Abstract
In higher organisms, epithelia separate compartments in order to guarantee their proper function. Such structures are able to seal but also to allow substances to pass. Within the paracellular pathway, a supramolecular structure, the tight junction transport is largely controlled by the temporospatial regulation of its major protein family called claudins. Besides the fact that the expression of claudins has been identified in different forms of human diseases like cancer, clearly defined mutations in the corresponding claudin genes have been shown to cause distinct human disorders. Such disorders comprise the skin and its adjacent structures, liver, kidney, the inner ear, and the eye. From the phenotype analysis, it has also become clear that different claudins can cause a complex phenotype when expressed in different organs. To gain deeper insights into the physiology and pathophysiology of claudin-associated disorders, several mouse models have been generated. In order to model human disorders in detail, they have been designed either as full knockouts, knock-downs or knock-ins by a variety of techniques. Here, we review human disorders caused by CLDN mutations and their corresponding mouse models that have been generated thus far and assess their usefulness as a model for the corresponding human disorder.
1. Introduction
In higher organisms epithelia, endothelia and mesothelia are essential to separate different compartments in order to guarantee their proper function. Such structures can be found ubiquitously, like in skin which separates the body from the surrounding environment, the lung (air/blood) and the intestine (gut lumen/blood). Further, epithelial structures form the blood-brain-barrier [1] and are also essential for liver and kidney function [2]. Epithelia consist of monolayer or multilayer structures [3] but a common prerequisite for their function is polarization, i.e., a clear orientation of apical to basolateral requiring a well-defined maintenance molecular machinery [4]. Moreover, besides providing a tight barrier (e.g., within the bladder), epithelial structures are able to regulate coordinated transport across cellular barriers. Transport across epithelial barriers is provided in general via two systems, the paracellular and the transcellular pathway. Whereas the first provides the organism with a maximum of resorptive capacity by minimal energy expenditure, the latter aims at fine-tuning depending on the actual needs of the organism, regulated also by a subset of various local, regional and global mechanisms [5].
In more detail, the transcellular pathway consists of apical uptake, intracellular buffering, transport and basolateral extrusion mechanisms. This pathway is highly energy dependent and mostly driven by the basolateral Na+-K+-ATPase. One of the advantages of such a pathway is its controllability on several of the steps, e.g., by hormones (e.g., 1,25(OH)2D3 or Parathyroid Hormone (PTH)) and therefore also aims at fine-tuning to provide the organism according to its actual needs and also on middle and long term regulation [6]. Especially for these pathways, it has been shown that mutations in human gene cause distinct monogenetic disorders, like Bartter’s [7] and Gitelman’s Syndrome [8] with a clear phenotype-genotype correlation. In order to study such genetic effects, mouse models have been generated to study successfully the consequences of human mutations [9,10,11,12,13].
Considering the paracellular pathway, enormous progress has been achieved during the last two decades as it became clear that this pathway, although basically driven by a given electrochemical gradient only, is regulated by several structures and mechanisms. In regions where concentration gradients of solutes across the epithelial layer are high, like the proximal tubule of the kidney, paracellular pathways provide the organism with maximal reabsorption by minimal energy expenditure. More distantly, crosstalk takes place before the transcellular pathway provides the major mechanism. A key component of the paracellular pathway is a supramolecular structure, called tight junction (TJ). The TJ consists of several membrane-bound proteins and their intracellular adapter and scaffolding proteins [14]. The major proteins essential for the TJ are claudins (lat. claudere, i.e., to seal). The family of claudins is currently included 27 members in eukaryotes. The claudin proteins are membrane-bound and span four times the plasma membrane with an intracellular C- and N-terminal part. Each of the four provides a functional entity, i.e., either to act as pore-forming or as a sealing component (for a detailed review: [15]). Classification of claudins has been made based on different factors such as sequence similarity and functionality. In this review, we used the latter one which was based on sealing or pore-forming capabilities of claudins reviewed by Krause et al. [16].
In 1999, the group of Lifton showed that the disorder Familial Hypercalciuria, Hypomagnesemia with Nephroclacinosis (FHHNC) is caused by mutations in the CLDN16 gene. This observation provided the first evidence that TJ disorders cause human disorders and diseases [17]. Since then, several other TJ disorders have been shown to cause, when mutated, (CLDN1, CLDN2 CLDN9 CLDN10, CLDN14, CLDN16, CLDN19), human disorders [18,19,20,21,22,23]. Furthermore, expression of claudins are influenced by many factors (e.g., smoking, diet changes, alcohol intake) [24,25,26] and numerous associations of disordered claudin expression and disease have been described. (for detail reviews see [27,28]).
To model such disorders, genetically modified mice have been generated. Initially, embryonic stem cell technology was used to create such models [29] and later, other approaches have been established successfully [30]. Besides global deletions, regional and local variations of murine gene expression have been established. Although not reported yet, based on current developments, it can be expected that CRISPR/Cas9 technology will take place as a standard procedure [31].
However, even though such models provide insights into physiology and pathophysiology and may open new avenues for the development of therapeutical interventions, they face limitations. Here, we provide an overview of current human claudin-associated disorders and their corresponding mouse models, their impact on physiology and pathophysiology.
2. Claudin Mutations Causing Human Disorders
2.1. Claudin 1
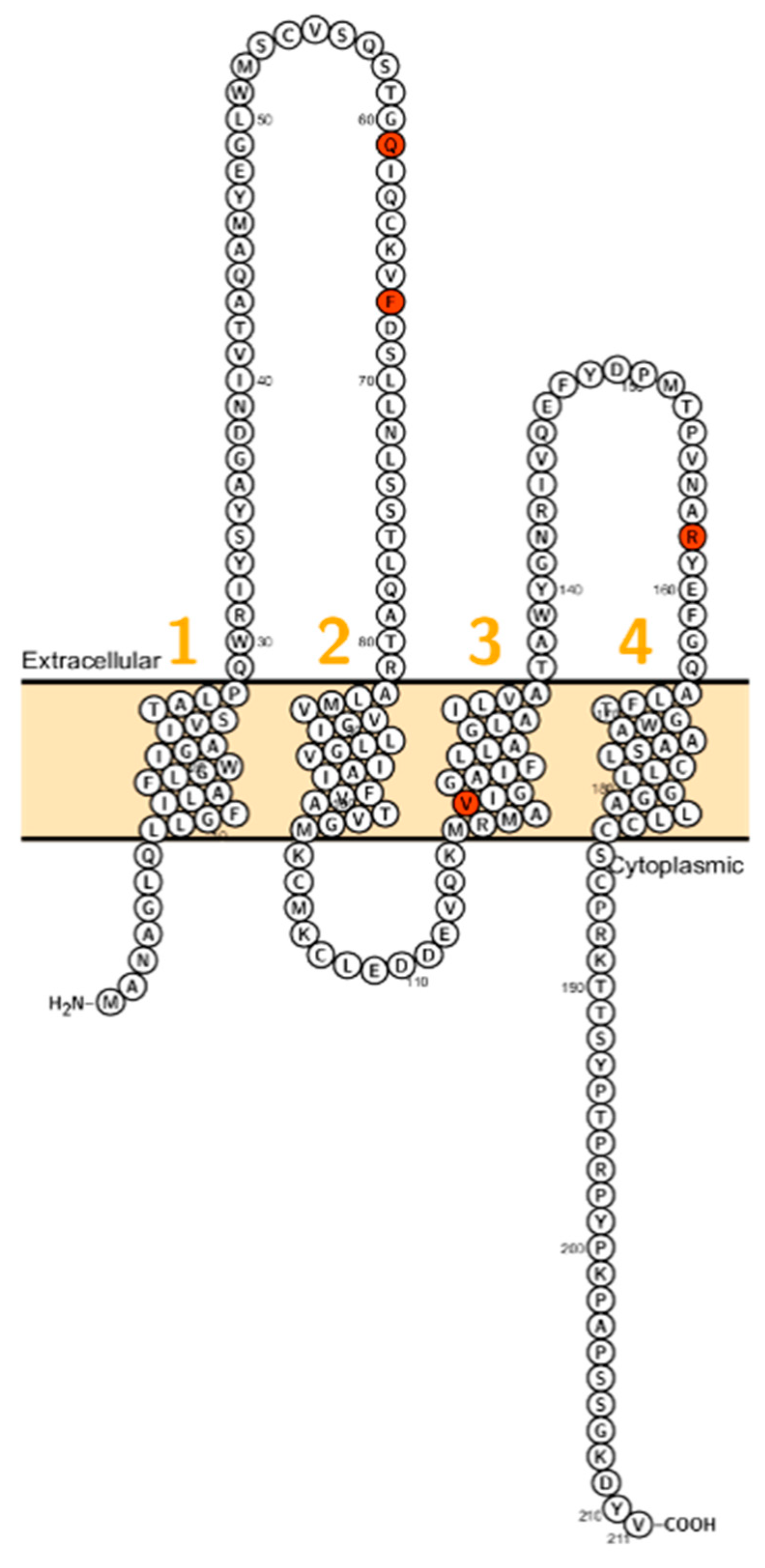
CLDN1 consists of four exons encoding a 211 amino acid protein that conveys barrier properties [16]. It has been shown that claudin-1 interacts with claudin-3 and claudin-5, which are also barrier–forming claudins [32,33]. In the skin, CLDN1 is expressed in the stratum corneum, stratum basale, stratum granulosum and stratum spinosum [34]. Further, it is expressed in the kidney [35], gall bladder [36], human ovarian epithelium [37] and the inner ear [38]. The expression of CLDN1 is increased in colorectal cancer [39], lung carcinoma [40], cervical cancer [41] and reduced in larynx cancer [42]. Evans et al. showed that CLDN1 is involved in Hepatitis C Virus entry into intestinal cells, which is presumably depending on the first extracellular loop (ECL, Figure 1) [43].
