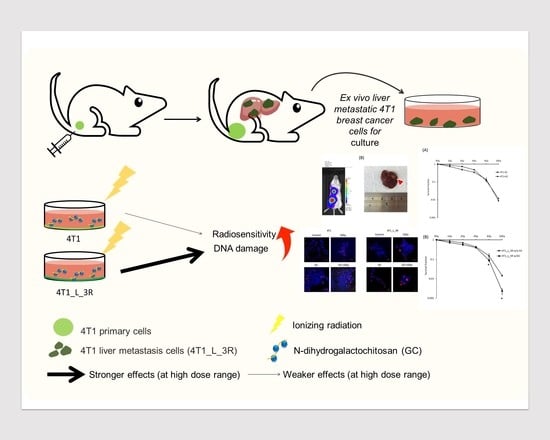
N-Dihydrogalactochitosan Potentiates the Radiosensitivity of Liver Metastatic Tumor Cells Originated from Murine Breast Tumors
Abstract
1. Introduction
2. Results
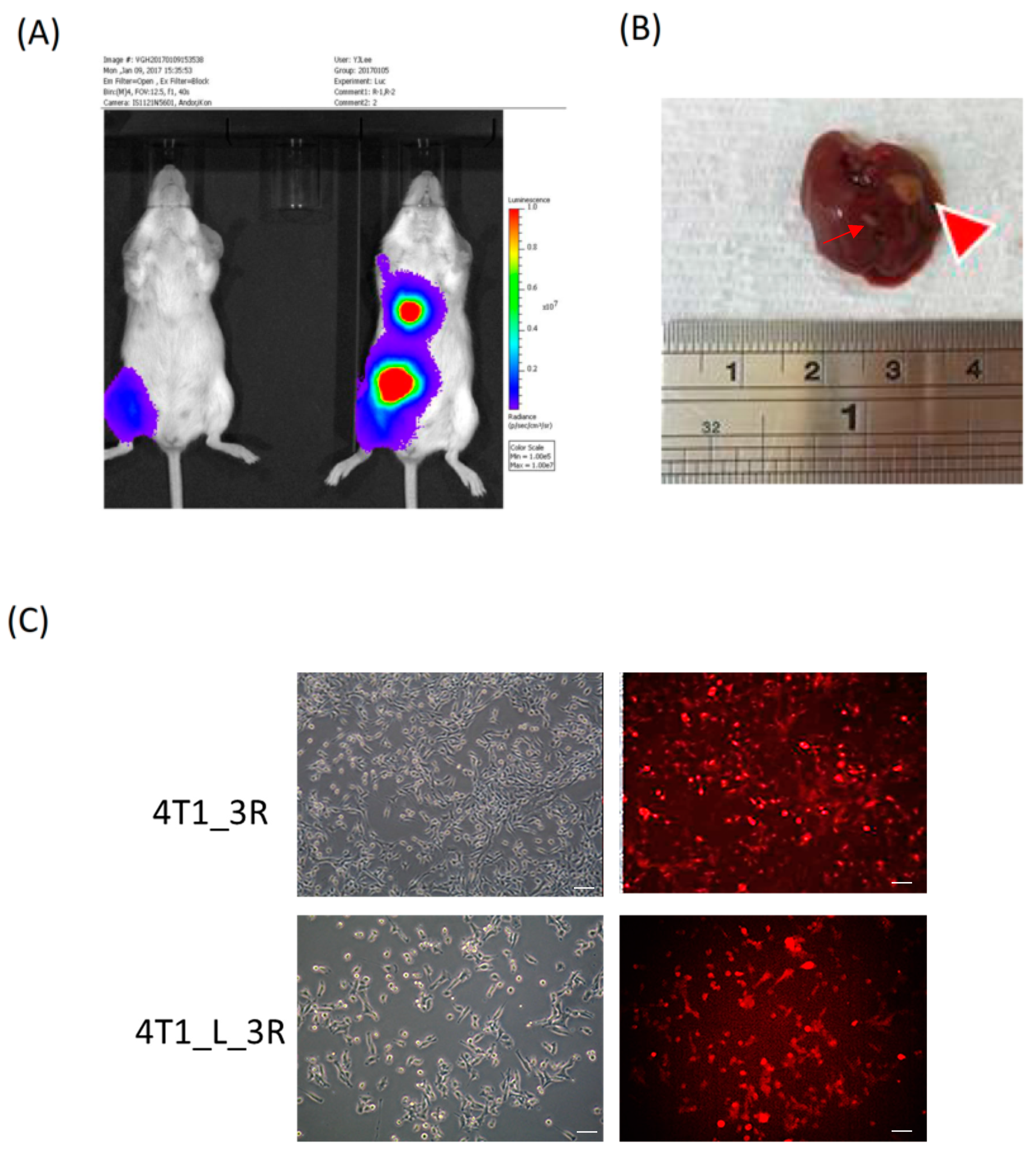
2.1. Isolation of Liver-Metastatic 4T1 Murine Breast Cancer Cells Based on Molecular Imaging
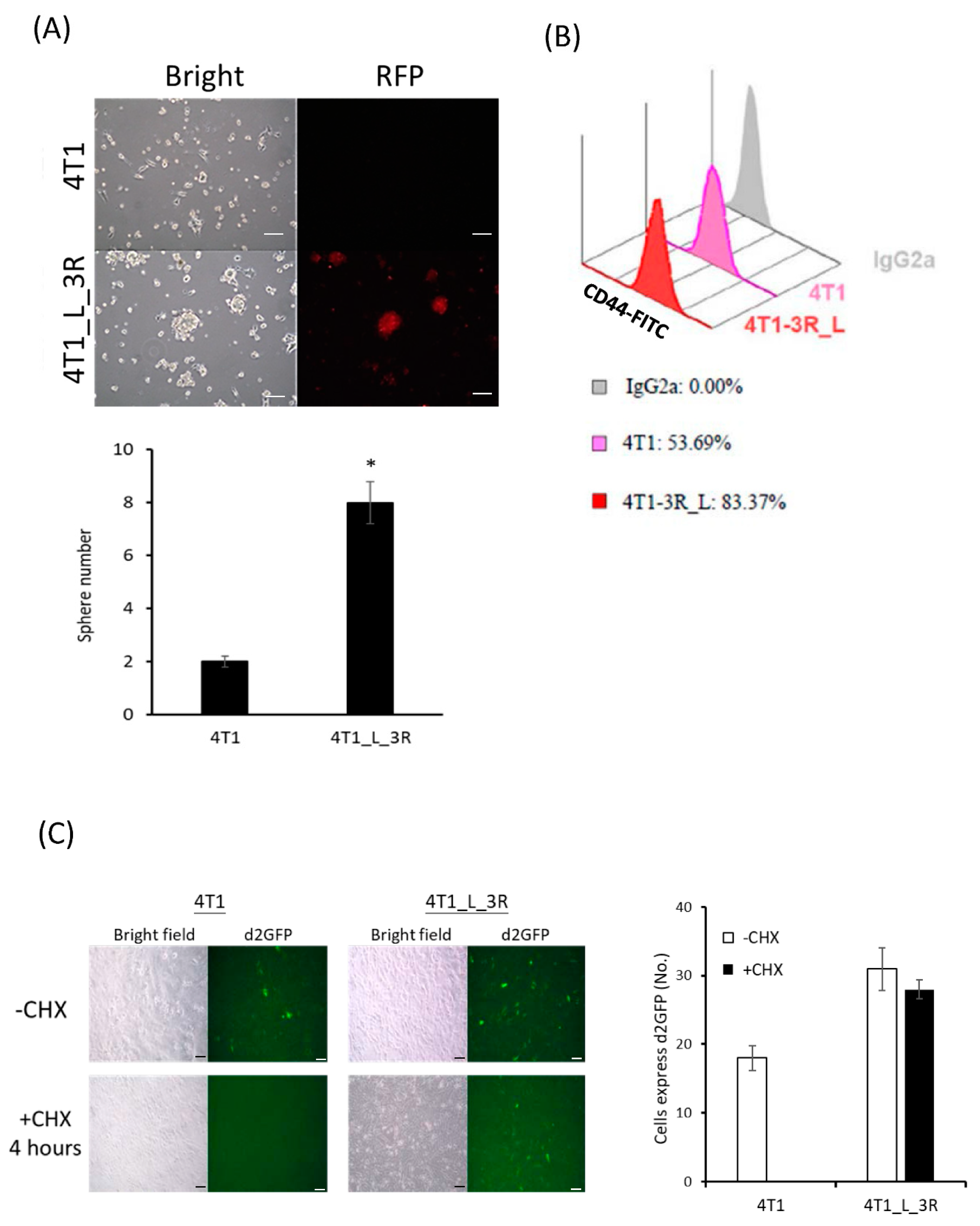
2.2. Liver-Metastatic 4T1 Cancer Cells Exhibit Properties of Tumor-Initiating Cells
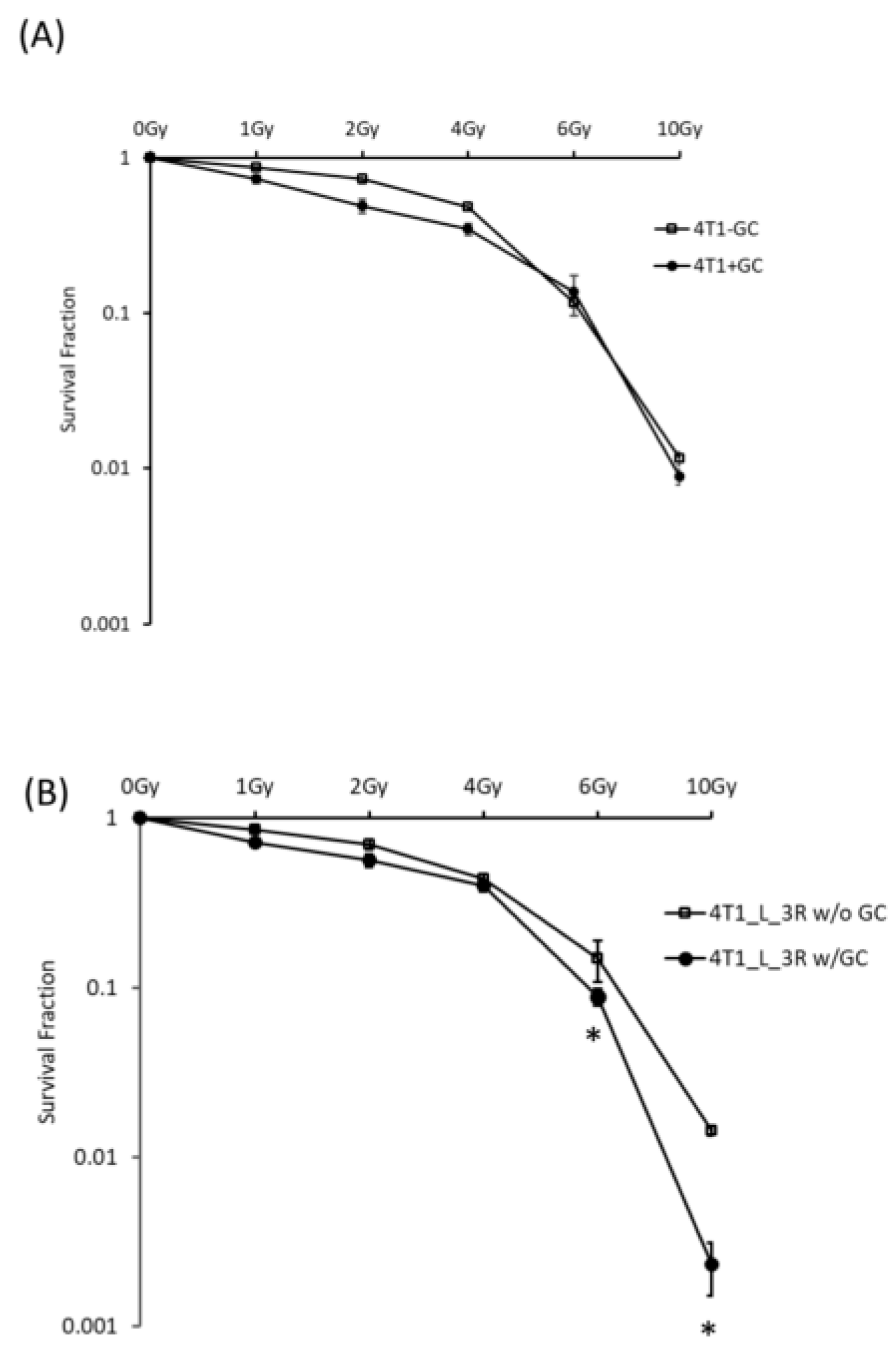
2.3. GC Enhances Radiosensitivity in Liver-Metastatic 4T1 Murine Breast Cancer Cells
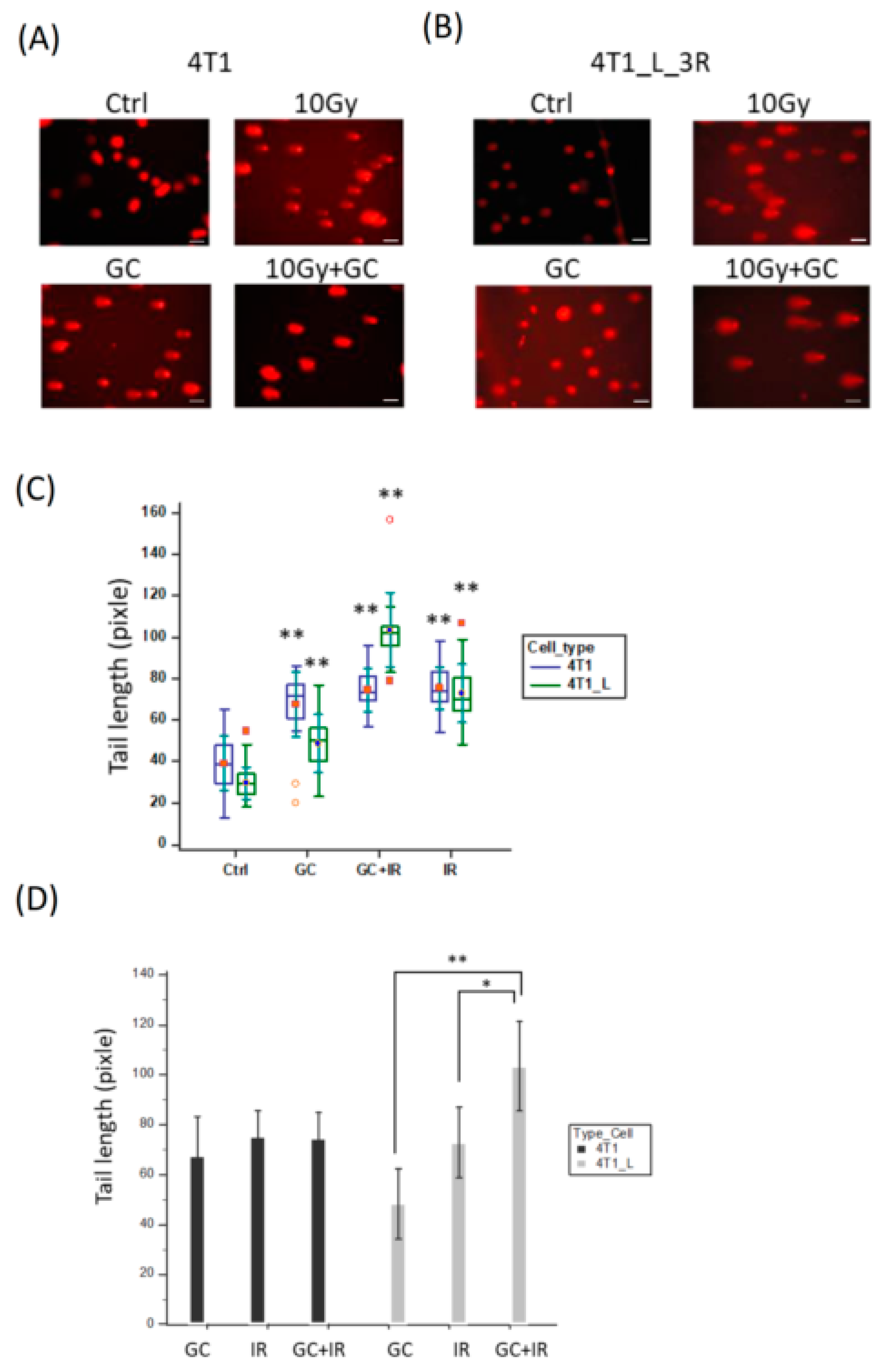
2.4. GC Enhances Ionizing Radiation-Induced DNA Damage in Liver-Metastatic 4T1 Cells
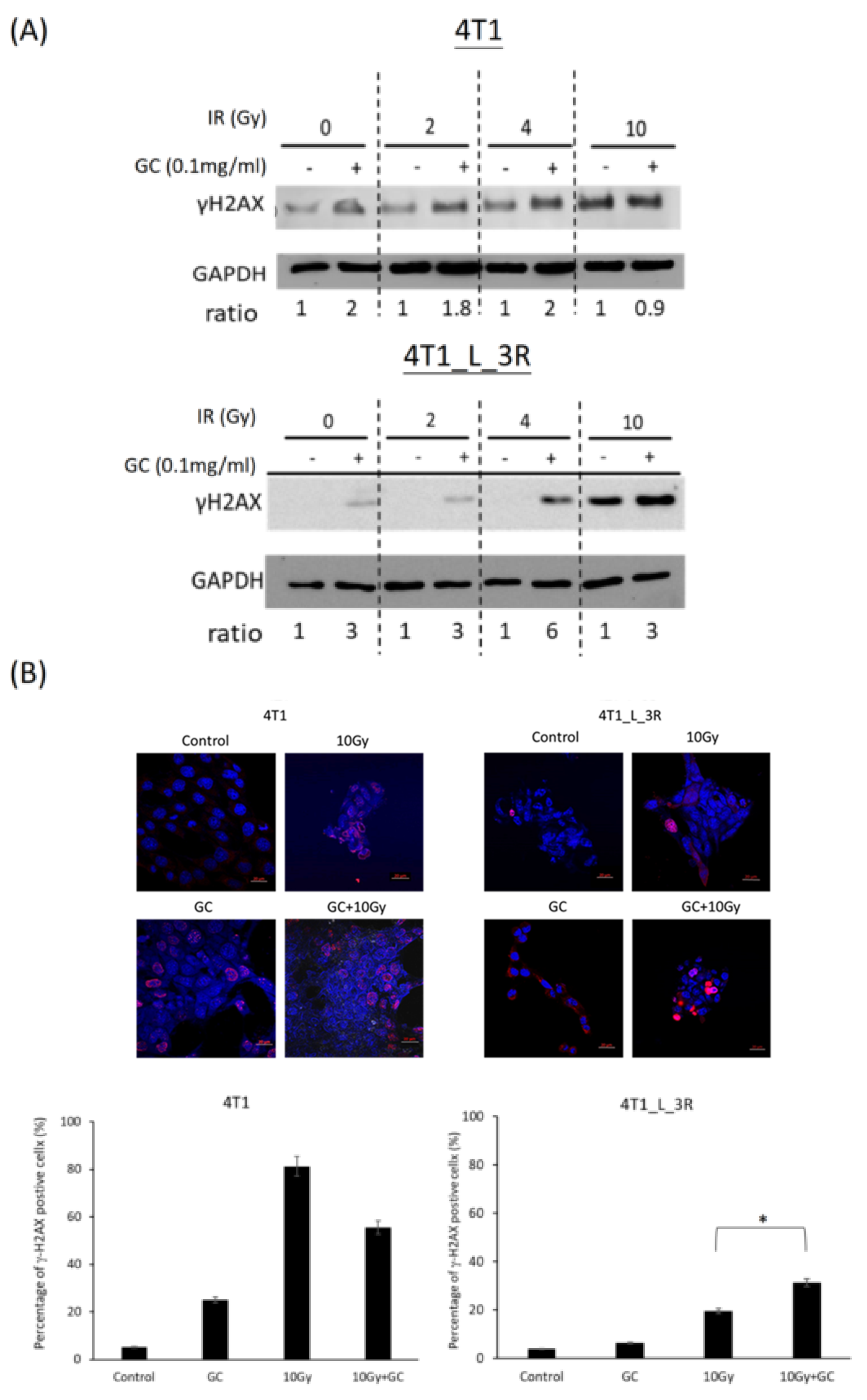
2.5. GC Combined to X-Rays Increases the Level of γ-H2AX in Liver-Metastatic 4T1 Cells More than in Parental 4T1 Cells
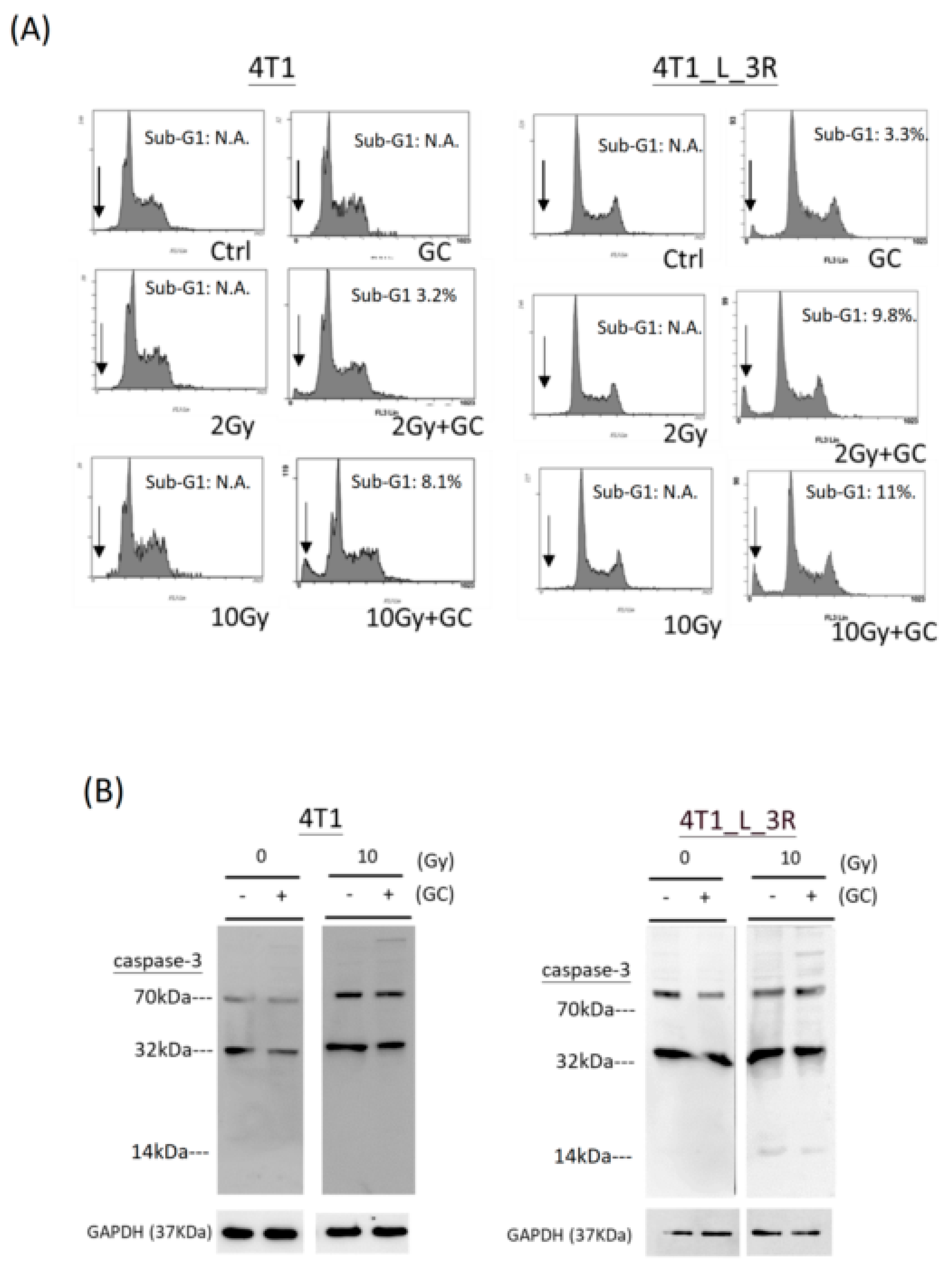
2.6. Effects of GC Combined with X-Rays on Apoptosis of Liver-Metastatic 4T1 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. GC and Ionizing Radiation Treatments
4.3. Establishment of a 4T1-3R Metastatic Animal Model
4.4. Colony Formation Assay
4.5. Comet Assay
4.6. Protein Extraction and Western Blotting
4.7. γ-H2AX Foci Assay
4.8. Flow Cytometric Analysis of DNA Histograms
