Targeting Cancer Resistance via Multifunctional Gold Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
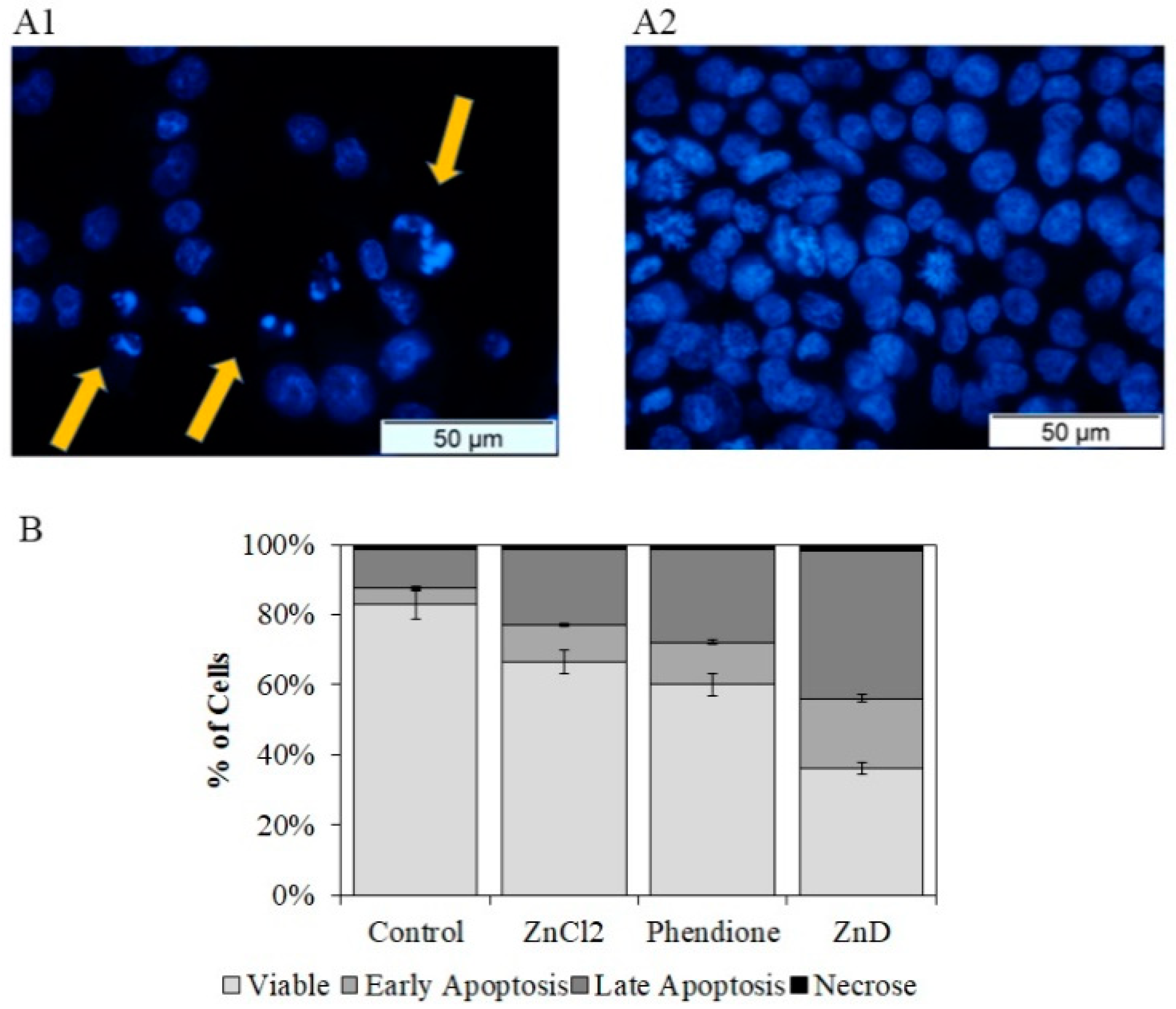
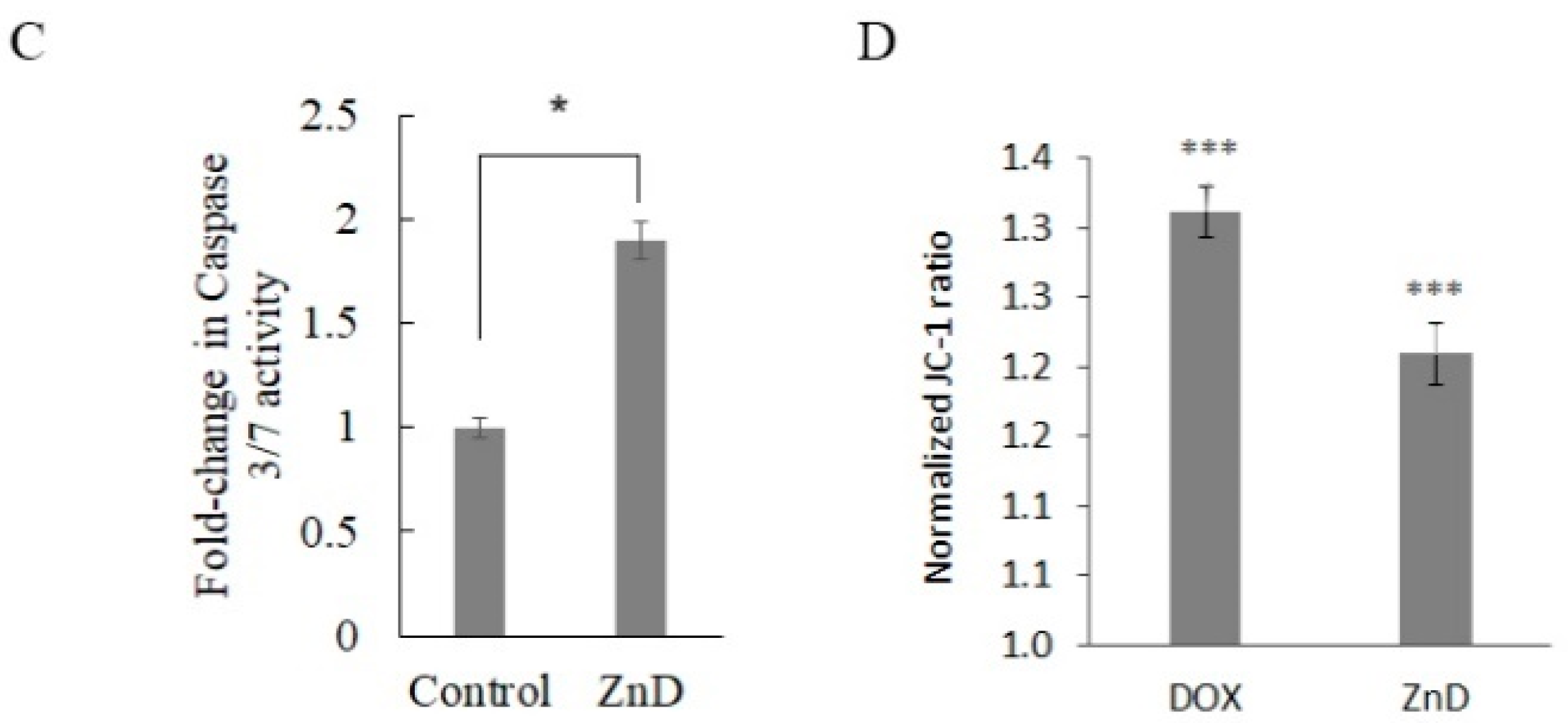
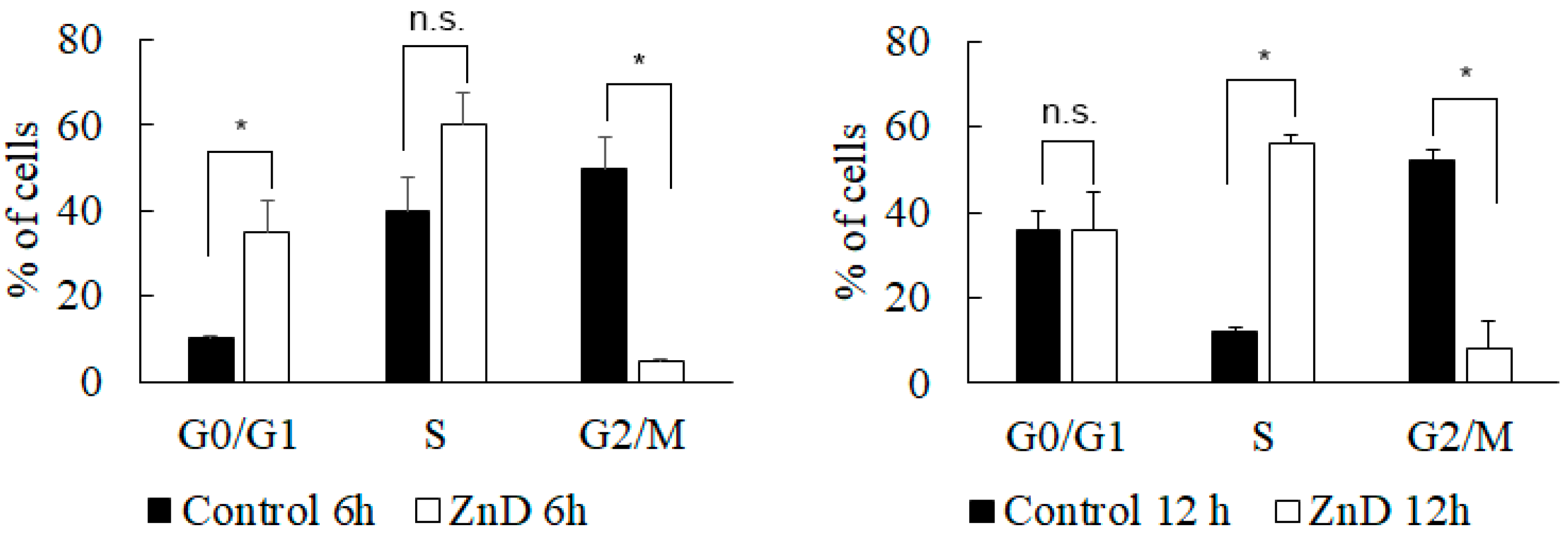
2.1. ZnD as a Powerful Anticancer Compound
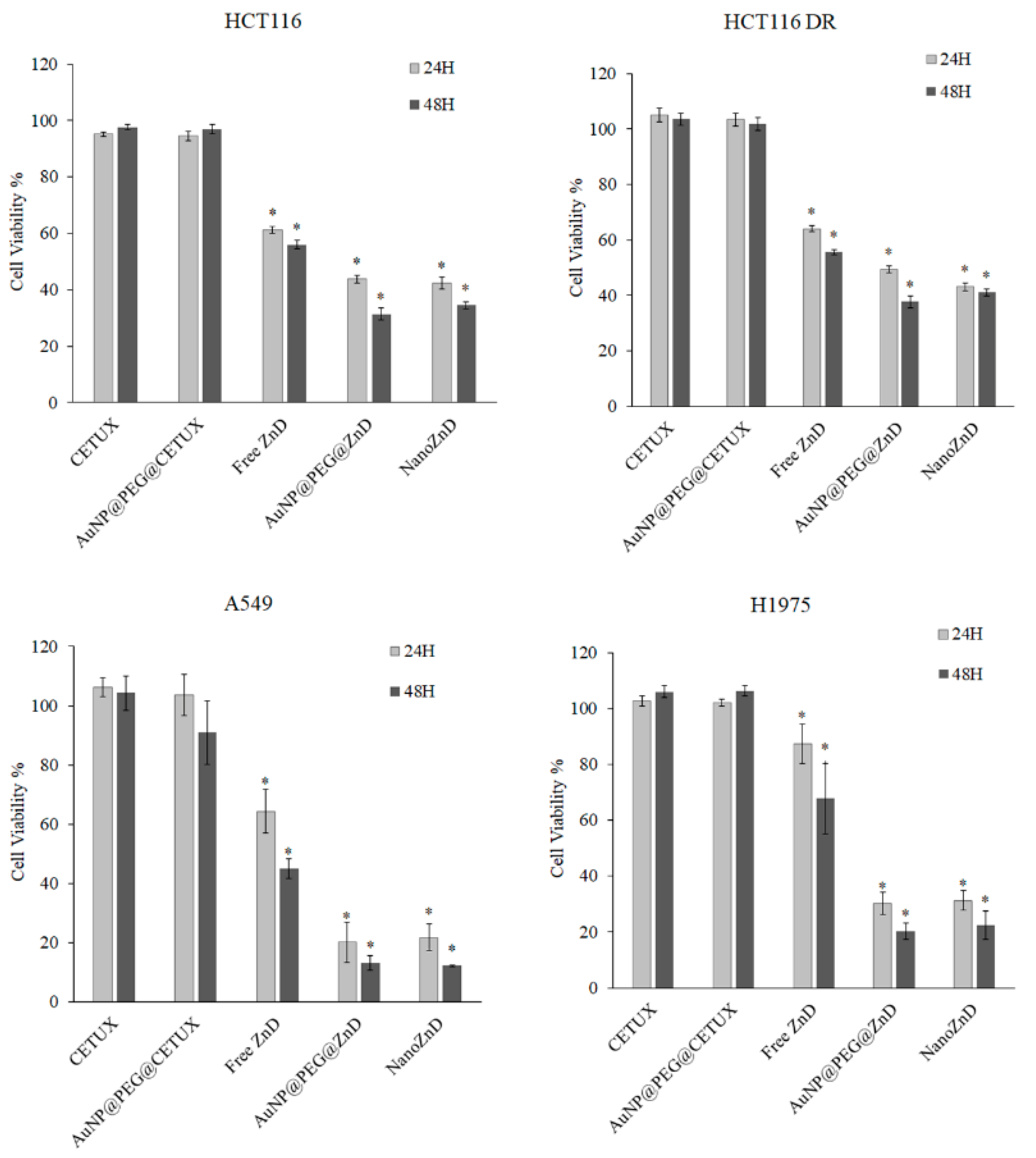
2.2. ZnD and DOX-Resistant Cancer Cells
2.3. Nanovectorization of ZnD: Increasing Cytotoxicity
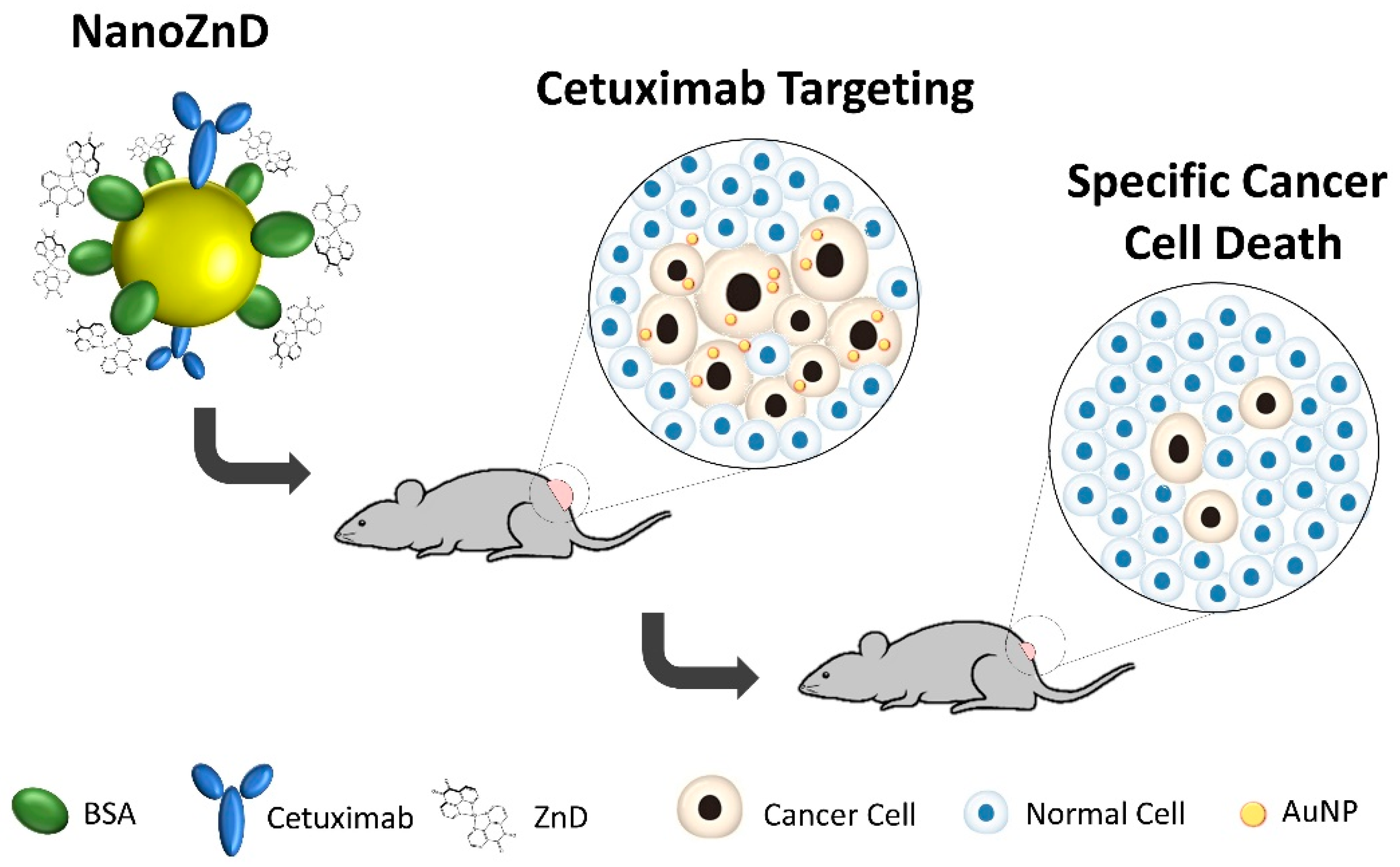
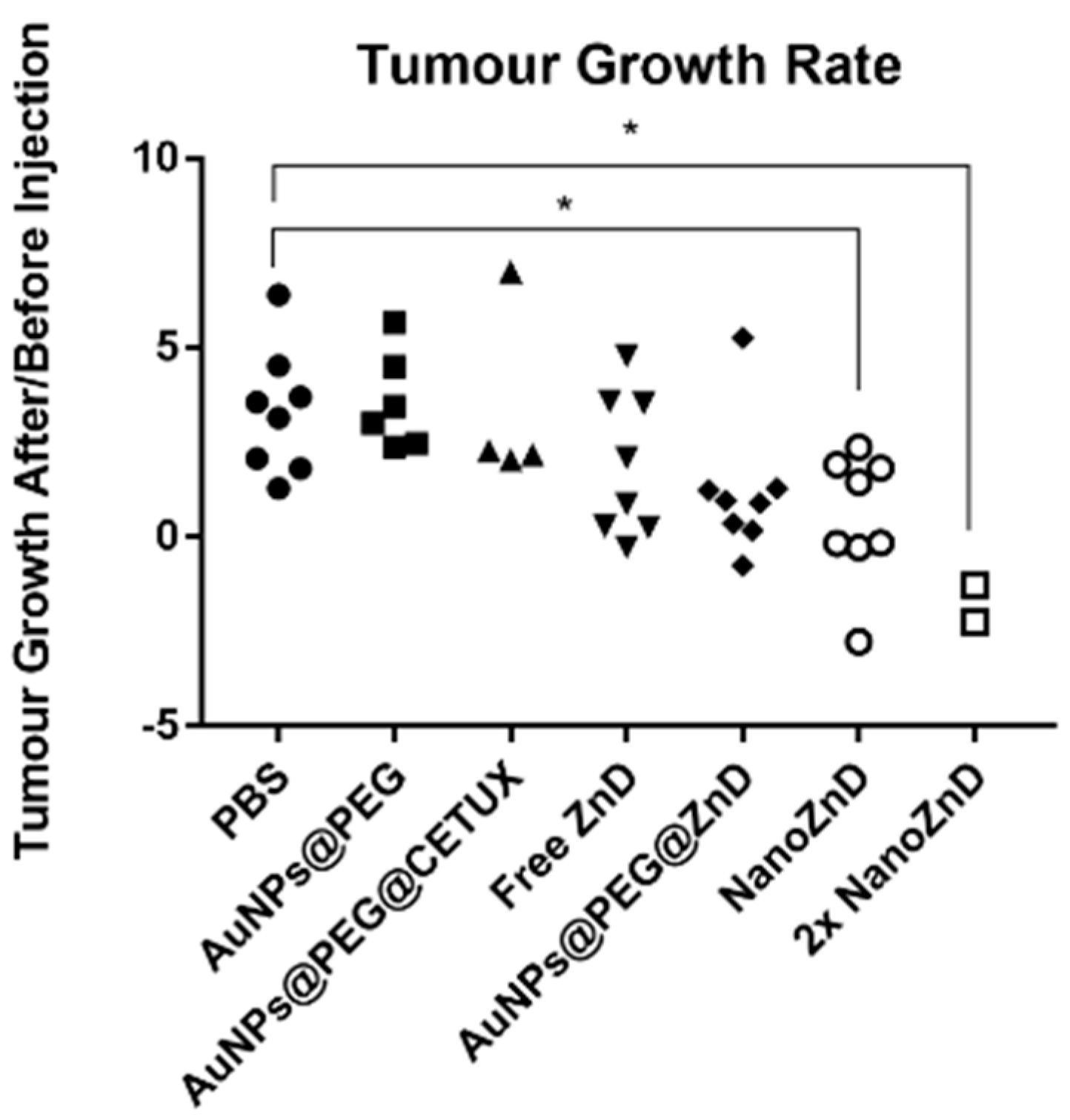
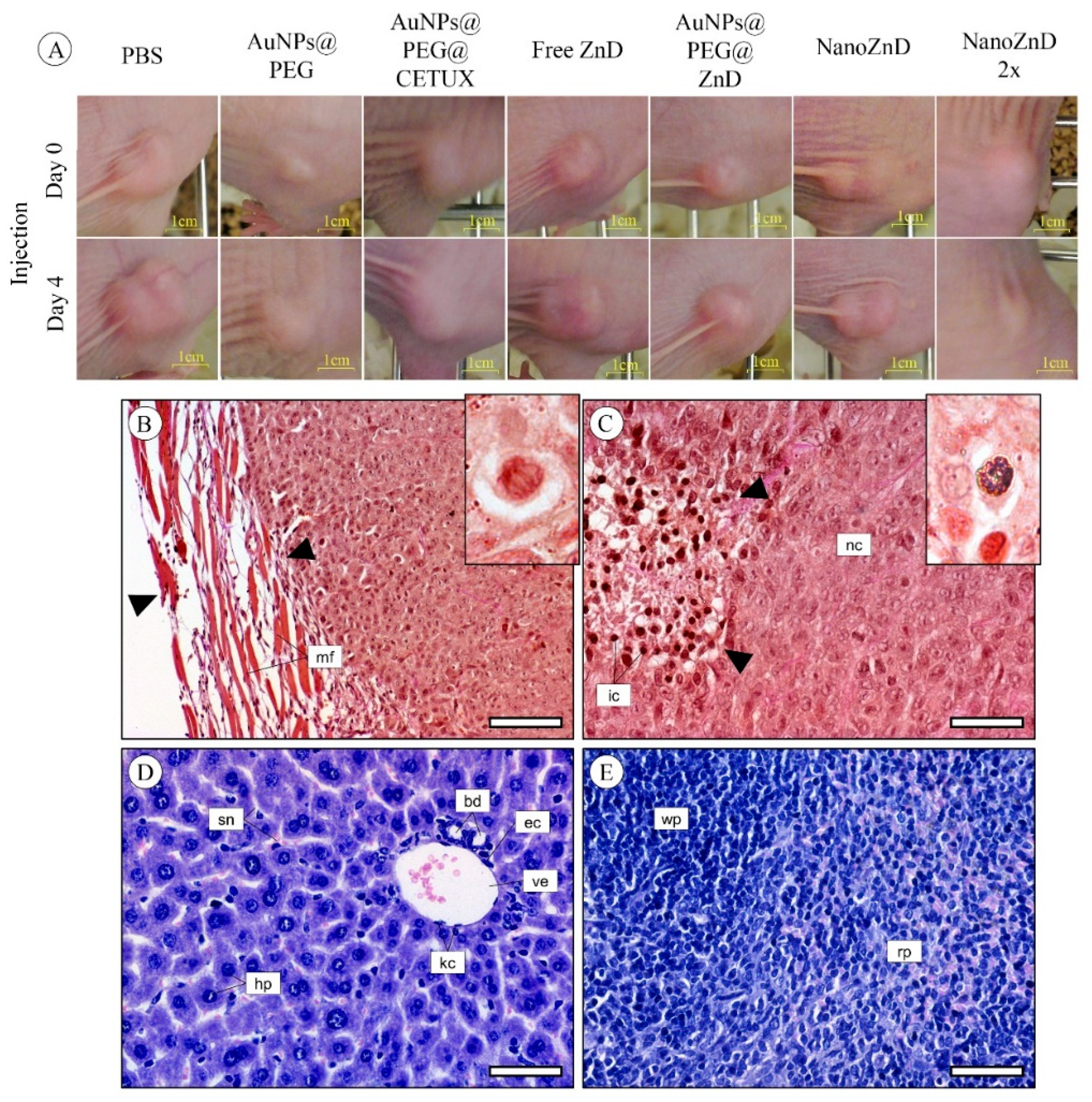
2.4. In Vivo Assays
3. Materials and Methods
3.1. Cell Culture
3.2. Cytotoxicity
3.3. Cell Death Mechanism
3.3.1. Hoechst 33258 Staining
3.3.2. Quantification of Cell Death by Flow Cytometry
3.3.3. Caspase-3/-7 Activity
3.3.4. Mitochondrial Membrane Potential
3.4. Analysis of Cell Cycle
3.5. Chromosomal DNA Alterations
3.5.1. Chromosomal Aberrations
3.5.2. Genotoxicity Assessment
3.6. Interaction Studies of ZnD with Albumin
3.7. Spectroscopic Measurements
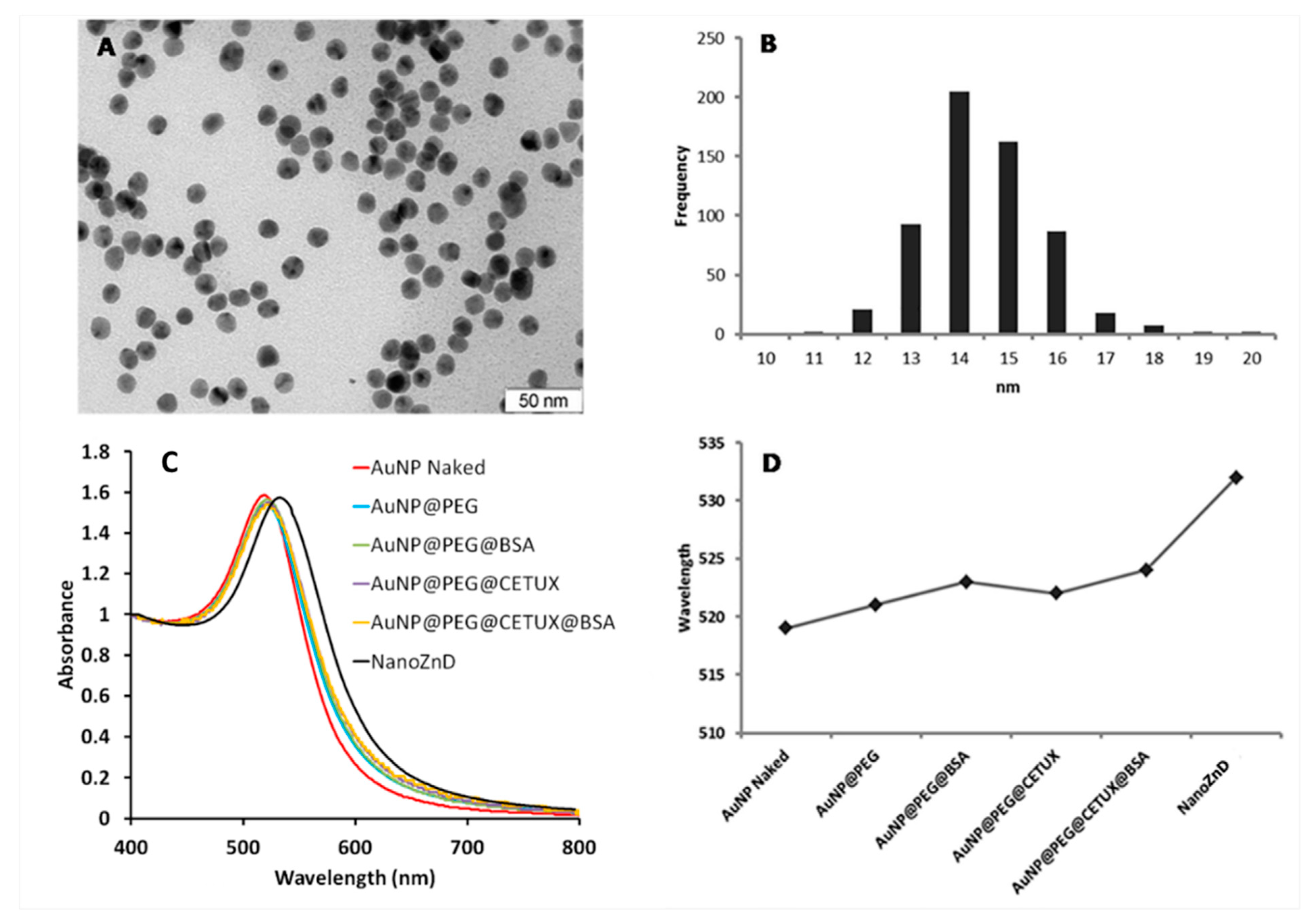
3.8. Gold Nanoparticles Synthesis and Assembly of Au Nanoconjugates
3.9. Au Nanoconjugates Characterization
3.10. AuNPs—Cell Interaction (Flow Cytometry)
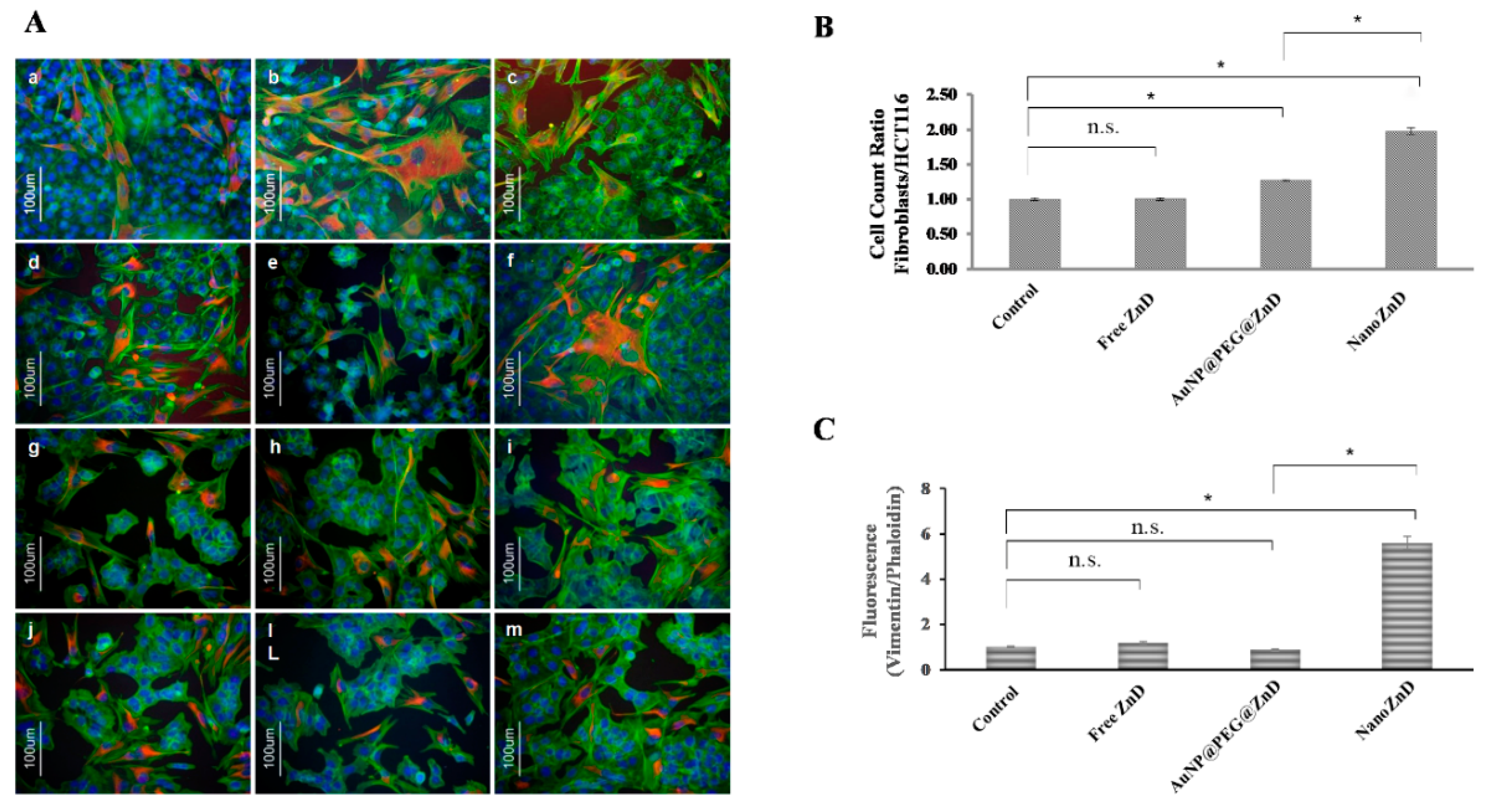
3.11. Co-Cultures—Selectivity Assay
3.12. Animal Model Assays
3.13. Histological Analysis
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | Gold Nanoparticles |
| BSA | Bovine Serum Albumin |
| DLS | Dynamic Light Scattering |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DOX | Doxorubicin |