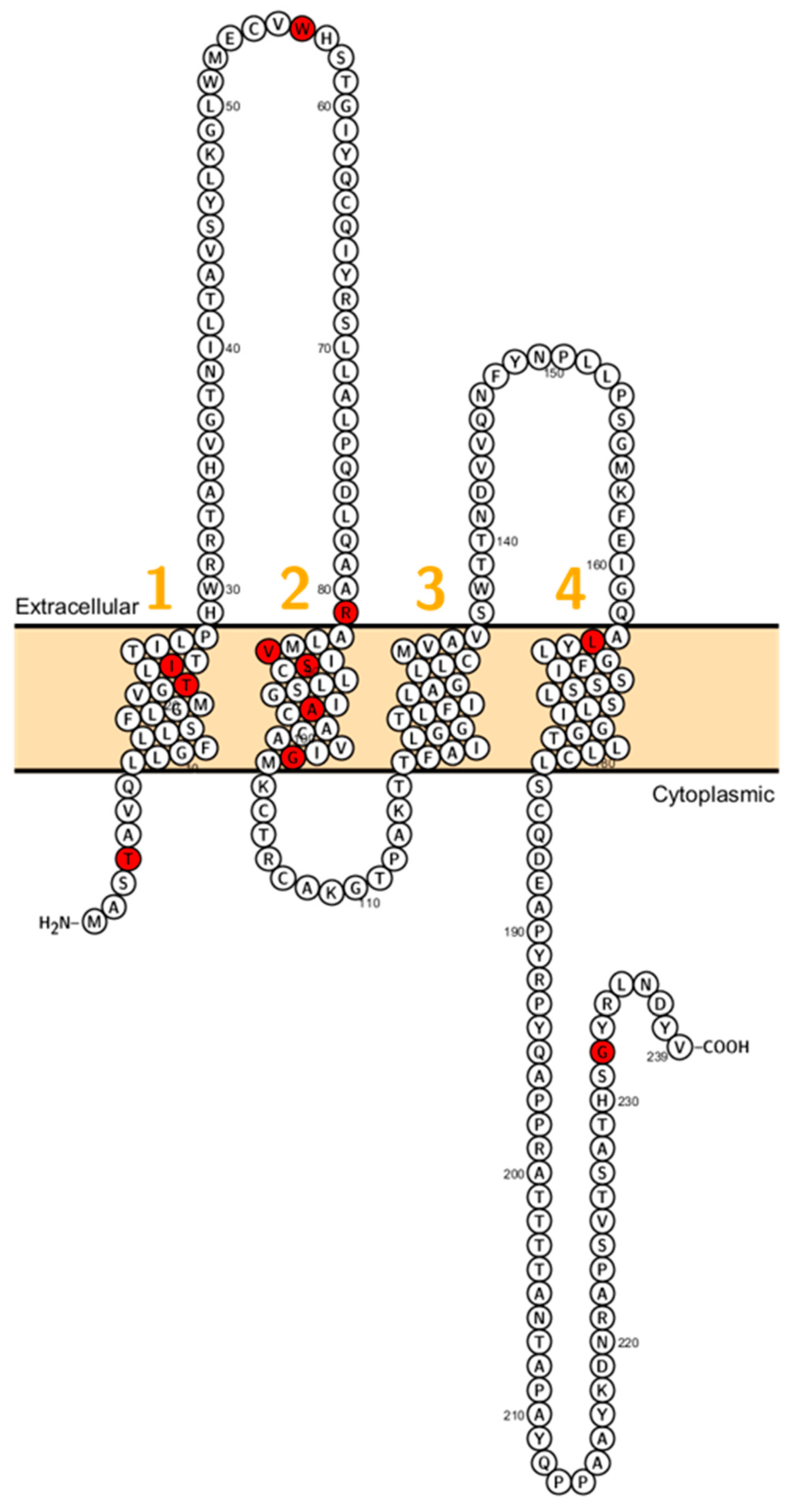
Figure 1.
Topology of CLDN1. FASTA sequence of CLDN1 (Uniprot accession no: O95832; plotted by Protter software http://wlab.ethz.ch/protter/). Each amino acid is shown as a single letter code and numbers (orange) indicate transmembrane domains. Mutations shown to be involved in human diseases are shown in red.
In 2002, Baala et al. described a family with autosomal dominant ichthyosis, alopecia, leucocytic vacuoles and sclerosing cholangitis (ILVASC; or neonatal ichthyosis with sclerosing cholangitis (NISCH-Syndrome OMIM 607626) assigned to chromosome 3q27–28 [44]. In 2004 Hadj-Rabia et al. identified in the same kindred a frameshift mutation in CLDN1 leading to a premature stop codon at position 67 [18] (Figure 1). This finding, that ILVASC/NISCH syndrome is caused by mutations in CLDN1 has been later confirmed by others [18,44,45,46]. In 2006, a neonate patient with erythroderma, massive lamellar desquamation and alopecia were reported. The hepatologic aspects were icterus, hyperbilirubinemia and increased biliary acids, liver biopsy showed panlobular cholestasis with acute hepatitis. All reported cases showed that CLDN1 mutations in humans were not lethal and did not affect fertility.
Furuse and colleagues generated Cldn1 deficient mice, which exhibited low body weight and died within the first day after birth, possibly attributable to an excessive trans-epithelial water loss (TEWL). Cldn1 deficient mice displayed also altered lipid composition and defects of the stratum corneum of the skin. On the other hand, CLDN1 deficient patients also displayed mild wrinkled skin and hyperproliferation of keratinocytes. However, only some patients had liver cell injury [18]. Whether knockout mice were affected by hepatic abnormalities has not been reported, probably due to the perinatal death.
Since the mouse model was limited because of its early lethality, a KD mouse approach was used, that showed reduced expression levels of Cldn1 and Cldn1 levels were associated with the severity of the phenotype [47]. Cldn1 KD mice were born with wrinkled skin similar to KO mice, however, morphological examinations at 8 weeks of age revealed a normal development, which might be explained by the low but still expressed Cldn1. The underlying mechanism of TEWL was further investigated by Hirano et al. by using tamoxifen-induced epidermis-specific deletion of Cldn1 in adult mice. Four days after induction, claudin-1 began depleting from basal layers and was undetectable in granular layers at day eight and tight junction leakage was observed. Neither TEWL nor stratum corneum defects were observed until day 18 suggesting that TJ deterioration is a prerequisite for stratum corneum defects [48].
2.2. Claudin 2
Claudin-2 is one of the two claudins that were initially identified by Furuse et al. in 1998 [49]. In humans, its gene contains two exons encoding a 203 amino acid protein that constitutes cation- selective pores [50]. It is expressed in rat brain [51], proximal tubules of the kidney [52] liver, and epididymis [53].
Its expression is increased in colorectal cancer [54], active ulcerative colitis [55], a severe form of the coeliac disease [56] and inflammatory bowel disease [57] whereas it is downregulated in breast tumors and osteosarcoma [58,59]. It has been shown that miR-16 modulates CLDN2 expression and causes dysfunctional barrier properties in inflammatory bowel disease [60].
Cldn2 KO mice have been generated by Muto et al. [61]. Mice were morphologically normal at birth. The authors did not observe renal morphological abnormalities under both light and electron microscope. However, further analysis in proximal tubules revealed reduced absorption of Na+ and higher urinary fractional excretion of Ca2+. The same mice were investigated by a different group, focusing on small intestine showing that Cldn2 deficient mice have slightly larger intestine and longer intestinal villi. They further demonstrated Cldn2 dependent Na+ selective intestinal paracellular permeability. Matsumoto et al. focused on liver and biliary tissues of Cldn2 deficient mice and similar to the kidney, no obvious morphological abnormalities were observed [62]. The detailed physiological analysis revealed a decreased bile flow in Cldn2 deficient mice and four weeks under a lithogenic diet, KO mice developed gallstones as a consequence of altered bile composition and flow rate [62]. As Cldn2 and Cldn15 play an important role in paracellular ion flow, Wada et al. generated Cldn2-/- Cldn15-/- double KO (dKO) mice [63]. Deficiency of both genes caused early death by week three, which was attributed to overt hypoglycemia of the dKO animals presumably caused by a disrupted Na+ flow, which is important for intestinal glucose absorption. Transgenic mice with colon specific overexpression of Cldn2 displayed an enlarged colon and RNAs of genes involved in cell proliferation were found to be increased. In contrast, for inflammation related RNAs, the opposite was shown, in line with the fact that mice were protected against experimentally induced colitis [64].
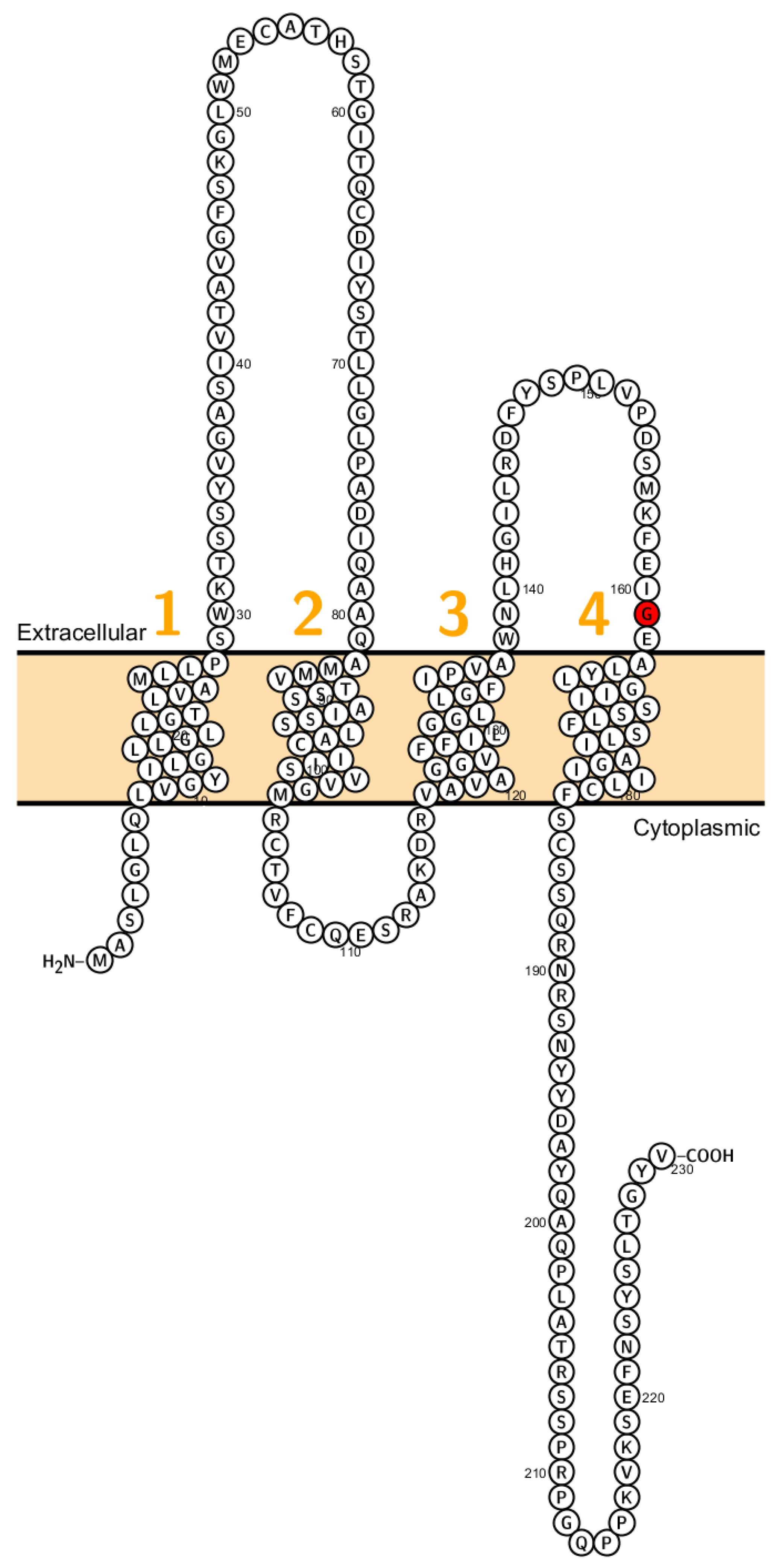
Recently a missense mutation of Cldn2 associated with obstructive azoospermia in a four generation spanning family has been identified (Figure 2). Further, it has been shown that different claudins (CLDN1, CLDN2, CLDN3, CLDN4 and CLDN7) are expressed in human epididymal tight junctions [65]. Except for CLDN2 all these claudins cause, when eliminated, a disruption of the epithelial barrier. Therefore, it has been assumed that malfunction of these claudins might cause infertility in men but has not been proven so far.