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Mostert, B.; Sleijfer, S.; Foekens, J.A.; Gratama, J.W. Circulating tumor cells (CTCs): Detection methods and their clinical relevance in breast cancer. Cancer Treat. Rev. 2009, 35, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.R.; Tiega Donfack, B.P.; Trumm, C.; Zech, C.J.; Michl, M.; Laubender, R.P.; Uebleis, C.; Bartenstein, P.; Heinemann, V.; Hacker, M. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J. Nucl. Med. 2012, 53, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.W.; Nakagawa, K.; Seto, T.; Crino, L.; Ahn, M.J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef]
- Kleeff, J.; Michl, P. Targeted therapy of pancreatic cancer: Biomarkers are needed. Lancet Oncol. 2017, 18, 421–422. [Google Scholar] [CrossRef]
- Glassman, D.; Hignett, S.; Rehman, S.; Linforth, R.; Salhab, M. Adjuvant Endocrine Therapy for Hormone-positive Breast Cancer, Focusing on Ovarian Suppression and Extended Treatment: An Update. Anticancer Res. 2017, 37, 5329–5341. [Google Scholar]
- Yousefi, H.; Yuan, J.; Keshavarz-Fathi, M.; Murphy, J.F.; Rezaei, N. Immunotherapy of cancers comes of age. Expert Rev. Clin. Immunol. 2017, 13, 1001–1015. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075. [Google Scholar] [CrossRef]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef]
