Therapeutic Properties of Mesenchymal Stem Cell on Organ Ischemia-Reperfusion Injury
Abstract
1. Introduction
2. Mesenchymal Stem Cells
2.1. Heart Treatment
2.2. Kidney Treatment
2.3. Liver Treatment
2.4. Lung Treatment
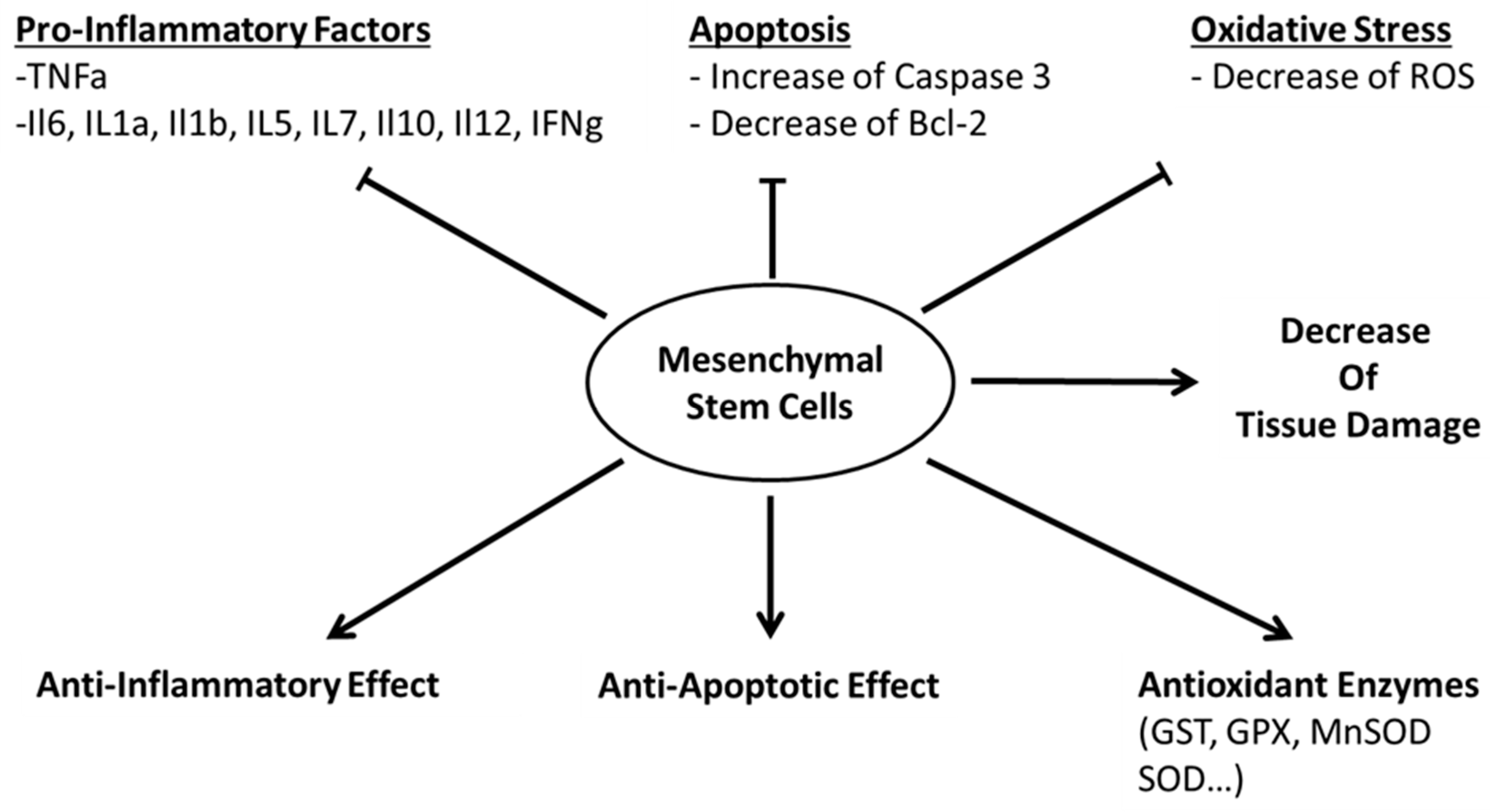
3. Mechanism of Action
(a) Paracrine Activity
(b) Cell-Cell Interaction
(c) Secretion of Vesicles
(d) Failed Treatment
4. Discussion and Perspectives
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCs | Adipose-Derived Stem Cells |
| ALP | Alkaline phosphatase |
| ALT | Alanine AmnioTransferase |
| AQP2 | Aquaporin 2 |
| AST | Aspartate AminoTransferase |
| ATP | Adenosine Triphosphate |
| Bad | Bcl-2-associated death |
| Bax | BCL2 Associated X, Apoptosis Regulator |
| Bcl-2 | B-Cell CLL/Lymphoma 2 |
| BMSCs | Bone Marrow Stem Cells |
| CCL4 | Carbon tetrachloride |
| CKMB | Creatine kinase-muscle/brain |
| CXCL2 | Chemokine ligand 2 |
| FGF | Fibroblast Growth Factor |
| G-CSF | Granulocyte-colony stimulating factor |
| GSH | Glutathione |
| HGF | Hepatocyte Growth Factor |
| HIF1a | Hypoxia-inducible factor |
| HMOX-1 | Heme oxygenase (decycling) 1 |
| HO-1 | Heme oxygenase-1 |
| I-CAM1 | Intercellular Adhesion Molecule 1 |
| IGFBP3 | Insulin-like growth factor-binding protein 3 |
| IFN | Interferon |
| IRI | Ischemia-Reperfusion Injury |
| LDH | Lactate dehydrogenase |
| LPSs | Lipopolysaccharides |
| MAPK | Mitogen Activated Protein Kinase |
| MDA | Malondialdehyde |
| MnSOD | Manganese Superoxide Dismutase |
| NKT | Natural Killer T |
| NLRP12 | NLR Family Pyrin Domain Containing 12 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PDGF | Platelet-derived growth factor |
| PECAM-1 | Platelet endothelial cell adhesion molecule |
| PGE2 | Prostaglandin E2 |
| PTX3 | Pentraxin 3 |
| SOD | Super Oxide Dismutase |
| MSCs | Mesenchymal Stem Cells |
| NFκB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| PARP | Poly (ADP-ribose) polymerase |
| SIRT | NAD-dependent deacetylase sirtuin |
| TIMP-1 | Metalloproteinase inhibitor 1 |
| TNF | Tumor Necrosis Factor |
| UCMSCs | Umbilical Cord Mesenchymal Stem Cells |
| VEGF | Vascular endothelial growth factor |
| XCl1 | X-C Motif Chemokine Ligand 1 |
References
- Murray, J. Interview with Dr Joseph Murray (by Francis L Delmonico). Am. J. Transplant. 2002, 2, 803–806. [Google Scholar] [PubMed]
- Knihs, N.D.S.; Roza, B.D.A.; Schirmer, J.; Ferraz, A.S. Application of Spanish quality instruments about organ donation and tranplants validated in pilot hospitals in Santa Catarina. Braz. J. Nephrol. 2015, 37, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.H.; Quigley, M. Opt-out organ donation: On evidence and public policy. J. R. Soc. Med. 2014, 107, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Miranda, B.; Fernandez Lucas, M.; de Felipe, C.; Naya, M.; Gonzalez-Posada, J.M.; Matesanz, R. Organ donation in Spain. Nephrol. Dial. Transplant. 1999, 14 (Suppl. S3), 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, P.; Guo, J.; Hu, N.; Wang, S.; Li, D.; Hu, S.; Ren, J.; Cao, F.; Chen, Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017, 13, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Braza, F.; Brouard, S.; Chadban, S.; Goldstein, D.R. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat. Rev. Nephrol. 2016, 12, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, L.; Li, L.; Pokhrel, G.; Qi, G.; Rong, R.; Zhu, T. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement. Altern. Med. 2014, 14, 19. [Google Scholar] [CrossRef]
- Funahashi, Y.; Yoshino, Y.; Sassa, N.; Matsukawa, Y.; Takai, S.; Gotoh, M. Comparison of Warm and Cold Ischemia on Renal Function After Partial Nephrectomy. Urology 2014, 84, 1408–1412. [Google Scholar] [CrossRef]
- Banner, N.R.; Thomas, H.L.; Curnow, E.; Hussey, J.C.; Rogers, C.A.; Bonser, R.S.; Steering Group of the United Kingdom Cardiothoracic Transplant Audit. The importance of cold and warm cardiac ischemia for survival after heart transplantation. Transplantation 2008, 86, 542–547. [Google Scholar] [CrossRef]
- Southard, J.H.; Belzer, F.O. Organ preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef]