| EGFR | Epidermal Growth Factor Receptor |
| EPR | Enhanced Permeability and Retention Effect |
| FITC | Fluorescein Isothiocyanate |
| FBS | Fetal Bovine Serum |
| HCT116 DR | HCT116 Doxorubicin-Resistant |
| ICP-AES | Inductively Coupled Plasma Atomic Emission Spectrometry |
| JC-1 | 5,5,6,6-tetrachloro-1,1,3,3 tetraethylbenzimidazolylcarbocyanine iodide |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| PEG | Polyethylene Glycol |
| PI | Propidium Iodide |
| RPMI | Roswell Park Memorial Institute |
| SPR | Surface Plasmon Resonance |
| TEM | Transmission Electron Microscopy |
| TC | Tetrachrome Stain |
| ZnD | DION - 1,10-phenanthroline-5,6-dione |
References
- Silva, T.F.S.; Smoleński, P.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Fernandes, A.R.; Luis, D.; Silva, A.; Santos, S.; Borralho, P.M.; Rodrigues, C.M.P.; et al. Cobalt and Zinc Compounds Bearing 1,10-Phenanthroline-5,6-dione or 1,3,5-Triaza-7-phosphaadamantane Derivatives—Synthesis, Characterization, Cytotoxicity, and Cell Selectivity Studies. Eur. J. Inorg. Chem. 2013, 2013, 3651–3658. [Google Scholar] [CrossRef]
- Martins, M.; Baptista, P.V.; Mendo, A.S.; Correia, C.; Videira, P.; Rodrigues, A.S.; Muthukumaran, J.; Santos-Silva, T.; Silva, A.; Guedes da Silva, M.F.C.; et al. In vitro and in vivo biological characterization of the anti-proliferative potential of a cyclic trinuclear organotin(IV) complex. Mol. BioSyst. 