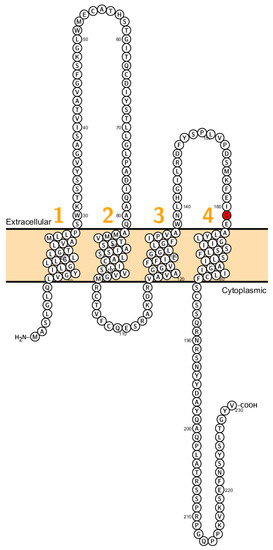
Figure 2.
Topology of CLDN2. FASTA sequence of CLDN2 (Uniprot accession no: P57739). Each amino acid is shown as a single letter code and numbers (orange) indicate transmembrane domains. Mutations shown to be involved in human diseases are shown in red.
2.3. Claudin 9
The Claudin-9 gene consists of a single exon encoding a 217 amino acid protein. It constitutes a barrier to K+ and Na+. Its expression was found in the inner ear, the liver [66] and the kidney [67].
Zhang et al. showed that claudin-9, together with claudin-6 mediates Hepatitis C Virus (HCV) entry into hepatoma cell lines [66]. Fofana et al. generated monoclonal antibodies against CLDN9 which inhibit HCVpp (HCV pseudo particles) entry to CLDN9 expressing cell lines [68].
The expression of CLDN9 is increased in gastric cancer [69] and promotes cell proliferation and migration of lung metastasis [70]. In cervical carcinoma, RNA expression levels were found to be decreased [71].
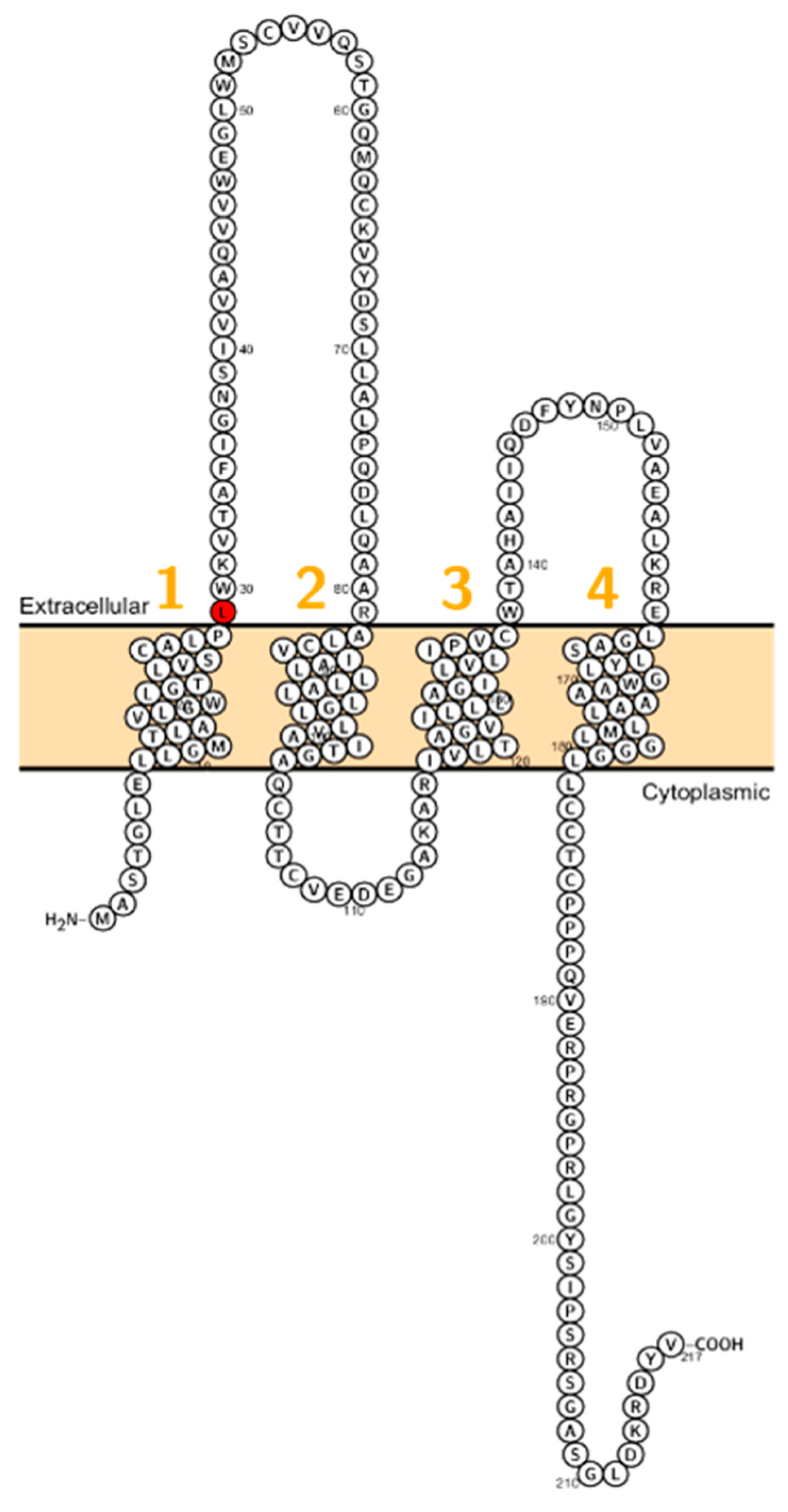
Sineni et al. described patients with inherited autosomal recessive hearing loss who had a truncated variant of claudin-9 (p.L29fs). The mutation is located at the beginning of the first ECL (Figure 3). The truncated protein was not detected at the plasma membrane, indicating a dysfunctional TJ and thereby affecting the peri- and endolymphatic ion composition in the inner ear as the cause of hearing impairment [19]. Patients did not display coordination disturbances. This pathophysiological principle has been demonstrated in the past when mutations in BSND have been identified in patients with Bartter syndrome Type 4 [72].
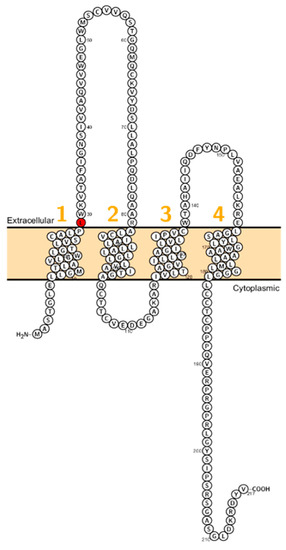
Figure 3.
Topology of CLDN9. FASTA sequence of CLDN9 (Uniprot accession no: O95484). Each amino acid is shown as a single letter code and numbers (orange) indicate transmembrane domains. Mutations shown to be involved in human diseases are shown in red.
Mice that carry a missense mutation in Cldn9 were shown to be deaf. Further analysis showed that Reissner’s membrane was morphologically normal when compared to wild type mice and that only the basal but not the apical part of the cochlea was morphologically affected. Inner ear development was normal until postnatal day 14 (P14) then degeneration of the organ of Corti was observed. Since claudin-9 acts as a barrier for Na+ and K+, heterologous expression of the mutant in MDCK cells did not affect membrane targeting, indicating that this protein was not functional even when properly targeted and inserted into the TJ. In both, patients and mice hearing loss are age dependent. In mice at P14, outer hair cells (OHC) appeared normal whereas severe degeneration of OHC was observed at P80. In patients, a younger affected sibling had moderate hearing loss when compared to the older sibling. Neither mutant mice nor human patients had balance problems. Although Cldn9 is expressed in various tissues, the authors did not report any abnormalities of other organs (e.g., kidney) yet.
2.4. Claudin 10
CLDN10 consists of five exons encoding a 228 amino acid protein. Initially, there were two isoforms described [73], later Günzel et al. described four different splice variants mostly localized at TJs except for the ones that lack exon 4 [74]. Claudin-10a constitutes a pore for anions whereas claudin-10b forms a pore for cations [16,73]. CLDN10b is expressed in many tissues including the brain, lung [75], salivary gland [76], mammary gland [77] but CLDN10a is exclusively expressed in the kidney [73]. There are no isoform-specific antibodies, but according to RNA hybridization data, Cldn10b is highly expressed in medulla and Cldn10a in the cortex [73] and based on RNA-Seq data, Cldn10a is expressed in the proximal tubule (PT) and Cldn10b in thick ascending limb (TAL) [11].
Both isoforms interact with claudin-18 and claudin-19 in a yeast two-hybrid analysis [78], whereas immunofluorescence experiments on kidney sections did not demonstrate colocalization [79]. Additionally, claudin-10 has been implicated in left-right-patterning as well as in tumor progression and invasiveness [80].