- Harrington, K.; Jankowska, P.; Hingorani, M. Molecular biology for the radiation oncologist: The 5Rs of radiobiology meet the hallmarks of cancer. Clin. Oncol. 2007, 19, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Lewensohn, R.; Zhivotovsky, B. Role of apoptosis in the response of lung carcinomas to anti-cancer treatment. Ann. N. Y. Acad. Sci. 2000, 926, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer Radiosensitizers. Trends Pharm. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Scicinski, J.; Ning, S.; Peehl, D.; Oronsky, A.; Cabrales, P.; Bednarski, M.; Knox, S. RRx-001, A novel dinitroazetidine radiosensitizer. Investig. New Drugs 2016, 34, 371–377. [Google Scholar] [CrossRef]
- Zhou, F.; Li, X.; Naylor, M.F.; Hode, T.; Nordquist, R.E.; Alleruzzo, L.; Raker, J.; Lam, S.S.; Du, N.; Shi, L.; et al. InCVAX--a novel strategy for treatment of late-stage, metastatic cancers through photoimmunotherapy induced tumor-specific immunity. Cancer Lett. 2015, 359, 169–177. [Google Scholar] [CrossRef]
- Chen, W.R.; Carubelli, R.; Liu, H.; Nordquist, R.E. Laser immunotherapy: A novel treatment modality for metastatic tumors. Mol. Biotechnol. 2003, 25, 37–44. [Google Scholar] [CrossRef]