2016, 12, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, B.; Guedes da Silva, M.F.C.; Silva, J.; Mendo, A.S.; Baptista, P.V.; Fernandes, A.R.; Pombeiro, A.J.L. Synthesis, characterization, thermal properties and antiproliferative potential of copper(II) 4′-phenyl-terpyridine compounds. Dalt. Trans. 2016, 45, 5339–5355. [Google Scholar] [CrossRef] [PubMed]
- Lenis-Rojas, O.A.; Roma-Rodrigues, C.; Fernandes, A.R.; Marques, F.; Pérez-Fernández, D.; Guerra-Varela, J.; Sánchez, L.; Vázquez-García, D.; López-Torres, M.; Fernández, A.; et al. Dinuclear Rull (bipy) 2 Derivatives: Structural, Biological, and in Vivo Zebrafish Toxicity Evaluation. Inorg. Chem. 2017, 56, 7127–7144. [Google Scholar] [CrossRef]
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Heterocyclic anticancer compounds: Recent advances and the paradigm shift towards the use of nanomedicine’s tool Box. Molecules 2015, 20, 16852–16891. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Jesus, J.; Martins, P.; Figueiredo, S.; Rosa, D.; Martins, L.M.R.D.R.S.; Corvo, M.L.; Carvalheiro, M.C.; Costa, P.M.; Baptista, P.V. Multifunctional gold-nanoparticles: A nanovectorization tool for the targeted delivery of novel chemotherapeutic agents. J. Control. Release 2017, 245, 52–61. [Google Scholar] [CrossRef]
- Panzarini, E.; Inguscio, V.; Anna Tenuzzo, B.; Carata, E.; Dini, L. Nanomaterials and autophagy: New insights in cancer treatment. Cancers 2013, 5, 296–319. [Google Scholar] [CrossRef]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug delivery to solid tumors: The predictive value of the multicellular tumor spheroid model for nanomedicine screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef]