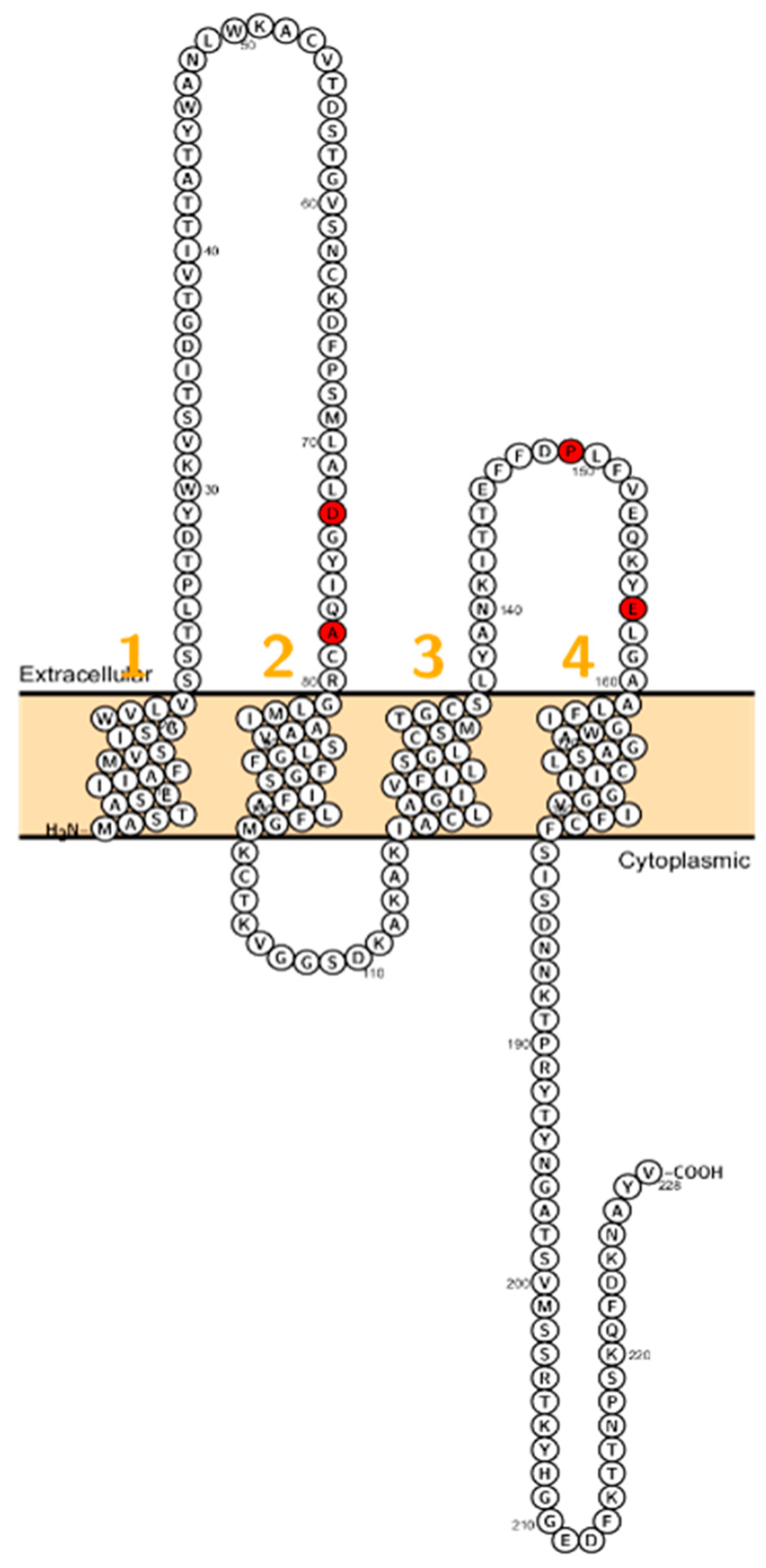
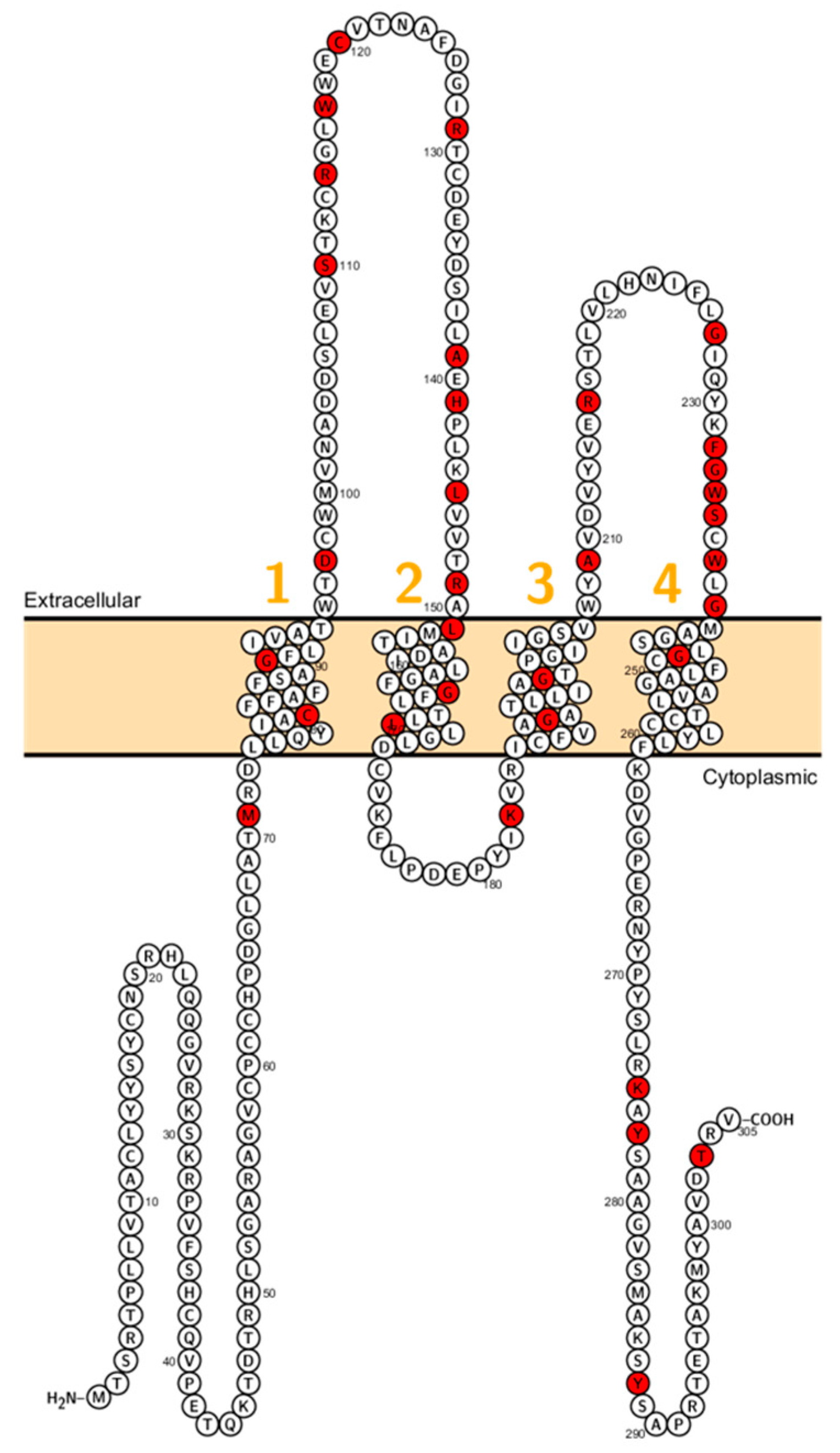
Patients with a homozygous mutation in CLDN10B (N48K) showed anhidrosis, alacrima, dry mouth, and kidney failure with hypermagnesemia, low urinary Mg2+ and Ca2+ [81] (Figure 4). Patients did not display overt hypokalemia indicating only a mild renal electrolyte wasting although Meyers et al. described a patient with CLDN10 mutation who initially presented with hypokalemia and follow-up examinations revealed a developing hypermagnesemia [82]. In fact, CLDN10 patients exhibit a considerable range of hypohidrosis, hypolacrymia, ichthyosis and xerostomia and a decreased amount of saliva. Functional testing revealed that patients had decreased NaCl absorption in the TAL, too [82].
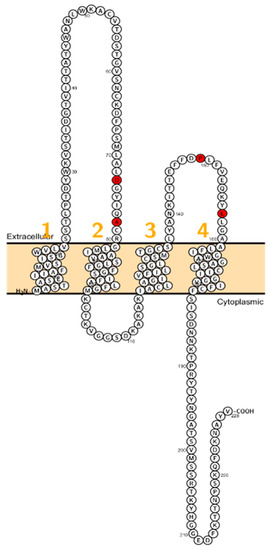
Figure 4.
Topology of CLDN10. FASTA sequence of CLDN10 (Uniprot accession no: P78369). Each amino acid is shown as a single letter code and numbers (orange) indicate transmembrane domains. Mutations shown to be involved in human diseases are shown in red.
TAL specific knockout of both isoforms in mice resulted in polyuria, polydipsia and hypermagnesemia. Moreover, acidic urine and calcium deposits in the kidney (nephrocalcinosis) were observed. Quantitative expression analysis of TAL tubules revealed that expression of Cldn10, Cldn16 and Cldn19 was increased in kidneys of mutant animals [11].
Mice lacking claudin-16 and claudin-10 in the kidney were found to have normal Mg2+ in serum and absence of nephrocalcinosis. The authors demonstrated that the deletion of Cldn10 and the loss of Cldn16 led to an expansion of the DCT, especially which eventually resulted in increased resorption of Mg2+ [83].
2.5. Claudin 14
CLDN14 is located on human chromosome 21 and consists of two exons encoding a 239 amino acid protein. It has five protein-coding transcript variants and is classified as a barrier forming claudin [15].
Overexpression of CLDN14 is associated with gastric and hepatocellular forms of cancer [84].
Cldn14 expression was found in outer and inner ear hair cells and also in the TAL, the DCT [85] and in the liver [12].
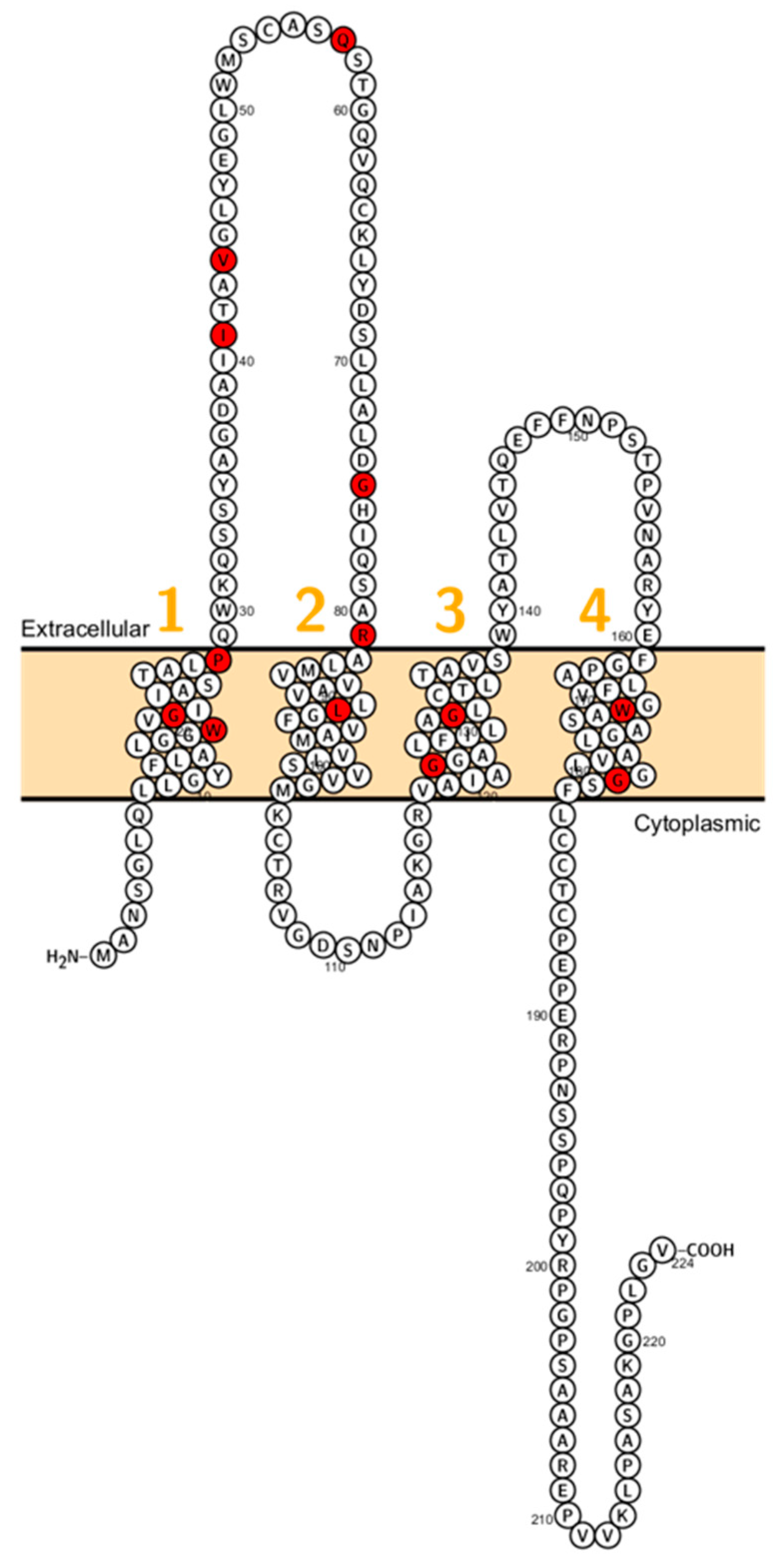
Wilcox et al. reported on deaf patients from two large consanguineous families revealing a mutation (V85D) in CLDN14. This mutation is predicted to interfere with a phosphorylation site and this residue is conserved between CLDN3 and CLDN9 which are expressed in the inner ear too [86]. Immunocytochemistry experiments showed that the mutant failed to localize on the membrane [87]. After this initial study, several other mutations (W56, R81H, G232R) have been reported (Figure 5).