- Vroemen, J.P.; van der Vliet, J.A.; Cohen, B.; Persijn, G.G.; Lansbergen, Q.; Kootstra, G. The Influence of Warm and Cold Ischemic Time on the Outcome of Cadaveric Renal Transplantation. Eur. Surg. Res. 1984, 16, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Kosieradzki, M.; Pratschke, J.; Kupiec-Weglinski, J.; Rowinski, W. Ischemia/Reperfusion Injury, Its Mechanisms, and Prevention. J. Transplant. 2012, 2012, 610370. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef]
- Kukan, M. Emerging roles of proteasomes in ischemia-reperfusion injury of organs. J. Physiol. Pharmacol. 2004, 55, 3–15. [Google Scholar]
- Kobayashi, M.; Takeyoshi, I.; Yoshinari, D.; Matsumoto, K.; Morishita, Y. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery 2002, 131, 344–349. [Google Scholar] [CrossRef]
- Ingram, T.E.; Fraser, A.G.; Bleasdale, R.A.; Ellins, E.A.; Margulescu, A.D.; Halcox, J.P.; James, P.E. Low-Dose Sodium Nitrite Attenuates Myocardial Ischemia and Vascular Ischemia-Reperfusion Injury in Human Models. J. Am. Coll. Cardiol. 2013, 61, 2534–2541. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, 264–277. [Google Scholar] [CrossRef]
- Kupatt, C.; Hinkel, R.; Lamparter, M.; Von Brühl, M.L.; Pohl, T.; Horstkotte, J.; Beck, H.; Müller, S.; Delker, S.; Gildehaus, F.J.; et al. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: Role of phosphatidylinositol 3-kinase/AKT kinase. Circulation 2005, 112, 117–122. [Google Scholar]
- Preda, M.B.; Rønningen, T.; Burlacu, A.; Simionescu, M.; Moskaug, J.O.; Valen, G. Remote Transplantation of Mesenchymal Stem Cells Protects the Heart Against Ischemia-Reperfusion Injury. Stem Cells 2014, 32, 2123–2134. [Google Scholar] [CrossRef]
- Takamura, M.; Usui, S.; Inoue, O.; Ootsuji, H.; Takashima, S.I.; Nomura, A.; Kato, T.; Murai, H.; Furusho, H.; Sakai, Y.; et al. Adipose-derived regenerative cells exert beneficial effects on systemic responses following myocardial ischemia/reperfusion. Cardiol. J. 2016, 23, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Yano, R.; Inadomi, C.; Luo, L.; Goto, S.; Hara, T.; Li, T.S. The effect of transient oxygenation on stem cell mobilization and ischemia/reperfusion heart injury. PLoS ONE 2018, 13, e0192733. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; He, Z.; Liang, Z.; Chen, Z.; Wang, H.; Zhang, J. Exosomes from Adipose-derived Mesenchymal Stem Cells Protect the Myocardium Against Ischemia/Reperfusion Injury Through Wnt/beta-Catenin Signaling Pathway. J. Cardiovasc. Pharmacol. 2017, 70, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, P.; Song, P.; Xiong, W.; Chen, H.; Peng, W.; Wang, S.; Li, S.; Fu, Z.; Wang, Y.; et al. Pretreatment of Adipose Derived Stem Cells with Curcumin Facilitates Myocardial Recovery via Antiapoptosis and Angiogenesis. Stem Cells Int. 2015, 2015, 638153. [Google Scholar] [CrossRef]
- Williams, A.R.; Hatzistergos, K.E.; Addicott, B.; McCall, F.; Carvalho, D.; Suncion, V.; Morales, A.R.; Da Silva, J.; Sussman, M.A.; Heldman, A.W.; et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 2013, 127, 213–223. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ahn, Y.; Kwon, J.S.; Cho, Y.K.; Jeong, M.H.; Cho, J.G.; Park, J.C.; Kang, J.C. Priming of Mesenchymal Stem Cells with Oxytocin Enhances the Cardiac Repair in Ischemia/Reperfusion Injury. Cells Tissues Organs 2012, 195, 428–442. [Google Scholar] [CrossRef]
- Sadek, E.M.; Afifi, N.M.; ElFattah, L.I.A.; Mohsen, M.A.A.E. Histological Study on Effect of Mesenchymal Stem Cell Therapy on Experimental Renal Injury Induced by Ischemia/Reperfusion in Male Albino Rat. Int. J. Stem Cells 2013, 6, 55–66. [Google Scholar] [CrossRef]
- Edelstein, C.L. Biomarkers of acute kidney injury. Adv. Chronic Kidney Dis. 2008, 15, 222–234. [Google Scholar] [CrossRef]
- Jang, M.J.; You, D.; Park, J.Y.; Kim, K.; Aum, J.; Lee, C.; Song, G.; Shin, H.C.; Suh, N.; Kim, Y.M.; et al. Hypoxic Preconditioned Mesenchymal Stromal Cell Therapy in a Rat Model of Renal Ischemia-reperfusion Injury: Development of Optimal Protocol to Potentiate Therapeutic Efficacy. Int. J. Stem Cells 2018, 11, 157–167. [Google Scholar] [CrossRef]
- Mias, C.; Trouche, E.; Seguelas, M.H.; Calcagno, F.; Sabatier, F.; Daniel, L.; Bianchi, P.; Calise, D.; Bourin, P.; Parini, A.; et al. Ex Vivo Pretreatment with Melatonin Improves Survival, Proangiogenic/Mitogenic Activity, and Efficiency of Mesenchymal Stem Cells Injected into Ischemic Kidney. Stem Cells 2008, 26, 1749–1757. [Google Scholar] [CrossRef]