- Sgambato, A.; Casaluce, F.; Maione, P.; Rossi, A.; Ciardiello, F.; Gridelli, C. Cetuximab in advanced non-small cell lung cancer (NSCLC): The showdown? J. Thorac. Dis. 2014, 6, 578–580. [Google Scholar]
- Jalal, S.; Waterhouse, D.; Edelman, M.J.; Nattam, S.; Ansari, R.; Koneru, K.; Clark, R.; Richards, A.; Wu, J.; Yu, M.; et al. Pemetrexed plus cetuximab in patients with recurrent non-small cell lung cancer (NSCLC): A phase I/II study from the Hoosier Oncology Group. J. Thorac. Oncol. 2009, 4, 1420–1424. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Tanno, S.; Fujita, Y.; Toyoshima, E.; Fujiuchi, S.; Nishigaki, Y.; Ishida, S.; Nagase, A.; Miyokawa, N.; Hirata, S.; et al. Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression. Oncol. Rep. 2000, 7, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Pabla, B. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J. Clin. Oncol. 2015, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Mauer, A.M.; William, W.N., Jr.; Tran, H.T.; Liu, D.; Lee, J.J.; Windt, P.; Hong, W.K.; Vokes, E.E.; Herbst, R.S. A Phase 2 Study of Cetuximab in Combination with Docetaxel in Chemotherapy-Refractory/Resistant Patients with Advanced Nonsmall Cell Lung Cancer. Cancer 2016, 95, 222–242. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Robinet, G.; Szczesna, A.; Ramlau, R.; Constenla, M.; Mennecier, B.C.; Pfeifer, W.; O’byrne, K.J.; Welte, T.; Kolb, R.; et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann. Oncol. 2008, 19, 362–369. [Google Scholar] [CrossRef]