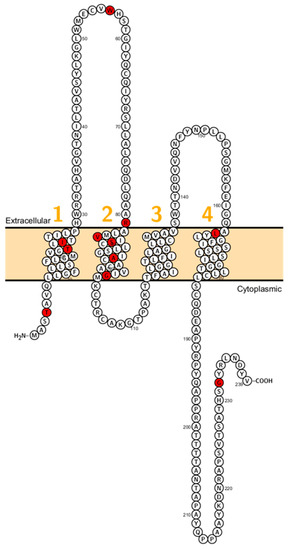
Figure 5.
Topology of CLDN14. FASTA sequence of CLDN14 (Uniprot accession no: O95500). Each amino acid is shown as a single letter code and numbers (orange) indicate transmembrane domains. Mutations shown to be involved in human diseases are shown in red.
Examination of Cldn14 mice that included kidney and liver along with 41 other tissues revealed no morphological differences between knockout and wild type animals. Inner hair cells and outer hair cells (OHC) seemed to develop normally. However, the rapid loss of OHC characterized by disorganization and loss of stereocilia was observed after the first week. Auditory brainstem response (ABR), tests revealed that knockout mice suffer from hearing loss by the third week of age [12].
Although initially no kidney abnormalities were observed neither in mice nor in patients, upon a high calcium diet, Cldn14 deficient mice developed hypomagnesemia and hypocalciuria. Moreover, it has been shown that two microRNA regulate extracellular Ca2+ levels through suppressing Cldn14 expression under normal diet conditions [85].
Transgenic overexpression of Cldn14 in TAL of kidney resulted in hypercalciuria and hypermagnesuria [88].
In humans, the hearing loss occurred at the prelingual stage and the severity of hearing loss depends on the mutation. A patient with non-common mutation (A163V) was reported with hearing ability until the age of three, in line with the observation in mice [89].
2.6. Claudin 16
Claudin-16 is a pore forming claudin encoded by CLDN16 gene [16]. It is expressed in duodenum, jejunum, ileum, colon [90], in tooth germ [91], in salivary glands [92], and also has been found in the endolymphatic duct of the inner ear [93]. The by far highest expression has been found in the thick ascending limb (TAL) of the kidney [94].
Several studies related overexpression of CLDN16 with different cancer types including ovarian cancer [95] and papillary thyroid carcinoma [96]. Also, claudin-16 might play a role in cell proliferation and differentiation, e.g., breast cancer [97,98].
In the kidney, Claudin-16 is crucial for TJ-specific ion transport in the TAL and handles approximately 25% Ca2+ and 70% of Mg2+ reabsorption [99]. However, the exact role of claudin-16 remains a matter of debate. Hou and colleagues showed that claudin-16 enhances the permeability of monovalent cations, including Na+, than that of divalent cations, as Mg2+ (<50%) [90]. When Cldn16 was deleted in mice, a cation-to-anion selectivity (PNa/PCl) but no divalent-to-monovalent cation selectivity (PMg/PNa) was observed [100]. Other groups have reported a selectivity of Claudin-16 for divalent cations Mg2+ and Ca2+ [101,102]. Hou et al. have described an interaction with claudin-19 as being necessary for correct TJ integration [78]. Additionally, it has been reported that claudin-14 reduces the cation permeability of claudin-16, but not for the claudin-19 in transfected LLC-PK1 cells. Based on this hypothesis, claudin-14 might act as a negative regulator of divalent cation permeation [85]. Split-ubiquitin yeast 2-hybrid (Y2H) membrane protein interaction assay showed that claudin-14 interacts with the claudin-16 [79]. Other known interactions reported are with syntaxin-8 [102] and with PDZ domain containing RING finger 1 (encoded by PDZRN) [103] by electrophysiological experiments [104].
Mutations in human CLDN16 cause an autosomal recessive disorder called Familial hypomagnesemia with hypercalcinuria and nephrocalcinosis (FHHNC) [105] (Figure 6). Other phenotypic features are incomplete distal tubular acidosis, impaired bone homeostasis [94], hypocitraturia and hyperuricemia which can be considered a secondary effect of renal insufficiency. To date, about 66 mutations including missense/nonsense, splicing, small deletion and small indels have been identified. Although still a matter of debate, it is believed that claudin-16 and claudin-19 form a heterodimer/tetramer essential for the divalent cation selectivity of the paracellular channels at the TAL and it has been demonstrated that some mutations disrupt this interaction [106].
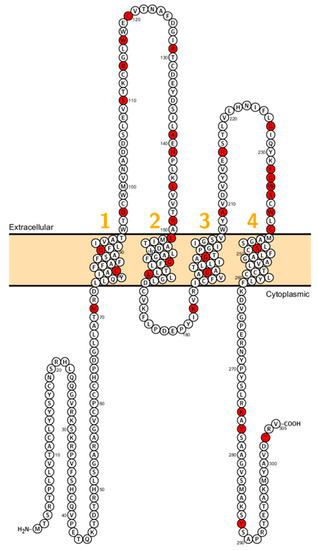
Figure 6.
Topology of CLDN16. FASTA sequence of CLDN16 (Uniprot accession no: Q9Y517). Each amino acid is shown as a single letter code and numbers (orange) indicate transmembrane domains. Mutations shown to be involved in human diseases are shown in red.
Cldn16 deficient mice exhibited hypomagnesia and hypercalciuria and a low urinary pH but did not show nephrocalcinosis which could be explained by the upregulation of several genes (e.g., Trpm6, Trpv5, Cnnm2) involved in calcium and magnesium transport or the altered pH. Moreover, mice did not show renal insufficiency [13].
Besides, knockdown mice were generated by RNA interference technology [100] which phenocopied the main features of FHHNC including hypercalcinuria, hypomagnesemia, nephrocalcinosis and urinary Ca2+ and Mg2+ wasting without showing nephrocalcinosis or renal insufficiency. Cldn16 KD animals showed increase of 1,25-dihydroxychlecalciferol [100].
For Cldn16 Patients, a genotype-phenotype correlation has been reported [94]. In sharp contrast to humans, although mice recapitulate many features of FHHNC like hypomagnesemia and hypercalciuria, they do not display nephrocalcinosis nor renal insufficiency [100]. Most of the patients with FHHNC nephrocalcinosis develop end stage renal disease with a need of renal transplantation [107]. Recently, amelogenesis imperfecta has been related to the absence of Claudin-16 in the ameloblasts in humans and mice [105] as an additional role of Cldn16 deficiency.
2.7. Claudin 19
The CLDN-19 gene encodes a 224 amino acid protein. Claudin-19 is a barrier forming claudin and is expressed in the thick ascending limb of the kidney and the retinal pigment epithelium (RPE) and the sheath of myelinated peripheral nerves. Additionally its expression was reported detected in the inner ear [38], stomach [108] and lung [109]. Like claudin-16, claudin-19 plays a major role in the permeability and selectivity of the TJ in TAL.
Claudin-19 interacts with claudin-16, both are involved in the reabsorption of divalent cations in the TAL [79,110]. Gong et al. isolated a stable dimer of claudin-16 with claudin-19 from transfected HEK293 cells and Sf9 cells [85]. The dimerization occurs through the cis-association of the third and the fourth transmembrane domain in both proteins [111]. This hypothesis is complemented by in vivo transgenic animal models, deletion of claudin-16 rendered claudin-19 delocalization from the TJ and vice versa [78]. Selective mutations disrupt the dimerization, triggering a loss of the transport function with FHHNC disease is the consequence [112].
Moreover, claudin-19 and ZO-1 are found by co-immunoprecipitation forming a complex in Madin-Darby canine kidney (MDCK) cells [97]. Meier et al. reported patients presenting by a phenotype of FHHNC disease but with the ocular disease [113,114]. All individuals of these families suffered from severe visual impairment, characterized by macular colobomata, significant myopia, and horizontal nystagmus. The genotype of these patients did not show a mutation in CLDN16, but Konrad and colleagues identified three mutations (G20D, Q57E) in CLDN19, recapitulating CLDN16 defects but, ocular defects, too. Other reported mutations (L90P and G123R) disrupt the interaction between claudin-16 and claudin-19 [23,112] (Figure 7).
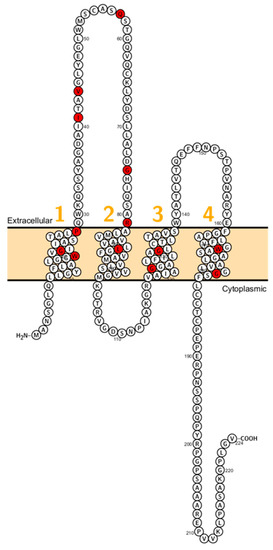
Figure 7.
Topology of CLDN19. FASTA sequence of CLDN19 (Uniprot accession no: Q8N6F1). Each amino acid is shown as a single letter code and numbers (orange) indicate transmembrane domains. Mutations shown to be involved in human diseases are shown in red.
Cldn19 KD mice showed a reduction of Mg2+ in plasma and excessive losses of Ca2+ and Mg2+ levels in kidney similar to Cldn16 KO mice [78]. These mutations are the cause of FHHNC diseases, in which interaction between claudin-16 and-19 is disrupted. The dissociation of these proteins can trigger a loss of the transport function of them and cause FHHNC [112]. On the other hand, knockout mouse of Cldn19 provokes renal reabsorption deficiency [79].
3. Discussion
Twenty years ago, the seminal work of Lifton’s group described mutations in a gene coding for a TJ protein (initially named paracellin-1 and thereafter classified as claudin-16) as being causative for a human disorder (FHHNC) [99]. CLDN16, being the first claudin of a continuously expanding group of claudins causing human disorders, has been subject to various investigations on expression, function and also on possibilities of pharmaceutical interventions [115]. To gain further insights, mouse models, either as knockdown or knock out have been established (Table 1). Such models recapitulated some (Hypercalciuria, Hypomagnesemia) but not all (Nephrocalcinosis and renal insufficiency) of the hallmarks of human FHHNC, demonstrating the benefits but also the limitations of such models of TJ disorders. Recently the association of claudin-16 and amelogenesis imperfecta in men and mice has been shown [91].

Table 1.
Overview of claudins causing human diseases and corresponding mouse models. The existence (by publication) is indicated by (+) while (−) indicates the absence of a corresponding model.