- Wang, B.; Wen, H.; Smith, W.; Hao, D.; He, B.; Kong, L. Regulation effects of melatonin on bone marrow mesenchymal stem cell differentiation. J. Cell. Physiol. 2019, 234, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Kadry, S.M.; El-Dakdoky, M.H.; Haggag, N.Z.; Rashed, L.A.; Hassen, M.T.; Zakaria, N. Melatonin improves the therapeutic role of mesenchymal stem cells in diabetic rats. Toxicol. Mech. Methods 2018, 28, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Oron, U.; Tuby, H.; Maltz, L.; Sagi-Assif, O.; Abu-Hamed, R.; Yaakobi, T.; Doenyas-Barak, K.; Efrati, S. Autologous Bone-Marrow Stem Cells Stimulation Reverses Post-Ischemic-Reperfusion Kidney Injury in Rats. Am. J. Nephrol. 2014, 40, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Murty, M.S.N.; Sharma, U.K.; Pandey, V.B.; Kankare, S.B. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J. Nephrol. 2013, 23, 180–183. [Google Scholar] [CrossRef]
- Haga, H.; Yan, I.K.; Borrelli, D.A.; Matsuda, A.; Parasramka, M.; Shukla, N.; Lee, D.D.; Patel, T.; Borelli, D. Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transplant. 2017, 23, 791–803. [Google Scholar] [CrossRef]
- El-Tahawy, A.; Ali, A.H.S.A. Possible Protective Effect of Bone Marrow-Mesenchymal Stem Cells (BM-MSCs) Against the Remote Liver Injury Induced by Renal Ischemia Reperfusion in Male Albino Rats. J. Cytol. Histol. 2017, 8, 1–14. [Google Scholar]
- Moussa, L.; Usunier, B.; Demarquay, C.; Benderitter, M.; Tamarat, R.; Sémont, A.; Mathieu, N. Bowel Radiation Injury: Complexity of the Pathophysiology and Promises of Cell and Tissue Engineering. Cell Transplant. 2016, 25, 1723–1746. [Google Scholar] [CrossRef]
- McHugh, S.M.; Diamant, N.E. Anal canal pressure profile: A reappraisal as determined by rapid pullthrough technique. Gut 1987, 28, 1234–1241. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylöstalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs Improve Myocardial Infarction in Mice because Cells Embolized in Lung Are Activated to Secrete the Anti-inflammatory Protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Feng, Z.; Ting, J.; Alfonso, Z.; Strem, B.M.; Fraser, J.K.; Rutenberg, J.; Kuo, H.C.; Pinkernell, K. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol. Dial. Transplant. 2010, 25, 3874–3884. [Google Scholar] [CrossRef]
- Li, S.; Zheng, X.; Li, H.; Zheng, J.; Chen, X.; Liu, W.; Tai, Y.; Zhang, Y.; Wang, G.; Yang, Y. Mesenchymal Stem Cells Ameliorate Hepatic Ischemia/Reperfusion Injury via Inhibition of Neutrophil Recruitment. J. Immunol. Res. 2018, 2018, 7283703. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kim, J.O.; Kim, S.J. Secretome from human adipose-derived stem cells protects mouse liver from hepatic ischemia–reperfusion injury. Surgery 2015, 157, 934–943. [Google Scholar] [CrossRef] [PubMed]
- La Francesca, S.; Ting, A.E.; Sakamoto, J.; Rhudy, J.; Bonenfant, N.R.; Borg, Z.D.; Cruz, F.F.; Goodwin, M.; Lehman, N.A.; Taggart, J.M.; et al. Multipotent adult progenitor cells decrease cold ischemic injury in ex vivo perfused human lungs: An initial pilot and feasibility study. Transplant. Res. 2014, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Si, Y.; Ding, J.; Chen, X.; Zhang, X.; Dong, Z.; Fu, W. Mesenchymal stem cells attenuate acute ischemia-reperfusion injury in a rat model. Exp. Ther. Med. 2015, 10, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Shologu, N.; Scully, M.; Laffey, J.G.; O’Toole, D. Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury. Int. J. Mol. Sci. 2018, 19, 2996. [Google Scholar] [CrossRef]
- Chu, X.; Xu, B.; Gao, H.; Li, B.Y.; Liu, Y.; Reiter, J.L.; Wang, Y. Lipopolysaccharides Improve Mesenchymal Stem Cell-Mediated Cardioprotection by MyD88 and stat3 Signaling in a Mouse Model of Cardiac Ischemia/Reperfusion Injury. Stem Cells Dev. 2019, 28, 620–631. [Google Scholar] [CrossRef]
- Pennella, S.; Lonardi, R.; Giuliani, E.; Farinetti, A.; Mattioli, A.V.; Bonetti, L.R.; Migaldi, M.; Barbieri, A.; Manenti, A. Does stem cell therapy induce myocardial neoangiogenesis? Histological evaluation in an ischemia/reperfusion animal model. J. Cardiovasc. Med. 2017, 18, 277–282. [Google Scholar] [CrossRef]