- Khambata-Ford, S.; Harbison, C.T.; Hart, L.L.; Awad, M.; Xu, L.A.; Horak, C.E.; Dakhil, S.; Hermann, R.C.; Lynch, T.J.; Weber, M.R. Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 918–927. [Google Scholar] [CrossRef]
- Devaiah, A.; Murchison, C. Analysis of 473 US Head and Neck Cancer Trials (1996–2014): Trends, Gaps, and Opportunities. Otolaryngol. Head. Neck Surg. 2016, 154, 309–314. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Faramarzi, M.A.; Nikfar, S.; Abdollahi, M. A comprehensive review of clinical trials on EGFR inhibitors such as cetuximab and panitumumab as monotherapy and in combination for treatment of metastatic colorectal cancer. Avicenna J. Med. Biotechnol. 2015, 7, 134–144. [Google Scholar]
- Deng, Y.; Kurland, B.F.; Wang, J.; Bi, J.; Li, W.; Rao, S.; Lan, P.; Lin, T.; Lin, E. High epidermal growth factor receptor expression in metastatic colorectal cancer lymph nodes may be more prognostic of poor survival than in primary tumor. Am. J. Clin. Oncol. Cancer Clin. Trials 2009, 32, 245–252. [Google Scholar] [CrossRef]
- Spano, J.P.; Lagorce, C.; Atlan, D.; Milano, G.; Domont, J.; Benamouzig, R.; Attar, A.; Benichou, J.; Martin, A.; Morere, J.F.; et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann. Oncol. 2005, 16, 102–108. [Google Scholar] [CrossRef]
- Inamura, K.; Ninomiya, H.; Ishikawa, Y.; Matsubara, O. Is the epidermal growth factor receptor status in lung cancers reflected in clinicopathologic features? Arch. Pathol. Lab. Med. 2010, 134, 66–72. [Google Scholar]