As the example of claudin-16 demonstrates, disorders that are caused by mutations in the corresponding claudin genes are not restricted to a given organ but rather should be considered as ‘claudinopathies’. This view would not restrict the consequences to tissue-specific local or regional expression but appreciates the function and loss in all of the organs and tissues where a given claudin is expressed. Moreover, in a given organ, multiple different claudins are expressed and can cause, when mutated a similar phenotype albeit having different underlying pathophysiology. For example, kidney stones are a worldwide problem that affects 12% of the world population regardless of gender [116,117]. Besides CLDN16 and CLDN19, in 2009, a genome-wide association study on an Icelandic and Dutch population revealed that a CLDN14 variant (rs219780[C]) is associated with kidney stones too.
In this perspective, animal models, especially mice with all their limitations, are indispensable to study the consequences of claudin deficiency.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG), Research Training Group GRK 2318 (to M.S. and D.M.), and the Open Access Publication Fund of the Charité—Universitätsmedizin Berlin. C.F.R. and L.A.M.C. acknowledge Ministerio Español de Ciencia e Innovación (MICINN) for funding under grants BFU2010-17857 and BFU2016-77408-R], as well as the Spanish Ministry of Economy and Competitiveness (MINECO/FEDER, UE) [REFsBES-2017-080435, BES-2014-068464] and the MICINN CONSOLIDER-INGENIO 2010 Program [grant number CSD2008-00005; BFU2015-70067-REDC]. We also thank MINECO for the Severo Ochoa Excellence Accreditation to CIC bioGUNE (SEV-2016-0644).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Baud, L. Renal Epithelial Cells: Differentiation and Plasticity. J. Am. Soc. Nephrol. 2003, 14, S1–S2. [Google Scholar] [CrossRef]
- Macara, I.G.; Guyer, R.; Richardson, G.; Huo, Y.; Ahmed, S.M. Epithelial homeostasis. Curr. Biol. 2014, 24, R815–R825. [Google Scholar] [CrossRef]
- Roignot, J.; Peng, X.; Mostov, K. Polarity in Mammalian Epithelial Morphogenesis. Cold Spring Harb. Perspect. Biol. 2013, 5, a013789. [Google Scholar] [CrossRef] [PubMed]
- Karasov, W.H. Integrative physiology of transcellular and paracellular intestinal absorption. J. Exp. Biol. 2017, 220, 2495–2501. [Google Scholar] [CrossRef]
- Sterling, T.M.; Nemere, I. Calcium uptake and membrane trafficking in response to PTH or 25(OH)D3 in polarized intestinal epithelial cells. Steroids 2007, 72, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.D.S.; Heilberg, I.P. Bartter syndrome: Causes, diagnosis, and treatment. Int. J. Nephrol. Renov. Dis. 2018, 11, 291–301. [Google Scholar] [CrossRef]
- Knoers, N.V.; Levtchenko, E.N. Gitelman syndrome. Orphanet J. Rare Dis. 2008, 3, 22. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Nakano, Y.; Kim, S.H.; Kim, H.M.; Sanneman, J.D.; Zhang, Y.; Smith, R.J.; Marcus, D.C.; Wangemann, P.; Nessler, R.A.; Banfi, B. A claudin-9-based ion permeability barrier is essential for hearing. PLoS Genet. 2009, 5, e1000610. [Google Scholar] [CrossRef]
- Breiderhoff, T.; Himmerkus, N.; Stuiver, M.; Mutig, K.; Will, C.; Meij, I.C.; Bachmann, S.; Bleich, M.; Willnow, T.E.; Müller, D. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc. Natl. Acad. Sci. USA 2012, 109, 14241–14246. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yosef, T.; Belyantseva, I.A.; Saunders, T.L.; Hughes, E.D.; Kawamoto, K.; Van Itallie, C.M.; Beyer, L.A.; Halsey, K.; Gardner, D.J.; Wilcox, E.R.; et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum. Mol. Genet. 2003, 12, 2049–2061. [Google Scholar] [CrossRef]
- Will, C.; Breiderhoff, T.; Thumfart, J.; Stuiver, M.; Kopplin, K.; Sommer, K.; Gunzel, D.; Querfeld, U.; Meij, I.C.; Shan, Q.; et al. Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am. J. Physiol. Ren. Physiol. 2010, 298, F1152–F1161. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann. N. Y. Acad. Sci. 2000, 915, 129–135. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta 2008, 1778, 631–645. [Google Scholar] [CrossRef]
- Sanjad, S.A.; Hariri, A.; Habbal, Z.M.; Lifton, R.P. A novel PCLN-1 gene mutation in familial hypomagnesemia with hypercalciuria and atypical phenotype. Pediatr. Nephrol. 2007, 22, 503–508. [Google Scholar] [CrossRef]
- Hadj-Rabia, S.; Baala, L.; Vabres, P.; Hamel-Teillac, D.; Jacquemin, E.; Fabre, M.; Lyonnet, S.; De Prost, Y.; Munnich, A.; Hadchouel, M.; et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: A tight junction disease. Gastroenterology 2004, 127, 1386–1390. [Google Scholar] [CrossRef]
- Sineni, C.J.; Yildirim-Baylan, M.; Guo, S.; Camarena, V.; Wang, G.; Tokgoz-Yilmaz, S.; Duman, D.; Bademci, G.; Tekin, M. A truncating CLDN9 variant is associated with autosomal recessive nonsyndromic hearing loss. Hum. Genet. 2019, 138, 1071–1075. [Google Scholar] [CrossRef]
- Hadj-Rabia, S.; Brideau, G.; Al-Sarraj, Y.; Maroun, R.C.; Figueres, M.-L.; Leclerc-Mercier, S.; Olinger, E.; Baron, S.; Chaussain, C.; Nochy, D.; et al. Multiplex epithelium dysfunction due to CLDN10 mutation: The HELIX syndrome. Genet. Med. 2017, 20, 190–201. [Google Scholar] [CrossRef]
- Wilcox, E.R.; Burton, Q.L.; Naz, S.; Riazuddin, S.; Smith, T.N.; Ploplis, B.; Belyantseva, I.; Ben-Yosef, T.; Liburd, N.A.; Morell, R.J.; et al. Mutations in the Gene Encoding Tight Junction Claudin-14 Cause Autosomal Recessive Deafness DFNB29. Cell 2001, 104, 165–172. [Google Scholar] [CrossRef]
- Hampson, G.; A Konrad, M.; Scoble, J. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC): Compound heterozygous mutation in the claudin 16 (CLDN16) gene. BMC Nephrol. 2008, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Schaller, A.; Seelow, D.; Pandey, A.V.; Waldegger, S.; Lesslauer, A.; Vitzthum, H.; Suzuki, Y.; Luk, J.M.; Becker, C.; et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am. J. Hum. Genet. 2006, 79, 949–957. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional Keys for Intestinal Barrier Modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Merikallio, H.; Kaarteenaho, R.; Pääkkö, P.; Lehtonen, S.; Hirvikoski, P.; Mäkitaro, R.; Harju, T.; Soini, Y. Impact of smoking on the expression of claudins in lung carcinoma. Eur. J. Cancer 2011, 47, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, A.; Wang, X.; Gabello, M.; Valenzano, M.C.; Soler, A.P.; Ko, A.; Morin, P.J.; Mullin, J.M. Dietary methionine restriction improves colon tight junction barrier function and alters claudin expression pattern. Am. J. Physiol. Physiol. 2010, 299, C1028–C1035. [Google Scholar] [CrossRef]
- Soini, Y. Claudins in lung diseases. Respir. Res. 2011, 12, 70. [Google Scholar] [CrossRef]
- Barmeyer, C.; Schulzke, J.D.; Fromm, M. Claudin-related intestinal diseases. Semin. Cell Dev. Biol. 2015, 42, 30–38. [Google Scholar] [CrossRef]
- Limaye, A.; Hall, B.; Kulkarni, A.B. Manipulation of mouse embryonic stem cells for knockout mouse production. Curr. Protoc. Cell Biol. 2009, 44, 19.13.1–19.13.24. [Google Scholar]
- Kooij, G.; Kopplin, K.; Blasig, R.; Stuiver, M.; Koning, N.; Goverse, G.; van der Pol, S.M.; van Het Hof, B.; Gollasch, M.; Drexhage, J.A.; et al. Disturbed function of the blood-cerebrospinal fluid barrier aggravates neuro-inflammation. Acta Neuropathol. 2014, 128, 267–277. [Google Scholar] [CrossRef]
- Adli, M. The CRISPR tool kit for genome editing and beyond. Nat. Commun. 2018, 9, 1911. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, B.L.; Ward, C.; Smith, T.; Ritzenthaler, J.D.; Koval, M. Regulation of Heterotypic Claudin Compatibility. J. Biol. Chem. 2007, 282, 30005–30013. [Google Scholar] [CrossRef] [PubMed]
- Piontek, J.; Fritzsche, S.; Cording, J.; Richter, S.; Hartwig, J.; Walter, M.; Yu, D.; Turner, J.R.; Gehring, C.; Rahn, H.-P.; et al. Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell. Mol. Life Sci. 2011, 68, 3903–3918. [Google Scholar] [CrossRef] [PubMed]
- Zorko, M.S.; Veraniä, P.; Leskovec, N.K.; Pavloviä, M.D.; Pavlović, M.D.; Veranič, P. Expression of tight-junction proteins in the inflamed and clinically uninvolved skin in patients with venous leg ulcers. Clin. Exp. Dermatol. 2009, 34, e949–e952. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Lamas, M.; Martin, D.; Namorado, M.D.C.; Islas, S.; Luna, J.; Tauc, M.; Gonzalez-Mariscal, L.; Gonz, L. The renal segmental distribution of claudins changes with development. Kidney Int. 2002, 62, 476–487. [Google Scholar] [CrossRef]
- Laurila, J.J.; Karttunen, T.; Koivukangas, V.; Laurila, P.A.; Syrjala, H.; Saarnio, J.; Soini, Y.; Ala-Kokko, T.I. Tight junction proteins in gallbladder epithelium: Different expression in acute acalculous and calculous cholecystitis. J. Histochem. Cytochem. 2007, 55, 567–573. [Google Scholar] [CrossRef]
- Zhu, Y.; Brannstrom, M.; Janson, P.O.; Sundfeldt, K. Differences in expression patterns of the tight junction proteins, claudin 1, 3, 4 and 5, in human ovarian surface epithelium as compared to epithelia in inclusion cysts and epithelial ovarian tumours. Int. J. Cancer 2006, 118, 1884–1891. [Google Scholar] [CrossRef]
- Kitajiri, S.-I.; Furuse, M.; Morita, K.; Saishin-Kiuchi, Y.; Kido, H.; Ito, J.; Tsukita, S. Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear. Res. 2004, 187, 25–34. [Google Scholar] [CrossRef]