- Song, L.; Yang, Y.J.; Dong, Q.T.; Qian, H.Y.; Gao, R.L.; Qiao, S.B.; Shen, R.; He, Z.X.; Lu, M.J.; Zhao, S.H.; et al. Atorvastatin Enhance Efficacy of Mesenchymal Stem Cells Treatment for Swine Myocardial Infarction via Activation of Nitric Oxide Synthase. PLoS ONE 2013, 8, e65702. [Google Scholar] [CrossRef]
- Swaminathan, M.; Stafford-Smith, M.; Chertow, G.M.; Warnock, D.G.; Paragamian, V.; Brenner, R.M.; Lellouche, F.; Fox-Robichaud, A.; Atta, M.G.; Melby, S.; et al. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J. Am. Soc. Nephrol. 2018, 29, 260–267. [Google Scholar] [CrossRef]
- Rosselli, D.D.; Mumaw, J.L.; Dickerson, V.; Brown, C.A.; Brown, S.A.; Schmiedt, C.W. Efficacy of allogeneic mesenchymal stem cell administration in a model of acute ischemic kidney injury in cats. Res. Vet. Sci. 2016, 108, 18–24. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Q.; Jiao, Z.; Li, H.; Bai, G.; Wang, H. Adipose-derived stem cells reduce liver oxidative stress and autophagy induced by ischemia-reperfusion and hepatectomy injury in swine. Life Sci. 2018, 214, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Qian, H.; Xu, W.; Zhu, W.; Zhang, X.; Chen, Y.; Wang, M.; Yan, Y.; Xie, Y. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol. Lett. 2010, 32, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.J.; Shen, W.C.; Chang, F.B.; Wu, V.C.; Wang, S.H.; Young, G.H.; Tsai, J.S.; Tseng, Y.C.; Peng, Y.S.; Chen, Y.L. Endothelial Progenitor Cells Derived from Wharton’s Jelly of Human Umbilical Cord Attenuate Ischemic Acute Kidney Injury by Increasing Vascularization and Decreasing Apoptosis, Inflammation, and Fibrosis. Cell Transplant. 2015, 24, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hong, L.; Huang, Z.; Na, N.; Hua, X.; Peng, Y.; Zhao, M.; Cao, R.; Sun, Q. Allogeneic mesenchymal stem cell as induction therapy to prevent both delayed graft function and acute rejection in deceased donor renal transplantation: Study protocol for a randomized controlled trial. Trials 2017, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Hale, S.L.; Martin, B.J.; Kuang, J.Q.; Dow, J.S.; Wold, L.E.; Kloner, R.A. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short-and long-term effects. Circulation 2005, 112, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Theise, N.D.; Collector, M.I.; Henegariu, O.; Hwang, S.; Gardner, R.; Neutzel, S.; Sharkis, S.J. Multi-Organ, Multi-Lineage Engraftment by a Single Bone Marrow-Derived Stem Cell. Cell 2001, 105, 369–377. [Google Scholar] [CrossRef]
- Wei, Y.; Xie, Z.; Bi, J.; Zhu, Z. Anti-inflammatory effects of bone marrow mesenchymal stem cells on mice with Alzheimer’s disease. Exp. Ther. Med. 2018, 16, 5015–5020. [Google Scholar] [CrossRef]
- Horton, J.A.; Hudak, K.E.; Chung, E.J.; White, A.O.; Scroggins, B.T.; Burkeen, J.F.; Citrin, D.E. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells 2013, 31, 2231–2241. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, S.J.; Park, G.; Cho, B.; Min, C.K.; Kim, T.Y.; Lee, J.S.; Oh, I.H. A Novel Function of Interleukin-10 Promoting Self-Renewal of Hematopoietic Stem Cells. Stem Cells 2007, 25, 1814–1822. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Leuning, D.G.; Beijer, N.R.M.; Du Fossé, N.A.; Vermeulen, S.; Lievers, E.; Van Kooten, C.; Rabelink, T.J.; De Boer, J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef] [PubMed]
- Van den Akker, F.; Vrijsen, K.R.; Deddens, J.C.; Buikema, J.W.; Mokry, M.; van Laake, L.W.; Doevendans, P.A.; Sluijter, J.P.G. Suppression of T cells by mesenchymal and cardiac progenitor cells is partly mediated via extracellular vesicles. Heliyon 2018, 4, e00642. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yue, W.; Le-Le, Z.; Bin, L.; Hu, X. Mesenchymal stem cells inhibit T cell activation by releasing TGF-beta1 from TGF-beta1/GARP complex. Oncotarget 2017, 8, 99784–99800. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Daniel, C.; Wagner, A.; Hohenstein, B.; Hugo, C. Thrombospondin-2 therapy ameliorates experimental glomerulonephritis via inhibition of cell proliferation, inflammation, and TGF-beta activation. Am. J. Physiol. Ren. Physiol. 2009, 297, 1299–1309. [Google Scholar] [CrossRef]
- Daniel, C.; Amann, K.; Hohenstein, B.; Bornstein, P.; Hugo, C. Thrombospondin 2 Functions as an Endogenous Regulator of Angiogenesis and Inflammation in Experimental Glomerulonephritis in Mice. J. Am. Soc. Nephrol. 2007, 18, 788–798. [Google Scholar] [CrossRef]