- Pirker, R.; Filipits, M. Cetuximab in non-small-cell lung cancer. Transl. Lung Cancer Res. 2012, 1, 54–60. [Google Scholar] [PubMed]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.S.; Zalcberg, J.R.; Tu, D.; Au, H.-J.; Berry, S.R.; Krahn, M.; Price, T.; Simes, R.J.; et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef]
- Bou-Assaly, W.; Mukherji, S. Cetuximab (Erbitux). Am. J. Neuroradiol. 2010, 31, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Master, A.M.; Sen Gupta, A. EGF receptor-targeted nanocarriers for enhanced cancer treatment. Nanomedicine 2012, 7, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, Z.; Hsie, A.W. Adriamycin induces large deletions as a major type of mutation in CHO cells. Mutat. Res. Lett. 1994, 325, 91–98. [Google Scholar] [CrossRef]
- Singh, B.; Gupta, R.S. Mutagenic Responses of Thirteen Anticancer Drugs on Mutation Induction at Multiple Genetic Loci and on Sister Chromatid Exchanges in Chinese Hamster Ovary Cells. Cancer Res. 1983, 43, 577–584. [Google Scholar] [PubMed]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Ngoune, R.; Peters, A.; von Elverfeldt, D.; Winkler, K.; Pütz, G. Accumulating nanoparticles by EPR: A route of no return. J. Control. Release 2016, 238, 58–70. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; What is the appropriate target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar] [PubMed]