- Mori, M.; Nakagawa, S.; Miyoshi, N.; Ishii, H.; Mimori, K.; Tanaka, F.; Sekimoto, M.; Doki, Y. Expression of CLDN1 in colorectal cancer: A novel marker for prognosis. Int. J. Oncol. 2011, 39, 791–796. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, A.; Sun, B.; Zhan, Z.; Chen, K.; Wang, C. Expression of CLDN1 and CLDN10 in lung adenocarcinoma in situ and invasive lepidic predominant adenocarcinoma. J. Cardiothorac. Surg. 2013, 8, 95. [Google Scholar] [CrossRef]
- Zhang, W.-N.; Li, W.; Wang, X.-L.; Hu, Z.; Zhu, D.; Ding, W.-C.; Liu, D.; Li, K.-Z.; Ma, D.; Wang, H. CLDN1 expression in cervical cancer cells is related to tumor invasion and metastasis. Oncotarget 2016, 7, 87449–87461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Piao, X.; Wang, C.; Wang, R.; Song, Z. Identification of claudin1, 3, 7 and 8 as prognostic markers in human laryngeal carcinoma. Mol. Med. Rep. 2019, 20, 393–400. [Google Scholar] [PubMed]
- Evans, M.J.; Von Hahn, T.; Tscherne, D.M.; Syder, A.J.; Panis, M.; Wölk, B.; Hatziioannou, T.; McKeating, J.A.; Bieniasz, P.D.; Rice, C.M. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 2007, 446, 801–805. [Google Scholar] [CrossRef]
- Baala, L.; Hadj-Rabia, S.; Hamel-Teillac, D.; Hadchouel, M.; Prost, C.; Leal, S.M.; Jacquemin, E.; Sefiani, A.; De Prost, Y.; Courtois, G.; et al. Homozygosity mapping of a locus for a novel syndromic ichthyosis to chromosome 3q27-q28. J. Investig. Dermatol. 2002, 119, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kirchmeier, P.; Sayar, E.; Hotz, A.; Hausser, I.; Islek, A.; Yilmaz, A.; Artan, R.; Fischer, J. Novel mutation in the CLDN1 gene in a Turkish family with neonatal ichthyosis sclerosing cholangitis (NISCH) syndrome. Br. J. Dermatol. 2014, 170, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, M.; Wagner, A.; Van Geel, M.; Nagtzaam, I.F.; Peeters, V.P.M.; Steijlen, P.M.; Van Steensel, M.A.M. Novel CLDN1 mutation in ichthyosis-hypotrichosis-sclerosing cholangitis syndrome without signs of liver disease. Br. J. Dermatol. 2018, 178, e202–e203. [Google Scholar]
- Tokumasu, R.; Yamaga, K.; Yamazaki, Y.; Murota, H.; Suzuki, K.; Tamura, A.; Bando, K.; Furuta, Y.; Katayama, I.; Tsukita, S. Dose-dependent role of claudin-1 in vivo in orchestrating features of atopic dermatitis. Proc. Natl. Acad. Sci. USA 2016, 113, E4061–E4068. [Google Scholar] [CrossRef]
- Hirano, T.; Yokouchi, M.; Atsugi, T.; Amagai, M.; Kubo, A. Epidermis-specific ablation of claudin-1 in adult mice demonstrates the essential role of a tight junction barrier in skin homeostasis. J. Dermatol. Sci. 2016, 84, e37. [Google Scholar] [CrossRef]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schöneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef]
- Lamas, M.; González-Mariscal, L.; Gutiérrez, R. Presence of claudins mRNA in the brain. Selective modulation of expression by kindling epilepsy. Mol. Brain Res. 2002, 104, 250–254. [Google Scholar] [CrossRef]
- Enck, A.H.; Berger, U.V.; Yu, A.S.L. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am. J. Physiol. Physiol. 2001, 281, F966–F974. [Google Scholar] [CrossRef]
- Guan, X.; Inai, T.; Shibata, Y. Segment-specific expression of tight junction proteins, claudin-2 and -10, in the rat epididymal epithelium. Arch. Histol. Cytol. 2005, 68, 213–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kinugasa, T.; Huo, Q.; Higashi, D.; Shibaguchi, H.; Kuroki, M.; Tanaka, T.; Futami, K.; Yamashita, Y.; Hachimine, K.; Maekawa, S.; et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007, 27, 3729–3734. [Google Scholar] [CrossRef]
- Oshima, T.; Miwa, H.; Joh, T. Changes in the expression of claudins in active ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23, S146–S150. [Google Scholar] [CrossRef]
- Szakál, D.N.; Győrffy, H.; Arató, A.; Cseh, Á.; Molnár, K.; Papp, M.; Dezsofi, A.; Veres, G. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease. Virchows Archiv. 2010, 456, 245–250. [Google Scholar] [CrossRef]
- Weber, C.R.; Nalle, S.C.; Tretiakova, M.; Rubin, D.T.; Turner, J.R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Investig. 2008, 88, 1110–1120. [Google Scholar] [CrossRef]
- Kim, T.H.; Huh, J.H.; Lee, S.; Kang, H.; I Kim, G.; An, H.J. Down-regulation of claudin-2 in breast carcinomas is associated with advanced disease. Histopathology 2008, 53, 48–55. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Li, Q.; Li, T. CLDN2 inhibits the metastasis of osteosarcoma cells via down-regulating the afadin/ERK signaling pathway. Cancer Cell Int. 2018, 18, 160. [Google Scholar] [CrossRef]
- Martínez, C.; Rodiño-Janeiro, B.K.; Lobo, B.; Stanifer, M.L.; Klaus, B.; Granzow, M.; González-Castro, A.M.; Salvo-Romero, E.; Alonso-Cotoner, C.; Pigrau, M.; et al. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut 2017, 66, 1537–1538. [Google Scholar] [CrossRef]
- Muto, S.; Hata, M.; Taniguchi, J.; Tsuruoka, S.; Moriwaki, K.; Saitou, M.; Furuse, K.; Sasaki, H.; Fujimura, A.; Imai, M.; et al. Claudin-2–deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc. Natl. Acad. Sci. USA 2010, 107, 8011–8016. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Imasato, M.; Yamazaki, Y.; Tanaka, H.; Watanabe, M.; Eguchi, H.; Nagano, H.; Hikita, H.; Tatsumi, T.; Takehara, T.; et al. Claudin 2 Deficiency Reduces Bile Flow and Increases Susceptibility to Cholesterol Gallstone Disease in Mice. Gastroenterology 2014, 147, 1134–1145.e10. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Tamura, A.; Takahashi, N.; Tsukita, S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 2013, 144, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Chaturvedi, R.; Olivares-Villagómez, D.; Habib, T.; Asim, M.; Shivesh, P.; Polk, D.B.; Wilson, K.T.; Washington, M.K.; Van Kaer, L.; et al. Targeted colonic claudin-2 expression renders resistance to epithelial injury, induces immune suppression, and protects from colitis. Mucosal Immunol. 2014, 7, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Dubé, É.; Dufresne, J.; Chan, P.T.; Hermo, L.; Cyr, D.G. Assessing the Role of Claudins in Maintaining the Integrity of Epididymal Tight Junctions Using Novel Human Epididymal Cell Lines1. Biol. Reprod. 2010, 82, 1119–1128. [Google Scholar] [CrossRef]
- Zheng, A.; Yuan, F.; Li, Y.; Zhu, F.; Hou, P.; Li, J.; Song, X.; Ding, M.; Deng, H. Claudin-6 and Claudin-9 Function as Additional Coreceptors for Hepatitis C Virus. J. Virol. 2007, 81, 12465–12471. [Google Scholar] [CrossRef]
- Abuazza, G.; Becker, A.; Williams, S.S.; Chakravarty, S.; Truong, H.-T.; Lin, F.; Baum, M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am. J. Physiol. Physiol. 2006, 291, F1132–F1141. [Google Scholar] [CrossRef]
- Fofana, I.; Zona, L.; Thumann, C.; Heydmann, L.; Durand, S.C.; Lupberger, J.; Blum, H.E.; Pessaux, P.; Gondeau, C.; Reynolds, G.M.; et al. Functional Analysis of Claudin-6 and Claudin-9 as Entry Factors for Hepatitis C Virus Infection of Human Hepatocytes by Using Monoclonal Antibodies. J. Virol. 2013, 87, 10405–10410. [Google Scholar] [CrossRef]
- Zavala-Zendejas, V.E.; Torres-Martinez, A.C.; Salas-Morales, B.; Fortoul, T.I.; Montano, L.F.; Rendon-Huerta, E.P. Claudin-6, 7, or 9 overexpression in the human gastric adenocarcinoma cell line AGS increases its invasiveness, migration, and proliferation rate. Cancer Investig. 2011, 29, 1–11. [Google Scholar] [CrossRef]
- Sharma, R.K.; Chheda, Z.S.; Das Purkayastha, B.P.; Gomez-Gutierrez, J.G.; Jala, V.R.; Haribabu, B. A spontaneous metastasis model reveals the significance of claudin-9 overexpression in lung cancer metastasis. Clin. Exp. Metastasis 2016, 33, 263–275. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, R.; Cao, H.; Zhang, H.; Xu, S.; Wang, A.; Liu, B.; Wang, Y.; Wang, R. Expression of claudin-5, -7, -8 and -9 in cervical carcinoma tissues and adjacent non-neoplastic tissues. Int. J. Clin. Exp. Pathol. 2015, 8, 9479–9486. [Google Scholar] [PubMed]
- Birkenhäger, R.; Otto, E.; Schürmann, M.J.; Vollmer, M.; Ruf, E.-M.; Maier-Lutz, I.; Beekmann, F.; Fekete, A.; Omran, H.; Feldmann, D.; et al. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat. Genet. 2001, 29, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Rogan, S.; Yu, A.; Vidal, L.S.; Holmes, J.; Anderson, J.M. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Physiol. 2006, 291, F1288–F1299. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Stuiver, M.; Kausalya, P.J.; Haisch, L.; Krug, S.; Rosenthal, R.; Meij, I.C.; Hunziker, W.; Fromm, M.; Muller, D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J. Cell Sci. 2009, 122, 1507–1517. [Google Scholar] [CrossRef]
- Zemke, A.C.; Snyder, J.C.; Brockway, B.L.; Drake, J.A.; Reynolds, S.D.; Kaminski, N.; Stripp, B.R. Molecular staging of epithelial maturation using secretory cell-specific genes as markers. Am. J. Respir. Cell Mol. Biol. 2009, 40, 340–348. [Google Scholar] [CrossRef]
- Hashizume, A.; Ueno, T.; Furuse, M.; Tsukita, S.; Nakanishi, Y.; Hieda, Y. Expression patterns of claudin family of tight junction membrane proteins in developing mouse submandibular gland. Dev. Dyn. 2004, 231, 425–431. [Google Scholar] [CrossRef]
- Jakab, C.; Halász, J.; Szasz, A.M.; Batmunkh, E.; Kiss, A.; Schaff, Z.; Rusvai, M.; Galfi, P.; Kulka, J. Expression and localisation of claudin-1,-2,-3,-4,-5,-7 and-10 proteins in the normal canine mammary gland. Acta Vet. Hung. 2008, 56, 341–352. [Google Scholar] [CrossRef]
- Hou, J.; Renigunta, A.; Gomes, A.S.; Hou, M.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. USA 2009, 106, 15350–15355. [Google Scholar] [CrossRef]