- Wang, Q.C.; Zhen, L. Establishment of A Serum-Free Culture System Based on Heparin and Anti-CD16 Antibody for Expansion of Human Cord Blood NK Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018, 26, 552–556. [Google Scholar]
- Yang, H.; Tian, W.; Wang, S.; Liu, X.; Wang, Z.; Hou, L.; Ge, J.; Zhang, X.; He, Z.; Wang, X. TSG-6 secreted by bone marrow mesenchymal stem cells attenuates intervertebral disc degeneration by inhibiting the TLR2/NF-kappaB signaling pathway. Lab. Investig. 2018, 98, 755–772. [Google Scholar] [CrossRef]
- Nikolic, A.; Simovic Markovic, B.; Gazdic, M.; Randall Harrell, C.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Lukic, M.L.; Stojkovic, M.; et al. Intraperitoneal administration of mesenchymal stem cells ameliorates acute dextran sulfate sodium-induced colitis by suppressing dendritic cells. Biomed. Pharmacother. 2018, 100, 426–432. [Google Scholar] [CrossRef]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Nasef, A.; Mazurier, C.; Bouchet, S.; François, S.; Chapel, A.; Thierry, D.; Gorin, N.C.; Fouillard, L.; Chapel, C. Leukemia inhibitory factor: Role in human mesenchymal stem cells mediated immunosuppression. Cell. Immunol. 2008, 253, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Djouad, F.; Bouffi, C.; Bony, C.; Apparailly, F.; Cantos, C.; Jorgensen, C.; Noël, D.; Charbonnier, L.M.; Louis-Plence, P.; Louis-Plence, P.; et al. Mesenchymal Stem Cells Inhibit the Differentiation of Dendritic Cells Through an Interleukin-6-Dependent Mechanism. Stem Cells 2007, 25, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Ġbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2, 3-dioxygenase–mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef]
- Cranford, T.L.; Enos, R.T.; Velazquez, K.T.; McClellan, J.L.; Davis, J.M.; Singh, U.P.; Nagarkatti, M.; Nagarkatti, P.S.; Robinson, C.M.; Murphy, E.A. Role of MCP-1 on inflammatory processes and metabolic dysfunction following high-fat feedings in the FVB/N strain. Int. J. Obes. 2016, 40, 844–851. [Google Scholar] [CrossRef]
- Angelo, L.S.; Kurzrock, R. Vascular Endothelial Growth Factor and Its Relationship to Inflammatory Mediators. Clin. Cancer Res. 2007, 13, 2825–2830. [Google Scholar] [CrossRef]
- Zittermann, S.I.; Issekutz, A.C. Basic Fibroblast Growth Factor (bFGF, FGF-2) Potentiates Leukocyte Recruitment to Inflammation by Enhancing Endothelial Adhesion Molecule Expression. Am. J. Pathol. 2006, 168, 835–846. [Google Scholar] [CrossRef]
- Bieback, K.; Ha, V.A.T.; Hecker, A.; Grassl, M.; Kinzebach, S.; Solz, H.; Sticht, C.; Klüter, H.; Bugert, P. Altered Gene Expression in Human Adipose Stem Cells Cultured with Fetal Bovine Serum Compared to Human Supplements. Tissue Eng. Part A 2010, 16, 3467–3484. [Google Scholar] [CrossRef]
- Eefting, F.; Rensing, B.; Wigman, J.; Pannekoek, W.J.; Liu, W.M.; Cramer, M.J.; Lips, D.J.; Doevendans, P.A. Role of apoptosis in reperfusion injury. Cardiovasc Res 2004, 61, 414–426. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, N.; Wang, J.A. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J. Endocrinol. Investig. 2008, 31, 103–110. [Google Scholar] [CrossRef]
- Hu, J.; Yan, Q.; Shi, C.; Tian, Y.; Cao, P.; Yuan, W. BMSC paracrine activity attenuates interleukin-1beta-induced inflammation and apoptosis in rat AF cells via inhibiting relative NF-kappaB signaling and the mitochondrial pathway. Am. J. Transl. Res. 2017, 9, 79–89. [Google Scholar] [PubMed]
- Mirotsou, M.; Zhang, Z.; Deb, A.; Zhang, L.; Gnecchi, M.; Noiseux, N.; Mu, H.; Pachori, A.; Dzau, V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc. Natl. Acad. Sci. USA 2007, 104, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Noiseux, N.; Gnecchi, M.; Zhang, L.; Solomon, S.D.; Deb, A.; Dzau, V.J.; Pratt, R.E.; Lopezilasaca, M.; Lopez-Ilasaca, M. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 2006, 14, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Cui, R.; Peng, L.; Ma, J.; Chen, X.; Xie, R.J.; Li, B. Mesenchymal stem cells, not conditioned medium, contribute to kidney repair after ischemia-reperfusion injury. Stem Cell Res. Ther. 2014, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Nwabo Kamdje, A.H.; Mosna, F.; Bifari, F.; Lisi, V.; Bassi, G.; Malpeli, G.; Ricciardi, M.; Perbellini, O.; Scupoli, M.T.; Pizzolo, G.; et al. Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood 2011, 118, 380–389. [Google Scholar] [CrossRef]