- Vlashi, E.; Kim, K.; Lagadec, C.; Donna, L.D.; McDonald, J.T.; Eghbali, M.; Sayre, J.W.; Stefani, E.; McBride, W.; Pajonk, F. In vivo imaging, tracking, and targeting of cancer stem cells. J. Natl. Cancer Inst. 2009, 101, 350–359. [Google Scholar] [CrossRef]
- Song, S.; Zhou, F.; Nordquist, R.E.; Carubelli, R.; Liu, H.; Chen, W.R. Glycated chitosan as a new non-toxic immunological stimulant. Immunopharmacol. Immunotoxicol. 2009, 31, 202–208. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, C.Y.; Yang, F.Y.; Wang, B.S.; Chen, J.Y.; Lin, L.T.; Leu, J.D.; Chiu, S.J.; Chen, F.D.; Lee, Y.J.; et al. Synergistic effects of glycated chitosan with high-intensity focused ultrasound on suppression of metastases in a syngeneic breast tumor model. Cell Death Dis. 2014, 5, e1178. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, J.; Zhang, Y.; Liu, M.; Lang, M.L.; Li, M.; Chen, W.R. Local Phototherapy Synergizes with Immunoadjuvant for Treatment of Pancreatic Cancer through Induced Immunogenic Tumor Vaccine. Clin. Cancer Res. 2018, 24, 5335–5346. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Leu, J.D.; Chang, C.Y.; Lee, Y.J.; Chen, W.R. Combination of Radiofrequency Ablation and Glycated Chitosan as Treatment on a Syngeneic Breast Tumor Model. Anticancer Res. 2017, 37, 2965–2974. [Google Scholar] [PubMed]