- Milatz, S.; Himmerkus, N.; Wulfmeyer, V.C.; Drewell, H.; Mutig, K.; Hou, J.; Breiderhoff, T.; Muller, D.; Fromm, M.; Bleich, M.; et al. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl. Acad. Sci. USA 2017, 114, e219–e227. [Google Scholar] [CrossRef]
- Schumann, S.; Buck, V.U.; Classen-Linke, I.; Wennemuth, G.; Grümmer, R. Claudin-3, claudin-7, and claudin-10 show different distribution patterns during decidualization and trophoblast invasion in mouse and human. Histochem. Cell Biol. 2015, 144, 571–585. [Google Scholar] [CrossRef]
- Klar, J.; Piontek, J.; Milatz, S.; Tariq, M.; Jameel, M.; Breiderhoff, T.; Schuster, J.; Fatima, A.; Asif, M.; Sher, M.; et al. Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. PLoS Genet. 2017, 13, e1006897. [Google Scholar] [CrossRef] [PubMed]
- Meyers, N.; Nelson-Williams, C.; Malaga-Dieguez, L.; Kaufmann, H.; Loring, E.; Knight, J.; Lifton, R.P.; Trachtman, H. Hypokalemia Associated with a Claudin 10 Mutation: A Case Report. Am. J. Kidney Dis. 2019, 73, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Breiderhoff, T.; Himmerkus, N.; Drewell, H.; Plain, A.; Gunzel, D.; Mutig, K.; Willnow, T.E.; Muller, D.; Bleich, M. Deletion of claudin-10 rescues claudin-16-deficient mice from hypomagnesemia and hypercalciuria. Kidney Int. 2018, 93, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, W.; Wang, H.; Wang, G. The distinct expression patterns of claudin-10, -14, -17 and E-cadherin between adjacent non-neoplastic tissues and gastric cancer tissues. Diagn. Pathol. 2013, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Renigunta, V.; Himmerkus, N.; Zhang, J.; Renigunta, A.; Bleich, M.; Hou, J. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 2012, 31, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ansar, M.; Andrade, P.B.; Khan, B.; Santos-Cortez, R.L.P.; Ahmad, W.; Leal, S.M. Novel CLDN14 mutations in Pakistani families with autosomal recessive non-syndromic hearing loss. Am. J. Med Genet. Part A 2012, 158, 315–321. [Google Scholar] [CrossRef]
- Wattenhofer, M.; Reymond, A.; Falciola, V.; Charollais, A.; Caille, D.; Borel, C.; Lyle, R.; Estivill, X.; Petersen, M.B.; Meda, P.; et al. Different mechanisms preclude mutant CLDN14 proteins from forming tight junctions in vitro. Hum. Mutat. 2005, 25, 543–549. [Google Scholar] [CrossRef]
- Gong, Y.; Hou, J. Claudin-14 underlies Ca(+)(+)-sensing receptor-mediated Ca(+)(+) metabolism via NFAT-microRNA-based mechanisms. J. Am. Soc. Nephrol. 2014, 25, 745–760. [Google Scholar] [CrossRef]
- Pater, J.A.; Benteau, T.; Griffin, A.; Penney, C.; Stanton, S.G.; Predham, S.; Kielley, B.; Squires, J.; Zhou, J.; Li, Q.; et al. A common variant in CLDN14 causes precipitous, prelingual sensorineural hearing loss in multiple families due to founder effect. Hum. Genet. 2017, 136, 107–118. [Google Scholar] [CrossRef]
- Hou, J. Claudins and mineral metabolism. Curr. Opin. Nephrol. Hypertens. 2016, 25, 308–313. [Google Scholar] [CrossRef]
- Bardet, C.; Courson, F.; Wu, Y.; Khaddam, M.; Salmon, B.; Ribes, S.; Thumfart, J.; Yamaguti, P.M.; Rochefort, G.Y.; Figueres, M.L.; et al. Claudin-16 Deficiency Impairs Tight Junction Function in Ameloblasts, Leading to Abnormal Enamel Formation. J. Bone Miner. Res. 2016, 31, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Kriegs, J.O.; Homann, V.; Kinne-Saffran, E.; Kinne, R.K.H. Identification and subcellular localization of paracellin-1 (claudin-16) in human salivary glands. Histochem. Cell Biol. 2007, 128, 45–53. [Google Scholar] [CrossRef]
- Runggaldier, D.; Pradas, L.G.; Neckel, P.H.; Mack, A.F.; Hirt, B.; Gleiser, C. Claudin expression in the rat endolymphatic duct and sac—First insights into regulation of the paracellular barrier by vasopressin. Sci. Rep. 2017, 7, 45482. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Schlingmann, K.P.; Peters, M.; Nejsum, L.N.; Nielsen, S.; Engel, H.; Grzeschik, K.H.; Seyberth, H.W.; Gröne, H.J.; Nüsing, R.; et al. Primary gene structure and expression studies of rodent paracellin-1. J. Am. Soc. Nephrol. 2001, 12, 2664–2672. [Google Scholar] [PubMed]
- Rangel, L.B.; Sherman-Baust, C.A.; Wernyj, R.P.; Schwartz, D.R.; Cho, K.R.; Morin, P.J. Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene 2003, 22, 7225–7232. [Google Scholar] [CrossRef] [PubMed]
- Fluge, Ø.; Bruland, O.; Akslen, L.A.; Lillehaug, J.R.; Varhaug, J.E. Gene Expression in Poorly Differentiated Papillary Thyroid Carcinomas. Thyroid 2006, 16, 161–175. [Google Scholar]
- Lee, N.P.; Tong, M.K.; Leung, P.P.; Chan, V.W.; Leung, S.; Tam, P.-C.; Chan, K.-W.; Lee, K.-F.; Yeung, W.S.; Luk, J.M. Kidney claudin-19: Localization in distal tubules and collecting ducts and dysregulation in polycystic renal disease. FEBS Lett. 2006, 580, 923–931. [Google Scholar] [CrossRef]
- Martin, T.A.; Harrison, G.M.; Watkins, G.; Jiang, W.G. Claudin-16 reduces the aggressive behavior of human breast cancer cells. J. Cell. Biochem. 2008, 105, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.B.; Lu, Y.; Choate, K.A.; Velazquez, H.; Al-Sabban, E.; Praga, M.; Casari, G.; Bettinelli, A.; Colussi, G.; Rodriguez-Soriano, J.; et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 1999, 285, 103–106. [Google Scholar] [CrossRef]
- Hou, J.; Shan, Q.; Wang, T.; Gomes, A.S.; Yan, Q.; Paul, D.L.; Bleich, M.; Goodenough, D.A. Transgenic RNAi Depletion of Claudin-16 and the Renal Handling of Magnesium. J. Biol. Chem. 2007, 282, 17114–17122. [Google Scholar] [CrossRef]
- Kausalya, P.J.; Amasheh, S.; Gunzel, D.; Wurps, H.; Muller, D.; Fromm, M.; Hunziker, W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J. Clin. Investig. 2006, 116, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Ikari, A.; Hirai, N.; Shiroma, M.; Harada, H.; Sakai, H.; Hayashi, H.; Suzuki, Y.; Degawa, M.; Takagi, K. Association of Paracellin-1 with ZO-1 Augments the Reabsorption of Divalent Cations in Renal Epithelial Cells. J. Biol. Chem. 2004, 279, 54826–54832. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, K.; Furukawa, C.; Fujii, N.; Kimura, T.; Furuta, T.; Matsunaga, T.; Endo, S.; Hasegawa, H.; Anzai, N.; Yamazaki, Y.; et al. The RING finger- and PDZ domain-containing protein PDZRN3 controls localization of the Mg(2+) regulator claudin-16 in renal tube epithelial cells. J. Biol. Chem. 2017, 292, 13034–13044. [Google Scholar] [CrossRef]
- Hou, J.; Renigunta, V.; Nie, M.; Sunq, A.; Himmerkus, N.; Quintanova, C.; Bleich, M.; Renigunta, A.; Wolf, M.T.F. Phosphorylated claudin-16 interacts with Trpv5 and regulates transcellular calcium transport in the kidney. Proc. Natl. Acad. Sci. USA 2019, 116, 19176–19186. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Mascarell, P.; Schirrmacher, C.E.; Martínez-Cruz, L.A.; Muller, D. Novel Aspects of Renal Magnesium Homeostasis. Front. Pediatr. 2018, 6, 77. [Google Scholar] [CrossRef]
- Yamaguti, P.M.; Dos Santos, P.A.C.; Leal, B.S.; Santana, V.B.B.D.M.; Mazzeu, J.F.; Acevedo, A.C.; Neves, F.D.A.R. Identification of the first large deletion in the CLDN16 gene in a patient with FHHNC and late-onset of chronic kidney disease: Case report. BMC Nephrol. 2015, 16, 92. [Google Scholar] [CrossRef]
- Konrad, M.; Hou, J.; Weber, S.; Dötsch, J.; Kari, J.A.; Seeman, T.; Kuwertz-Bröking, E.; Peco-Antic, A.; Tasic, V.; Dittrich, K.; et al. CLDN16 genotype predicts renal decline in familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J. Am. Soc. Nephrol. 2008, 19, 171–181. [Google Scholar] [CrossRef]
- Niimi, T.; Nagashima, K.; Ward, J.M.; Minoo, P.; Zimonjic, D.B.; Popescu, N.C.; Kimura, S. claudin-18, a Novel Downstream Target Gene for the T/EBP/NKX2.1 Homeodomain Transcription Factor, Encodes Lung- and Stomach-Specific Isoforms through Alternative Splicing. Mol. Cell. Biol. 2001, 21, 7380–7390. [Google Scholar] [CrossRef]
- Wolburg, H.; Wolburg-Buchholz, K.; Liebner, S.; Engelhardt, B. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci. Lett. 2001, 307, 77–80. [Google Scholar] [CrossRef]
- Fromm, M.; Piontek, J.; Rosenthal, R.; Gunzel, D.; Krug, S.M. Tight junctions of the proximal tubule and their channel proteins. Pflüg. Arch. 2017, 469, 877–887. [Google Scholar] [CrossRef]
- Gong, Y.; Renigunta, V.; Zhou, Y.; Sunq, A.; Wang, J.; Yang, J.; Renigunta, A.; Baker, L.A.; Hou, J. Biochemical and biophysical analyses of tight junction permeability made of claudin-16 and claudin-19 dimerization. Mol. Biol. Cell 2015, 26, 4333–4346. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Renigunta, A.; Konrad, M.; Gomes, A.S.; Schneeberger, E.E.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Investig. 2008, 118, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Meier, W.; Blumberg, A.; Imahorn, W.; De Luca, F.; Wildberger, H.; Oetliker, O. Idiopathic hypercalciuria with bilateral macular colobomata: A new variant of oculo-renal syndrome. Helvetica Paediatr. Acta 1979, 34, 257–269. [Google Scholar]
- Rodriguez-Soriano, J.; Vallo, A. Pathophysiology of the renal acidification defect present in the syndrome of familial hypomagnesaemia-hypercalciuria. Pediatr. Nephrol. 1994, 8, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kondoh, M.; Suzuki, H.; Yagi, K. Claudin as a target for drug development. Curr. Med. Chem. 2011, 18, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.S.; Thongprayoon, C.; Cheungpasitporn, W.; Sakhuja, A.; Mao, M.A.; Erickson, S.B. Incidence and characteristics of kidney stones in patients with horseshoe kidney: A systematic review and meta-analysis. Urol. Ann. 2018, 10, 87–93. [Google Scholar]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).