- Zhang, M.; Mal, N.; Kiedrowski, M.; Chacko, M.; Askari, A.T.; Popovic, Z.B.; Koc, O.N.; Penn, M.S. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007, 21, 3197–3207. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Q.; Chen, F.; Zhang, J.; Guo, J.; Wang, H.; Gu, J.; Ma, L.; Ho, G. Human umbilical cord matrix-derived stem cells exert trophic effects on beta-cell survival in diabetic rats and isolated islets. Dis. Models Mech. 2015, 8, 1625–1633. [Google Scholar] [CrossRef]
- Ma, J.; Sawai, H.; Matsuo, Y.; Ochi, N.; Yasuda, A.; Takahashi, H.; Wakasugi, T.; Funahashi, H.; Sato, M.; Takeyama, H. IGF-1 Mediates PTEN Suppression and Enhances Cell Invasion and Proliferation via Activation of the IGF-1/PI3K/Akt Signaling Pathway in Pancreatic Cancer Cells. J. Surg. Res. 2010, 160, 90–101. [Google Scholar] [CrossRef]
- Hau, S.; Reich, D.M.; Scholz, M.; Naumann, W.; Emmrich, F.; Kamprad, M.; Boltze, J. Evidence for neuroprotective properties of human umbilical cord blood cells after neuronal hypoxia in vitro. BMC Neurosci. 2008, 9, 30. [Google Scholar] [CrossRef]
- Mittal, R.; Karhu, E.; Wang, J.S.; Delgado, S.; Zukerman, R.; Mittal, J.; Jhaveri, V.M. Cell communication by tunneling nanotubes: Implications in disease and therapeutic applications. J. Cell. Physiol. 2019, 234, 1130–1146. [Google Scholar] [CrossRef]
- Spees, J.L.; Olson, S.D.; Whitney, M.J.; Prockop, D.J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. USA 2006, 103, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Khryapenkova, T.G.; Vasileva, A.K.; Marey, M.V.; Galkina, S.I.; Isaev, N.K.; Sheval, E.V.; Polyakov, V.Y.; Sukhikh, G.T.; Zorov, D.B. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J. Cell. Mol. Med. 2008, 12, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.F.; Shuai, J.; Parker, I. Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys. J. 2011, 100, 37–39. [Google Scholar] [CrossRef]
- Wang, X.; Veruki, M.L.; Bukoreshtliev, N.V.; Hartveit, E.; Gerdes, H.H. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc. Natl. Acad. Sci. USA 2010, 107, 17194–17199. [Google Scholar] [CrossRef]
- Wang, X.; Schwarz, T.L. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 2009, 136, 163–174. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Yeung, S.C.; Liang, Y.; Liang, X.; Ding, Y.; Ip, M.S.M.; Tse, H.F.; Mak, J.C.W.; Lian, Q. Mitochondrial Transfer of Induced Pluripotent Stem Cell–Derived Mesenchymal Stem Cells to Airway Epithelial Cells Attenuates Cigarette Smoke–Induced Damage. Am. J. Respir. Cell Mol. Biol. 2014, 51, 455–465. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Tomasoni, S.; Longaretti, L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013, 22, 772–780. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019, 33, 1695–1710. [Google Scholar] [CrossRef]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.; Liles, W.C.; Waworuntu, R.; Mulligan, M.S. Pretreatment with bone marrow-derived mesenchymal stromal cell-conditioned media confers pulmonary ischemic tolerance. J Thorac. Cardiovasc. Surg. 2016, 151, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Yip, H.K.; Shao, P.L.; Wu, S.C.; Chen, K.H.; Chen, Y.T.; Yang, C.C.; Sun, C.K.; Kao, G.S.; Chen, S.Y.; et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia–reperfusion injury. Int. J. Cardiol. 2016, 216, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.L.; Zhao, Y.; Robert Smith, J.; Weiss, M.L.; Kron, I.L.; Laubach, V.E.; Sharma, A.K. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 2017, 18, 212. [Google Scholar] [CrossRef]
- Chen, M.; Xiang, Z.; Cai, J. The anti-apoptotic and neuro-protective effects of human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) on acute optic nerve injury is transient. Brain Res 2013, 1532, 63–75. [Google Scholar] [CrossRef]
- Burger, D.; Gutsol, A.; Carter, A.; Allan, D.S.; Touyz, R.M.; Burns, K.D. Human cord blood CD133+cells exacerbate ischemic acute kidney injury in mice. Nephrol. Dial. Transplant. 2012, 27, 3781–3789. [Google Scholar] [CrossRef]
- Li, W.Y.; Choi, Y.J.; Lee, P.H.; Huh, K.; Kang, Y.M.; Kim, H.S.; Ahn, Y.H.; Lee, G.; Bang, O.Y. Mesenchymal stem cells for ischemic stroke: Changes in effects after ex vivo culturing. Cell Transplant. 2008, 17, 1045–1059. [Google Scholar] [CrossRef]