- Borrego-Soto, G.; Ortiz-Lopez, R.; Rojas-Martinez, A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet. Mol. Biol. 2015, 38, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, K.; Almasan, A. Histone H2AX phosphorylation: A marker for DNA damage. Methods Mol. Biol. 2012, 920, 613–626. [Google Scholar] [PubMed]
- Ko, Y.S.; Jin, H.; Lee, J.S.; Park, S.W.; Chang, K.C.; Kang, K.M.; Jeong, B.K.; Kim, H.J. Radioresistant breast cancer cells exhibit increased resistance to chemotherapy and enhanced invasive properties due to cancer stem cells. Oncol. Rep. 2018, 40, 3752–3762. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Nie, B.; Pienta, K.J.; Morgan, T.M.; Taichman, R.S. Cancer stem cells and their role in metastasis. Pharmacol. Ther. 2013, 138, 285–293. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, S.Y.; Liu, R.S.; Wang, H.E.; Chen, J.C.; Chiou, S.H.; Chang, C.A.; Lin, L.T.; Tan, D.T.; Lee, Y.J. Remnant living cells that escape cell loss in late-stage tumors exhibit cancer stem cell-like characteristics. Cell Death Dis. 2012, 3, e399. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef]
- McMahon, S.J. The linear quadratic model: Usage, interpretation and challenges. Phys. Med. Biol. 2018, 64, 01TR01. [Google Scholar] [CrossRef]