- Hu, T.; Li, Z.; Gao, C.-Y.; Cho, C.H. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J. Gastroenterol. 2016, 22, 6876. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Ambrosone, A.; Sanz, V.; Hernandez, Y.; Marchesano, V.; Tian, F.; Child, H.; Berry, C.C.; Ibarra, M.R.; Baptista, P.V.; et al. Design of multifunctional gold nanoparticles for in vitro and in vivo gene silencing. ACS Nano 2012, 6, 8316–8324. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Larguinho, M.; Cordeiro, A.; Raposo, L.R.; Costa, P.M.; Santos, S.; Diniz, M.S.; Fernandes, A.R.; Baptista, P.V. Gold-nanobeacons for gene therapy: Evaluation of genotoxicity, cell toxicity and proteome profiling analysis. Nanotoxicology 2014, 8, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, P.; Mendes, R.; Cabral, R.; Martins, L.M.D.R.S.; Baptista, P.V.; Fernandes, A.R. Combination of chemotherapy and Au-nanoparticle photothermy in the visible light to tackle doxorubicin resistance in cancer cells. Sci. Rep. 2018, 8, 11429. [Google Scholar] [CrossRef]
- Costa, P.M. Chapter 4—Staining Protocols; Academic Press: Cambridge, MA, USA, 2018; pp. 83–117. ISBN 978-0-12-812032-3. [Google Scholar]
| Human Cell Line | Relative IC50 ± SEM |
|---|---|
| HCT116 | 0.215 ± 0.01 |
| HCT116 DR | 0.108 ± 0.01 |
| A549 | 0.714 ± 0.09 |
| H1975 | 0.355 ± 0.04 |
| Fibroblasts | 0.600 ± 0.13 |
| AuNP Conjugate | Proteins per AuNP | ZnD per AuNP | DLS (nm) |
|---|---|---|---|
| AuNPs citrate | - | - | 15.3 ± 0.2 |
| AuNP@PEG | - | - | 18.6 ± 0.3 |
| AuNP@PEG@BSA | 7.0 ± 0.5 | - | 27.4 ± 0.4 |
| AuNP@PEG@CETUX | 1.6 ± 0.2 | - | 78.3 ± 0.7 |