- Doster, D.L.; Jensen, A.R.; Khaneki, S.; Markel, T.A. Mesenchymal stromal cell therapy for the treatment of intestinal ischemia: Defining the optimal cell isolate for maximum therapeutic benefit. Cytotherapy 2016, 18, 1457–1470. [Google Scholar] [CrossRef]
- Park, J.S.; Bae, S.H.; Jung, S.; Lee, M.; Choi, D. Enrichment of vascular endothelial growth factor secreting mesenchymal stromal cells enhances therapeutic angiogenesis in a mouse model of hind limb ischemia. Cytotherapy 2019, 21, 433–443. [Google Scholar] [CrossRef]
- Li, L.; Du, G.; Wang, D.; Zhou, J.; Jiang, G.; Jiang, H. Overexpression of Heme Oxygenase-1 in Mesenchymal Stem Cells Augments Their Protection on Retinal Cells In Vitro and Attenuates Retinal Ischemia/Reperfusion Injury In Vivo against Oxidative Stress. Stem Cells Int. 2017, 2017, 4985323. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakagawa, K.; Tomiyasu, M.; Nakashima, A.; Katayama, K.; Imura, T.; Herlambang, B.; Okubo, T.; Arihiro, K.; Kawahara, Y.; et al. Mesenchymal Stem Cell-Based Therapy Improves Lower Limb Movement After Spinal Cord Ischemia in Rats. Ann. Thorac. Surg. 2018, 105, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Yuan, X.; Ou, Y.; Zhu, X.; Cheng, Z.; Wu, X.; Meng, Y. The Relationship between the Bcl-2/Bax Proteins and the Mitochondria-Mediated Apoptosis Pathway in the Differentiation of Adipose-Derived Stromal Cells into Neurons. PLoS ONE 2016, 11, e0163327. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K. Aging of mesenchymal stem cells: Implication in regenerative medicine. Regen. Ther. 2018, 9, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Kern, S.; Kocaömer, A.; Ferlik, K.; Bugert, P. Comparing mesenchymal stromal cells from different human tissues: Bone marrow, adipose tissue and umbilical cord blood. Biomed. Mater. Eng. 2008, 18, 71–76. [Google Scholar]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yang, L.; Zhang, Y.; Gao, W.; Yao, Z.; Song, Y.; Wang, Y. Effects of age on biological and functional characterization of adiposederived stem cells from patients with endstage liver disease. Mol. Med. Rep. 2017, 16, 3510–3518. [Google Scholar] [CrossRef]
- Marędziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 2152435. [Google Scholar] [CrossRef]
- Zhuo, Y.; Li, S.H.; Chen, M.S.; Wu, J.; Kinkaid, H.Y.M.; Fazel, S.; Weisel, R.D.; Li, R.K. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: Combined consequences for cell therapy in older recipients. J. Thorac. Cardiovasc. Surg. 2010, 139, 1286–1294. [Google Scholar] [CrossRef]
- Fabian, C.; Naaldijk, Y.; Leovsky, C.; Johnson, A.A.; Rudolph, L.; Jaeger, C.; Arnold, K.; Stolzing, A. Distribution pattern following systemic mesenchymal stem cell injection depends on the age of the recipient and neuronal health. Stem Cell Res. Ther. 2017, 8, 85. [Google Scholar] [CrossRef]
- Lopez-Santalla, M.; Mancheno-Corvo, P.; Escolano, A.; Menta, R.; Delarosa, O.; Redondo, J.M.; Bueren, J.A.; Dalemans, W.; Lombardo, E.; Garin, M.I. Comparative Analysis between the In Vivo Biodistribution and Therapeutic Efficacy of Adipose-Derived Mesenchymal Stromal Cells Administered Intraperitoneally in Experimental Colitis. Int. J. Mol. Sci. 2018, 19, 1853. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensebé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.E.; Capcha, J.M.C.; De Bragança, A.C.; Sanches, T.R.; Gouveia, P.Q.; De Oliveira, P.A.F.; Malheiros, D.M.A.C.; Volpini, R.A.; Santinho, M.A.R.; Santana, B.A.A.; et al. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res. Ther. 2017, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.; Planat-Benard, V.; Bel, A.; Puymirat, E.; Geha, R.; Pidial, L.; Nematalla, H.; Bellamy, V.; Bouaziz, P.; Peyrard, S.; et al. Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections. Cardiovasc Res 2011, 91, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. In StemBook; Harvard Stem Cell Institute: Cambridge, MA, USA, 2008. [Google Scholar]