- Franken, N.A.; Oei, A.L.; Kok, H.P.; Rodermond, H.M.; Sminia, P.; Crezee, J.; Stalpers, L.J.; Barendsen, G.W. Cell survival and radiosensitisation: Modulation of the linear and quadratic parameters of the LQ model (Review). Int. J. Oncol. 2013, 42, 1501–1515. [Google Scholar] [CrossRef]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Chitosan promotes ROS-mediated apoptosis and S phase cell cycle arrest in triple-negative breast cancer cells: Evidence for intercalative interaction with genomic DNA. RSC Adv. 2017, 7, 43141–43150. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Munoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Song, S.; Chen, W.R.; Xing, D. Immunostimulatory properties of glycated chitosan. J. Xray Sci. Technol. 2011, 19, 285–292. [Google Scholar] [PubMed]
- McIlwrath, A.J.; Vasey, P.A.; Ross, G.M.; Brown, R. Cell cycle arrests and radiosensitivity of human tumor cell lines: Dependence on wild-type p53 for radiosensitivity. Cancer Res. 1994, 54, 3718–3722. [Google Scholar] [PubMed]
- Naderi, S.; Hunton, I.C.; Wang, J.Y. Radiation dose-dependent maintenance of G(2) arrest requires retinoblastoma protein. Cell Cycle 2002, 1, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Aslakson, C.J.; Miller, F.R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992, 52, 