| AuNP@PEG@CETUX@BSA | 6.9 ± 0.5 | - | 110.4 ± 0.8 |
| AuNP@PEG@BSA@ZnD | 7.0 ± 0.5 | 402 ± 32 | 118.7 ± 0.9 |
| NanoZnD | 6.9 ± 0.5 | 438 ± 19 | 126.3 ± 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, P.; Corvo, M.L.; Ferreira-Silva, M.; Martins, P.; Carvalheiro, M.C.; Costa, P.M.; Martins, C.; Martins, L.M.D.R.S.; Baptista, P.V.; Fernandes, A.R. Targeting Cancer Resistance via Multifunctional Gold Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5510. https://doi.org/10.3390/ijms20215510
Pedrosa P, Corvo ML, Ferreira-Silva M, Martins P, Carvalheiro MC, Costa PM, Martins C, Martins LMDRS, Baptista PV, Fernandes AR. Targeting Cancer Resistance via Multifunctional Gold Nanoparticles. International Journal of Molecular Sciences. 2019; 20(21):5510. https://doi.org/10.3390/ijms20215510
Chicago/Turabian StylePedrosa, Pedro, M. Luísa Corvo, Margarida Ferreira-Silva, Pedro Martins, Manuela Colla Carvalheiro, Pedro M. Costa, Carla Martins, L. M. D. R. S. Martins, Pedro V. Baptista, and Alexandra R. Fernandes. 2019. "Targeting Cancer Resistance via Multifunctional Gold Nanoparticles" International Journal of Molecular Sciences 20, no. 21: 5510. https://doi.org/10.3390/ijms20215510
APA StylePedrosa, P., Corvo, M. L., Ferreira-Silva, M., Martins, P., Carvalheiro, M. C., Costa, P. M., Martins, C., Martins, L. M. D. R. S., Baptista, P. V., & Fernandes, A. R. (2019). Targeting Cancer Resistance via Multifunctional Gold Nanoparticles. International Journal of Molecular Sciences, 20(21), 5510. https://doi.org/10.3390/ijms20215510