- Kim, K.; Grainger, D.W.; Okano, T. Utah’s cell sheet tissue engineering center. Regen. Ther. 2019, 11, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Florentino, A.; Bardag-Gorce, F.; Niihara, Y. Engineering, differentiation and harvesting of human adipose-derived stem cell multilayer cell sheets. Regen. Med. 2019, 14, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.; Berthiaume, F.; Dardik, A.; Hsia, H. Stem Cells and Engineered Scaffolds for Regenerative Wound Healing. Bioengineering 2018, 5, 23. [Google Scholar] [CrossRef]
- Kim, J.; Lin, B.; Kim, S.; Choi, B.; Evseenko, D.; Lee, M. TGF-beta1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J. Biol. Eng. 2015, 9, 1. [Google Scholar] [CrossRef]
- Yao, Q.; Cosme, J.G.; Xu, T.; Miszuk, J.M.; Picciani, P.H.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef]
- Iwai, S.; Sakonju, I.; Okano, S.; Teratani, T.; Kasahara, N.; Yokote, S.; Yokoo, T.; Kobayash, E. Impact of Ex Vivo Administration of Mesenchymal Stem Cells on the Function of Kidney Grafts from Cardiac Death Donors in Rat. Transplant. Proc. 2014, 46, 1578–1584. [Google Scholar] [CrossRef]
- Wakao, S.; Kuroda, Y.; Ogura, F.; Shigemoto, T.; Dezawa, M. Regenerative Effects of Mesenchymal Stem Cells: Contribution of Muse Cells, a Novel Pluripotent Stem Cell Type that Resides in Mesenchymal Cells. Cells 2012, 1, 1045–1060. [Google Scholar] [CrossRef]
- Sohni, A.; Verfaillie, C.M. Mesenchymal Stem Cells Migration Homing and Tracking. Stem Cells Int. 2013, 2013, 130763. [Google Scholar] [CrossRef] [PubMed]
| Targeted Organ | Animal Model/Human Study | Cells per Dose/Administration Protocol/Location of the Injection | Length of the Subjects Follow Up | Effects Reported Due to Stem Cells Treatment |
|---|---|---|---|---|
| Kidney [30] | Rat | 1 × 106/3 times BMSC pretreated with melatonin/Renal parenchymal | Up to 2 months |
|
| Heart [46] | Mouse | 1 × 106/1 time/Coronary Injection before Ischemia | 75 min |
|
| Heart [47] | Rabbit | 4 × 106/1 time/intramuscularly or intravenously | 20 days |
|
| Heart [48] | Swine | 3 × 107 Cells (Pretreated with Atorvastatin)/1 time/In the Infarct or Peri-infarct area | 4 weeks |
|
| Lung [43] | Human Study (4 patients) | 10 × 107/1 time/Intra-bronchial Injection | N/A |
|
| Kidney [49] | Human Study (135 patients) | 2 × 106 Cells per 1 kg/1 time/Unknown | Long term Follow up |
|
| Targeted Organ | Animal Model/Human Study | Cells per Dose/Administration Protocol/Location of the Injection | Length of the Subjects Follow Up | Effects Reported due to Stem Cells Treatment |
|---|---|---|---|---|
| Kidney [40] | Rat | 5× 106/1 time/Intra-Arterial | Up to 72 h |
|
| Heart [20] | Mouse | 1 × 106 ASC or 1 × 106 ASC (overexpressing HMOX-1)/1 time/sub-cutaneously | 1 h |
|
| Kidney [50] | Cat | 2 × 106 of ASC or BMSC or Fibroblasts/1 time/intra-parenchymal | 6 days |
|
| Liver [51] | Bama miniature pigs | 1 × 106/kg/1 time/liver parenchyma | Up to 7 days | All the difference occurs at 1 day (not 7 days after injection)
|
| Targeted Organ | Animal Model/Human Study | Cells per Dose/Administration Protocol/Location of the Injection | Length of the Subjects Follow Up | Effects Reported due to Stem Cells Treatment |
|---|---|---|---|---|
| Kidney [52] | Rat | 1 × 106/1 time/Left Carotid Artery | 72 h |
|
| Kidney [53] | Mouse | 5 × 105/1 time/Renal artery | 7 days |
|
| Kidney [54] | Human study for allograft | 2 × 106 per kilogram before graft 1 time, vein injection/and 5 × 106 per during surgery, renal arterial injection | 1 Year follow up | End points (Results not reported yet NCT02490020): Allograft rejection, kidney function, post-operatives’ complications, infection, pneumonia, bleeding. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, J. Therapeutic Properties of Mesenchymal Stem Cell on Organ Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2019, 20, 5511. https://doi.org/10.3390/ijms20215511
Oliva J. Therapeutic Properties of Mesenchymal Stem Cell on Organ Ischemia-Reperfusion Injury. International Journal of Molecular Sciences. 2019; 20(21):5511. https://doi.org/10.3390/ijms20215511
Chicago/Turabian StyleOliva, Joan. 2019. "Therapeutic Properties of Mesenchymal Stem Cell on Organ Ischemia-Reperfusion Injury" International Journal of Molecular Sciences 20, no. 21: 5511. https://doi.org/10.3390/ijms20215511
APA StyleOliva, J. (2019). Therapeutic Properties of Mesenchymal Stem Cell on Organ Ischemia-Reperfusion Injury. International Journal of Molecular Sciences, 20(21), 5511. https://doi.org/10.3390/ijms20215511