1399–1405. [Google Scholar] [PubMed]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Lee, T.J.; Kim, E.J.; Kim, S.; Jung, E.M.; Park, J.W.; Jeong, S.H.; Park, S.E.; Yoo, Y.H.; Kwon, T.K. Caspase-dependent and caspase-independent apoptosis induced by evodiamine in human leukemic U937 cells. Mol. Cancer 2006, 5, 2398–2407. [Google Scholar] [CrossRef]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Leu, J.D.; Chiu, Y.W.; Lo, C.C.; Chiang, P.H.; Chiu, S.J.; Tsai, C.H.; Hwang, J.J.; Chen, R.C.; Gorbunova, V.; Lee, Y.J. Enhanced cellular radiosensitivity induced by cofilin-1 over-expression is associated with reduced DNA repair capacity. Int. J. Radiat. Biol. 2013, 89, 433–444. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lin, L.T.; Wang, C.Y.; Chiu, Y.W.; Chou, Y.T.; Chiu, S.J.; Wang, H.E.; Liu, R.S.; Wu, C.Y.; Chan, P.C.; et al. Over-expression of cofilin-1 suppressed growth and invasion of cancer cells is associated with up-regulation of let-7 microRNA. Biochim. Biophys. Acta. 2015, 1852, 851–861. [Google Scholar] [CrossRef]
- Friesner, J.D.; Liu, B.; Culligan, K.; Britt, A.B. Ionizing radiation-dependent gamma-H2AX focus formation requires ataxia telangiectasia mutated and ataxia telangiectasia mutated and Rad3-related. Mol. Biol. Cell 2005, 16, 2566–2576. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-Y.; Chang, C.-Y.; Wang, C.-Y.; Liu, K.; Kang, C.-Y.; Lee, Y.-J.; Chen, W.R. N-Dihydrogalactochitosan Potentiates the Radiosensitivity of Liver Metastatic Tumor Cells Originated from Murine Breast Tumors. Int. J. Mol. Sci. 2019, 20, 5581. https://doi.org/10.3390/ijms20225581
Wang C-Y, Chang C-Y, Wang C-Y, Liu K, Kang C-Y, Lee Y-J, Chen WR. N-Dihydrogalactochitosan Potentiates the Radiosensitivity of Liver Metastatic Tumor Cells Originated from Murine Breast Tumors. International Journal of Molecular Sciences. 2019; 20(22):5581. https://doi.org/10.3390/ijms20225581
Chicago/Turabian StyleWang, Chung-Yih, Chun-Yuan Chang, Chun-Yu Wang, Kaili Liu, Chia-Yun Kang, Yi-Jang Lee, and Wei R. Chen. 2019. "N-Dihydrogalactochitosan Potentiates the Radiosensitivity of Liver Metastatic Tumor Cells Originated from Murine Breast Tumors" International Journal of Molecular Sciences 20, no. 22: 5581. https://doi.org/10.3390/ijms20225581
APA StyleWang, C.-Y., Chang, C.-Y., Wang, C.-Y., Liu, K., Kang, C.-Y., Lee, Y.-J., & Chen, W. R. (2019). N-Dihydrogalactochitosan Potentiates the Radiosensitivity of Liver Metastatic Tumor Cells Originated from Murine Breast Tumors. International Journal of Molecular Sciences, 20(22), 5581. https://doi.org/10.3390/ijms20225581