 | 218.36 ± 21.65 | |
| 100 | 1.95 ± 0.27 * | 0.79 ± 0.18 | 227.64 ± 30.21 * | 174.18 ± 21.36 * | 0.87 ± 0.28 * | 236.14 ± 17.69 * | |
| Name | Accession Number | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|---|
| Gapdh | NM_001289726.1 | CCCATCACCATCTTCCAGGAGC | CCAGTGAGCTTCCCGTTCAGC |
| Ccl2 | NM_011333.3 | GCAGCAGGTGTCCCAAAGAA | ATTTACGGGTCAACTTCACATTCAA |
| Il2 | NM_008366.3 | TGAGTCAGCAACTGTGGTGG | GCCCTTGGGGCTTACAAAAAG |
| Il10 | NM_010548.2 | ATAACTGCACCCACTTCCCA | GGGCATCACTTCTACCAGGT |
| Il1b | NM_008361.4 | CCTTCCAGGATGAGGACATGA | TGAGTCACAGAGGATGGGCTC |
| Nos2 | NM_001313922.1 | CGAAACGCTTCACTTCCAA | TGAGCCTATATTGCTGTGGCT |
| Ptgs2 | NM_011198.4 | CAGACAACATAAACTGCGCCTT | GATACACCTCTCCACCAATGACC |
| Tnf | NM_001278601.1 | ACCCTCACACTCACAAACCA | ACCCTGAGCCATAATCCCCT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeer, R.S.; Hammad, S.F.; Leheta, O.F.; Abdel Moneim, A.E.; Amin, H.K. Anti-Inflammatory and Anti-Hyperuricemic Functions of Two Synthetic Hybrid Drugs with Dual Biological Active Sites. Int. J. Mol. Sci. 2019, 20, 5635. https://doi.org/10.3390/ijms20225635
Almeer RS, Hammad SF, Leheta OF, Abdel Moneim AE, Amin HK. Anti-Inflammatory and Anti-Hyperuricemic Functions of Two Synthetic Hybrid Drugs with Dual Biological Active Sites. International Journal of Molecular Sciences. 2019; 20(22):5635. https://doi.org/10.3390/ijms20225635
Chicago/Turabian StyleAlmeer, Rafa S., Sherif F. Hammad, Ola F. Leheta, Ahmed E. Abdel Moneim, and Hatem K. Amin. 2019. "Anti-Inflammatory and Anti-Hyperuricemic Functions of Two Synthetic Hybrid Drugs with Dual Biological Active Sites" International Journal of Molecular Sciences 20, no. 22: 5635. https://doi.org/10.3390/ijms20225635
APA StyleAlmeer, R. S., Hammad, S. F., Leheta, O. F., Abdel Moneim, A. E., & Amin, H. K. (2019). Anti-Inflammatory and Anti-Hyperuricemic Functions of Two Synthetic Hybrid Drugs with Dual Biological Active Sites. International Journal of Molecular Sciences, 20(22), 5635. https://doi.org/10.3390/ijms20225635






